Abstract
Purpose
The risk of thermal damage increases with the introduction of high-power lasers during holmium laser lithotripsy. This study aimed to quantitatively evaluate the temperature change of renal calyx in the human body and the 3D printed model during high-power flexible ureteroscopic holmium laser lithotripsy and map out the temperature curve.
Methods
The temperature was continuously measured by a medical temperature sensor secured to a flexible ureteroscope. Between December 2021 and December 2022, willing patients with kidney stones undergoing flexible ureteroscopic holmium laser lithotripsy were enrolled. High frequency and high-power settings (24 W, 80 Hz/0.3 J and 32 W, 80 Hz/0.4 J) were performed for each patient with room temperature (25 °C) irrigation. In the 3D printed model, we studied more holmium laser settings (24 W, 80 Hz/0.3 J, 32 W, 80 Hz/0.4 J and 40 W, 80 Hz/0.4 J) with warmed (37 °C) and room temperature (25 °C) irrigation.
Results
Twenty-two patients were enrolled in our study. With 30 ml/min or 60 ml/min irrigation, the local temperature of the renal calyx did not reach 43 °C in any patient under 25 °C irrigation after 60 s laser activation. There were similar temperature changes in the 3D printed model with the human body under the irrigation of 25 °C. Under the irrigation of 37 °C, the temperature rise slowed down, but the temperature in the renal calyces was close to or even exceeded the 43 °C at the setting of 32 W, 30 ml/min and 40 W, 30 ml/min after continuing laser activation.
Conclusion
In the irrigation of 60 ml/min, the temperature in the renal calyces can still be maintained within a safe range after continuous activation of a holmium laser up to 40 W. However, continuous activation of 32 W or higher power holmium laser in the renal calyces for more than 60 s in the limited irrigation of 30 ml/min can cause excessive local temperature, in such situation room temperature perfusion at 25 ℃ may be a relatively safer option.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The holmium:yttrium–aluminum-garnet (Ho:YAG) laser is the most frequently used device for laser lithotripsy of urinary stones [1]. With the ever-increasing power, pulse frequency capabilities and the addition of pulse modulation, especially the emergence of a high-power 120W Holmium laser generator with Moses technology which has less energy loss and lower stone retropulsion, the application scenario of holmium laser lithotripsy has been expanded [2,3,4,5], which improves the efficiency and effect of lithotripsy and makes it possible to treat renal stones larger than 2 cm (cm) in diameter with a flexible ureteroscopy [6,7,8].
Dusting and popcorning are common principles in ureteroscopic lithotripsy, which can create stone dust and tiny fragments (usually less than 1–2 mm/mm in diameter) to enable spontaneous passage of the small particles [9]. Their basic requirements are high frequency and long laser action time (usually longer than 2 min) [10, 11].
However, the risk of thermal damage increases with the introduction of high-power lasers and the lasting time of laser activation [12]. The primary mechanism of thermal injury is protein denaturation, leading to severe complications such as ureteral stenosis. Higher temperature irrigation fluid, continuous laser activation and limited irrigation flow can promote this injury [13,14,15]. It is generally believed that a temperature of 43 °C lasting 120 min can cause thermal damage to various tissues [16]. Teng et al. found in the human body that the fluid temperature after a 20-W laser for 60 s can stay below 43 °C with 37 °C irrigation at 15 or 30 ml/min [17]. High-power holmium settings can induce potentially injurious temperature elevations in laboratory "caliceal" models and the porcine in vivo model [13,14,15, 18]. However, there are no studies on the holmium laser's real intraoperative calyceal temperature change with power above 20 watts (W). In this study, we aim to use Moses holmium laser with more than 20W in the human body and the 3D printed model to study the safety of lithotripsy.
Material and methods
Temperature measurement
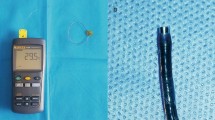
The temperature measurement device consists of a medical temperature sensor (Jingpu, China) (Fig. 1) and a medical monitor (Philips, The Netherlands). The temperature of the surgical area was measured with the sensor attached to Olympus fiber-optic flexible ureteroscope (Olympus, Japan), with its tip 5 mm from the distal end (Fig. 1) and inserted into the renal pelvis with the flexible ureteroscope through a 12/14 Fr single lumen ureteral access sheath (Cook, USA).
In the human body
After obtaining ethics approval (Approval no. K2021127 and no.2022-0893), the study included patients who underwent flexible ureteroscopic and holmium laser lithotripsy between December 2021 and October 2022. Inclusion criteria were as follows: (1) 18–70 years of age; (2) American Society of Anesthesiologists (ASA) grade I or II; (3) clinically confirmed calyceal stones. Exclusion criteria were as follows: (1) preoperative fever; (2) preoperative urinary tract infection; (3) preoperative ureteral stricture.
In this study, we used high frequency and high-power settings (24 W, 80 Hz/0.3 J and 32 W, 80 Hz/0.4 J) with a 200 μm laser fiber (Lumenis, USA) and Lumenis Pulse 120H Holmium Laser System with MOSES Technology (Lumenis, USA) to perform lithotripsy. The flow rate of 30 ml/min and 60 ml/min saline irrigation (controlled at 25 °C to reduce the risk of fluid overheating during lithotripsy) was delivered using a pumping system (Yida, China).
We continuously irrigated of 30 or 60 ml/min during each trial. We first tried to reach a stable temperature in the renal calyx where the stones were located through irrigation, after which the laser was activated for 60 s and temperature variations were documented every 5 s.
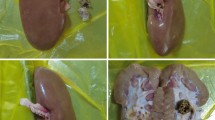
In the 3D printed model
The kidney model (Fig. 2) was obtained by 3D printing, and its data was from CT imaging files of the normal urinary system, available from a standard library (www.slicer.org). In the model, we used more holmium laser settings (24 W, 80 Hz/0.3 J, 32 W, 80 Hz/0.4 J and 40 W, 80 Hz/0.4 J), two different irrigation temperatures (25 °C and 37 °C) and two irrigation rates (30 ml/min and 60 ml/min). We conducted experiments and recorded temperatures on the kidney model's upper, middle and lower calyces. All experiments were repeated three times. In each experiment, we placed the laser fiber in the renal calyx and released the laser continuously for 60 s after the temperature was stabilized through continuous irrigation.
Statistical analysis
Statistical analyses were performed with GraphPad Prism version 9 (GraphPad Software Inc., USA) and SPSS 22.0 (IBM Corporation, USA) software packages. Data were presented as the mean ± SD. Paired t test was used to analyze the differences between group. p < 0.05 was considered to be statistically significant.
Results
Effect of flexible ureteroscopic holmium laser lithotripsy on the calyceal temperature in the human body
Twenty-two patients who experienced all four parameter settings were included in our study. The clinical and perioperative characteristics are summarized in Tables 1 and 2.
The overall temperature change curve is shown in Fig. 3. Under the irrigation setting of 60 ml/min, the intrarenal temperatures increased sharply during the first 10 s of laser activation. After 20 s, the temperature tended to stabilize (Fig. 3A). Under the irrigation setting of 30 ml/min, the intrarenal temperatures increased sharply during the first 20 s of laser activation. After 30 s, the temperature tended to stabilize (Fig. 3B).
With the same irrigation flow rate, the group with higher laser power setting flow had higher temperature rise (24W 30 ml/min vs. 32W 30 ml/min: 10.03 ± 0.88 vs. 13.32 ± 1.17, 24W 60 ml/min vs. 32W 60 ml/min: 6.91 ± 0.95 vs. 8.33 ± 0.85) (Fig. 4A, B). With the same laser power setting, the group with lower irrigation flow had higher temperature rise (24W 30 ml/min vs. 24W 60 ml/min: 10.03 ± 0.88 vs. 6.91 ± 0.95, 32W 30 ml/min vs. 32W 60 ml/min: 13.32 ± 1.17 vs. 8.33 ± 0.85) (Fig. 4C, D), and the difference between groups was also significant. The local temperature of the renal calyx did not reach 43 °C in any of these groups under irrigated conditions at 25 °C. If a higher irrigation temperature is used, the local temperature may exceed 43 °C due to the higher starting temperature. Therefore, choosing the appropriate perfusion temperature is advisable when laser activation for more than 60 s is required. Otherwise, the laser activation has to be stopped promptly.
Temperature changes after 60 s of laser activation (A Mean temperature changes after 60-s laser activation at 30 ml/min. B Mean temperature changes after 60-s laser activation at 60 ml/min. C Mean temperature changes after 60-s laser activation at 24 W. D Mean temperature changes after 60-s laser activation at 32 W) ***p < 0.001
Effect of flexible ureteroscopic holmium laser lithotripsy on the calyceal temperature in the 3D printed model
We tested more parameter settings in the 3D printed model. Under saline irrigation at 25 °C, there were similar temperature changes within the model and in vivo (Figs. 3, 5). None group reached the alert temperature after 60 s of laser activation (Table 3).
Under saline irrigation at 37 °C, the temperature rise slowed down (Fig. 6). However, the temperature in the renal calyces was close to or even exceeded the alert temperature at the setting of 32 W, 30 ml/min and 40 W, 30 ml/min after continuing laser activation (Table 4). When the laser power of over 32 W is continuously released, the 37 °C irrigation is unsuitable.
Discussion
Our study is the first study of temperature changes under the release of high-power holmium laser in the renal calyces of patients who have undergone flexible ureteroscopy and reveals an accurate temperature change curve. The high-power laser platform made continuous dusting and popcorn possible and improved the efficiency of flexible ureteroscope lithotripsy. However, it has raised concerns about the increased temperature of the calyces during laser activation, which increases the risk of thermal injury and has been the subject of many studies [12,13,14,15, 17, 19,20,21,22,23,24]. The cumulative equivalent minutes at 43 °C (CEM43) is a good indicator of the degree of thermal damage to the tissue. CEM43 over 120 min can commonly cause thermal damage in various tissues [16]. Animal experiments have shown that CEM 43 longer than 80 min causes significant bladder damage, and CEM 43 longer than 70 min causes considerable kidney damage [25,26,27]. Hein et al. found that high-power laser settings, particularly higher than 30 W, have an elevated risk of liquid overheating in postmortem porcine kidneys [28]. High irrigation was required for safety in the porcine when the high-power holmium laser was continuously released [14]. Previous studies were conducted in vitro, ex vivo, or in a small number of live Yorkshire pigs, which all pointed out that high-power lasers may induce injurious temperatures and prompted the desire to go further in the human body to clarify thermal safety envelope and accurately simulate clinical situations [13, 14, 19, 21, 23, 24].
The temperature change in renal calyces is affected by the temperature of irrigation. In our study, under 25 °C room temperature irrigation, the fluid in the kidney could still be maintained at a safe temperature with the continuous activation of holmium laser power up to 32W. However, saline irrigation at 37 °C may increase the risk of overheating at the same power, according to the model and in vivo data. It's essential for surgeons to carefully consider the temperature of the irrigation liquid when planning to release the laser to improve efficiency continuously. In a randomized controlled study, He et al. found that room temperature irrigation did not increase the risk of postoperative infection, which was consistent with our findings [29]. Therefore, in the case of continuous laser activation, room temperature irrigation is a better choice.
It is well known that the slow down of irrigation and the increase of holmium laser power can accelerate and increase the rise of temperature, as confirmed in our study, and a numeric reference was given [13, 14]. Saline irrigation can clear vision and is essential to prevent liquid overheating. In vivo and in vitro experiments have proved that the temperature can rise rapidly to a dangerous level without irrigation or insufficient irrigation [13, 14, 17, 23]. Lower power and higher irrigation rates are feasible when overheating the fluid is a concern. Otherwise, room temperature or chilled irrigation, which can provide a lower starting temperature, may also be a better choice [22]. In our study, we chose the constant irrigation rate instead of the constant irrigation pressure used in previous studies to map out the temperature curve, because constant pressure would show different irrigation speeds when encountering different intrarenal pressures.
Power determines the temperature rather than the frequency or energy in the laser lithotripsy [12, 13]. Our study is instructive for working conditions at the same power. At high power, such as 40 W, the temperature in the renal calyx can still be within a safe range for a short time. Our study within the model was consistent with that in vivo, and more research can be carried out in the future, as the model showed convenience and security.
Our study had certain limitations. Firstly, due to the limited number of patients and safety concerns, no additional attempts were made to reveal the temperature variations at different holmium laser parameters in more detail. Secondly, we did not compare the difference in temperature change between the presence and absence of stones during laser release. Thirdly, we did not use more thermometers or thermal imaging instruments to observe the temperature of multiple sites in the renal calyces during laser release. Fourthly, this research was lacking in the comparison for patients with different frequency and power setting and a randomized controlled trial should be designed in the future to evaluate the postoperative complication between the different frequency and power setting.
Conclusion
In the irrigation of 60 ml/min, the temperature in the renal calyces can still be maintained within a safe range after continuous activation of a holmium laser up to 40 W. However, continuous activation of 32W or higher power holmium laser in the renal calyces for more than 60 s in the limited irrigation of 30 ml/min can cause excessive local temperature, in such situation room temperature perfusion at 25 ℃ may be a relatively safer option.
Data statement
The data used and analyzed in the current study are available from the corresponding author on reasonable request.
References
Terry RS, Whelan PS, Lipkin ME (2020) New devices for kidney stone management. Curr Opin Urol 302:144–148. https://doi.org/10.1097/MOU.0000000000000710
Aldoukhi AH, Roberts WW, Hall TL, Ghani KR (2017) Holmium laser lithotripsy in the new stone age: dust or bust? Front Surg 4:57. https://doi.org/10.3389/fsurg.2017.00057
Elhilali MM, Badaan S, Ibrahim A, Andonian S (2017) Use of the moses technology to improve holmium laser lithotripsy outcomes: a preclinical study. J Endourol 31(6):598–604. https://doi.org/10.1089/end.2017.0050
Pietropaolo A, Hughes T, Mani M, Somani B (2021) Outcomes of ureteroscopy and laser stone fragmentation (URSL) for kidney stone disease (KSD): comparative cohort study using MOSES technology 60 W laser system versus regular holmium 20 W laser. J Clin Med 10(13):2742. https://doi.org/10.3390/jcm10132742
Pietropaolo A, Jones P, Whitehurst L, Somani BK (2019) Role of “dusting and pop-dusting” using a high-powered (100 W) laser machine in the treatment of large stones (>/= 15 mm): prospective outcomes over 16 months. Urolithiasis 47(4):391–394. https://doi.org/10.1007/s00240-018-1076-4
Hu W, Li J (2020) Advances in laser techniques for stone treatment. J Clin Surg 28:183–185. https://doi.org/10.3969/j.issn.1005-6483.2020.02.027
Aboumarzouk OM, Monga M, Kata SG, Traxer O, Somani BK (2012) Flexible ureteroscopy and laser lithotripsy for stones > 2 cm: a systematic review and meta-analysis. J Endourol 26(10):1257–1263. https://doi.org/10.1089/end.2012.0217
Cohen J, Cohen S, Grasso M (2013) Ureteropyeloscopic treatment of large, complex intrarenal and proximal ureteral calculi. BJU Int 111(3 Pt B):E127-131. https://doi.org/10.1111/j.1464-410X.2012.11352.x
Weiss B, Shah O (2016) Evaluation of dusting versus basketing—can new technologies improve stone-free rates? Nat Rev Urol 13(12):726–733. https://doi.org/10.1038/nrurol.2016.172
Aldoukhi AH, Roberts WW, Hall TL, Teichman JMH, Ghani KR (2018) Understanding the popcorn effect during holmium laser lithotripsy for dusting. Urology 122:52–57. https://doi.org/10.1016/j.urology.2018.08.031
Klaver P, de Boorder T, Rem AI, Lock T, Noordmans HJ (2017) In vitro comparison of renal stone laser treatment using fragmentation and popcorn technique. Lasers Surg Med 49(7):698–704. https://doi.org/10.1002/lsm.22671
De Coninck V, Defraigne C, Traxer O (2021) Watt determines the temperature during laser lithotripsy. World J Urol 40(5):1257–1258. https://doi.org/10.1007/s00345-021-03848-6
Aldoukhi AH, Ghani KR, Hall TL, Roberts WW (2017) Thermal response to high-power holmium laser lithotripsy. J Endourol 31(12):1308–1312. https://doi.org/10.1089/end.2017.0679
Aldoukhi AH, Hall TL, Ghani KR, Maxwell AD, MacConaghy B, Roberts WW (2018) Caliceal fluid temperature during high-power holmium laser lithotripsy in an in vivo porcine model. J Endourol 32(8):724–729. https://doi.org/10.1089/end.2018.0395
Maxwell AD, MacConaghy B, Harper JD, Aldoukhi AH, Hall TL, Roberts WW (2019) Simulation of laser lithotripsy-induced heating in the urinary tract. J Endourol 33(2):113–119. https://doi.org/10.1089/end.2018.0485
Sapareto SA, Dewey WC (1984) Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys 10(6):787–800. https://doi.org/10.1016/0360-3016(84)90379-1
Teng J, Wang Y, Jia Z, Guan Y, Fei W, Ai X (2021) Temperature profiles of calyceal irrigation fluids during flexible ureteroscopic Ho:YAG laser lithotripsy. Int Urol Nephrol 53(3):415–419. https://doi.org/10.1007/s11255-020-02665-x
Taratkin M, Laukhtina E, Singla N, Kozlov V, Abdusalamov A, Ali S et al (2020) Temperature changes during laser lithotripsy with Ho:YAG laser and novel Tm-fiber laser: a comparative in-vitro study. World J Urol 38(12):3261–3266. https://doi.org/10.1007/s00345-020-03122-1
Aldoukhi AH, Black KM, Hall TL, Ghani KR, Maxwell AD, MacConaghy B et al (2020) Defining thermally safe laser lithotripsy power and irrigation parameters. In Vitro Model J Endourol 34(1):76–81. https://doi.org/10.1089/end.2019.0499
Aldoukhi AH, Dau JJ, Majdalany SE, Hall TL, Ghani KR, Hollingsworth JM et al (2021) Patterns of laser activation during ureteroscopic lithotripsy: effects on caliceal fluid temperature and thermal dose. J Endourol 35(8):1217–1222. https://doi.org/10.1089/end.2020.1067
Belle JD, Chen R, Srikureja N, Amasyali AS, Keheila M, Baldwin DD (2022) Does the novel thulium fiber laser have a higher risk of urothelial thermal injury than the conventional holmium laser in an in vitro study? J Endourol 36(9):1249–1254. https://doi.org/10.1089/end.2021.0842
Dau JJ, Hall TL, Maxwell AD, Ghani KR, Roberts WW (2021) Effect of chilled irrigation on caliceal fluid temperature and time to thermal injury threshold during laser lithotripsy. In Vitro Model J Endourol 35(5):700–705. https://doi.org/10.1089/end.2020.0896
Rezakahn Khajeh N, Hall TL, Ghani KR, Roberts WW (2022) Pelvicaliceal volume and fluid temperature elevation during laser lithotripsy. J Endourol 36(1):22–28. https://doi.org/10.1089/end.2021.0383
Williams JG, Goldsmith L, Moulton DE, Waters SL, Turney BW (2021) A temperature model for laser lithotripsy. World J Urol 39(6):1707–1716. https://doi.org/10.1007/s00345-020-03357-y
Haveman J, Smals OA, Rodermond HM (2003) Effects of hyperthermia on the rat bladder: a pre-clinical study on thermometry and functional damage after treatment. Int J Hyperth 19(1):45–57. https://doi.org/10.1080/02656730210158455
He X, McGee S, Coad JE, Schmidlin F, Iaizzo PA, Swanlund DJ et al (2004) Investigation of the thermal and tissue injury behaviour in microwave thermal therapy using a porcine kidney model. Int J Hyperth 20(6):567–593. https://doi.org/10.1080/0265673042000209770
Yarmolenko PS, Moon EJ, Landon C, Manzoor A, Hochman DW, Viglianti BL et al (2011) Thresholds for thermal damage to normal tissues: an update. Int J Hyperth 27(4):320–343. https://doi.org/10.3109/02656736.2010.534527
Hein S, Petzold R, Suarez-Ibarrola R, Muller PF, Schoenthaler M, Miernik A (2020) Thermal effects of Ho:YAG laser lithotripsy during retrograde intrarenal surgery and percutaneous nephrolithotomy in an ex vivo porcine kidney model. World J Urol 38(3):753–760. https://doi.org/10.1007/s00345-019-02808-5
He Y, Feng YG, He J, Liang B, Jiang MD, Liu J et al (2021) Effects of irrigation fluid temperature during flexible ureteroscopic holmium laser lithotripsy on postoperative fever and shivering: a randomized controlled trial. BMC Urol 21(1):72. https://doi.org/10.1186/s12894-021-00841-4
Funding
All authors report that this work was supported by the National Natural Science Foundation of China (No. 81871153 to Jiaming Wen, No. 81970601 to Bohan Wang and No. 82200850 to Jingchao Wei).
Author information
Authors and Affiliations
Contributions
Conception and design: ZW, JW and JW. Collection and assembly of data: GH, KQ, ZJ, WZ and BW. Analysis and interpretation of the data: CS and QH. Drafting of the manuscript: ZW and JW. Revision of the manuscript: JW. Supervision: JW.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conficts of interest.
Ethical approval
The study procedures were approved by the institutional ethics committee of the Second Affiliated Hospital of Zhejiang University (No. 2022-0893) and the institutional ethics committee of the Fourth Affiliated Hospital of Zhejiang University (No. K2021127). This research was registered in National Health Security Information Platform, medical research registration, and filing information system in November 2021, and the unique identification number is MR-33-22-003044.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, Z., Wei, J., Sun, C. et al. Temperature changes of renal calyx during high-power flexible ureteroscopic Moses holmium laser lithotripsy: a case analysis study. Int Urol Nephrol 55, 1685–1692 (2023). https://doi.org/10.1007/s11255-023-03611-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03611-3