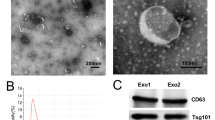
Abstract
Purpose
To determine expression differences of urine exosomal miR-19b1-5p, 21-5p, 136-3p, 139-5p, 210-3p and concentration differences of urinary BLCA-4, NMP22, APE1/Ref1, CRK, VIM between bladder cancer, follow-up patients, and control samples, to evaluate diagnostic importance of these differences and establish a diagnostic panel for bladder cancer.
Methods
Urine samples of 59 bladder cancer patients, 34 healthy controls, and 12 follow-up patients without recurrence were enrolled to this study. Real-time PCR and ELISA were performed to determine urine exosomal miR-19b1-5p, 21-5p, 136-3p, 139-5p, 210-3p expressions and urinary BLCA-4, NMP22, APE1/Ref1, CRK, VIM, creatinine concentrations. Logistic regression analyses were performed to determine the diagnostic panel, the sensitivity, and specificity of the panel assessed by the ROC curve analysis. p values < 0.05 were considered statistically significant.
Results
In bladder cancer risk groups, mir-139, -136, -19 and 210 expressions or positivity were found to be different and concentrations of urinary Ape1/Ref1, BLCA4, CRK, and VIM increased by twofold on average compared to healthy controls. Logistic regression and ROC analyses revealed that panel could differentiate bladder cancer patients from healthy controls with 80% sensitivity and 88% specificity (AUC = 0.899), low-risk patients from controls with 93% sensitivity and 95.5% specificity (AUC = 0.976). Despite the low number of samples, our findings suggest that urine exosomal miR-19b1-5p, 136-3p, 139-5p expression, and urinary APE1/Ref1, BLCA-4, CRK concentrations are promising candidates in terms of bladder cancer diagnosis.
Conclusions
Although our panel has great sensitivity for early detection of BC, it needs to be validated in larger populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bladder cancer (BC) is the ninth most lethal malignancy in men [1]. Cystoscopy, with a sensitivity of 90%, is the golden standard for the diagnosis of bladder cancer, and it is an invasive method that can cause small tumors to be overlooked [2]. There are currently six Food and Drug Administration (FDA)-approved tests for clinical use, sensitivity of these kits for BC diagnosis ranges from 57–82 to 74–88% and the sensitivity increases as the stage and degree of cancer increases [3]. Although these tests have been approved by the FDA for clinical use, they are not sufficiently informative for clinical use, since their specificity and sensitivities are too low [4].
Exosomal miRNAs are strong candidates for diagnosis, prognosis, and clinical follow-up for BC since they are stable and resistant to various storage conditions and could be isolated from a wide range of biological samples non-invasively [5].
Urinary miR-136-3p, which targets the Notch3 gene, expressed only in urine of BC patients, it was absent in healthy controls (HC) [6]. The expression of miR-210-3p and miR-96 was increased in urine samples of BC patients compared to HC. Combination of HYAL1, lncRNA-UCA1, miR-210-3p, and miR-96 had 100% sensitivity and 89% specificity in the diagnosis of BC (AUC = 0.981) [7]. miR-21 targets number of genes associated with apoptosis and cell cycle such as PTEN and BCL2. It is expressed in tumor tissues, white blood cells, urine supernatants, and urine exosomes of BC patients and may have a diagnostic importance for BC [8, 9]. miR-139-5p/3p is downregulated in BC tissues, causing an increased invasion and migration [10, 11]. miR-19b1-5p, an oncogenic miRNA that targets the PTEN gene, is expressed in the urine supernatant and tissue samples of BC patients more than HC [12,13,14]. miR-16-5p is thought to be a tumor-suppressor miRNA that targets the CCND1 gene [12]. Its expression was found to be increased in total urine, urine supernatant, and tissue samples of BC patients, compared to controls, but different normalization methods were used in studies [9, 15].
Ape1/Ref1 activates many proteins in apoptosis, angiogenesis, and survival pathways, which causes a more aggressive phenotype in cancer. Urinary Ape1/Ref1 concentration of BC patients was higher than healthy controls (HC), and it is correlated with stage and grade of tumor, indicating a diagnostic biomarker potential [16]. BLCA4 is a nuclear matrix protein that specifically targets BC tissues, unaffected by the presence of infection, cystitis [17]. A proto-oncogene product, Crk, is an adapter protein that regulates cell adhesion and migration, induces EMT and metastasis in BC cells via the HGF/c-Met loop [18]. Urinary Crk concentration in BC patients has not been studied yet and since it is involved in the molecular pathogenesis of BC, it has been included in this study. Nuclear matrix proteins are structural components of the cell nucleus. NMP22, an FDA-approved diagnostic molecule for BC, is more common in malignant urothelial cells than healthy urothelium and is released into the urine as a result of apoptosis [19]. Upregulation of vimentin (VIM) and fibronectin, the epithelial mesenchymal transition markers, are associated with MIBC diagnosis, resistance to treatment and poor prognosis [20]. However, urinary VIM concentration in BC samples was not evaluated before.
In the present study, our main goal was to establish a diagnostic panel for bladder cancer diagnosis. For this purpose, we determined expression differences of urine exosomal miR-19b1-5p, 21-5p, 136-3p, 139-5p, 210-3p and concentration differences of urinary BLCA-4, NMP22, APE1/Ref1, CRK, VIM between bladder cancer patients, follow-up patients and control samples. Then, we examined the molecules that differ between the groups by logistic regression analysis.
Materials and methods
Patients and samples
All samples were obtained from the Marmara University Research and Educational Hospital Urology department, between 2016 and 2018. All patients were diagnosed with cystoscopy and risk stratification was done according to European and American Urology Association Guidelines (Table S1, S2). Urine samples of 59 BC patients, 34 HC, and 12 follow-up patients without recurrence were enrolled to this study (Table 1). Due to insufficient urine sample, urinary protein concentrations of five BC patients and two follow-up patients could not been investigated.
Sample collection and processing
30 mL urine was collected prior to any surgical intervention. After centrifugation, upper phase was used for exosomal miRNA isolation and urinary protein quantification according to manufacturers’ recommendations. (see supplementary material for detailed information). Spot urine protein concentrations were normalized according to creatinine to rule out physiological changes such as dehydration [21].
Statistical analysis
Statistical analysis was performed using SPSS, GraphPad and MedCalc programs. All analyses with p < 0.05 were considered statistically significant. miRNA expression levels and also presence/absence status were analyzed. In RT-PCR experiments, statistical evaluations were made with ΔCt values and fold change was expressed using the 2−∆∆Ct values if significant difference was observed. The photometric values obtained as a result of ELISA test were evaluated with four-parameter logistic regression analysis. Urinary protein concentrations were normalized to creatinine to rule out physiological changes such as dehydration [21, 22]. Concentration differences between groups were analyzed by Kruskal–Wallis test and the Mann–Whitney U test was used to compare two groups. Logistic regression analysis was performed to determine the diagnostic panel, the sensitivity, and specificity of the panel assessed by the receiver operation curve (ROC) analysis. The logistic regression model was analyzed with Hosmer and Lemeshow tests. If the result is greater than 0.05, it shows that the fit between model and data is sufficient [23].
Results
Properties of sample groups and determination of parameters to be included in logistic regression
Sample properties are summarized in Table 1. miRNA expressions and urinary protein concentrations were compared between HC, BC, and follow-up patients. In addition, in terms of expression and concentration, bladder cancer risk groups were analyzed if they differ from each other or from healthy controls.
Exosomal miR-16-5p and -21-5p expressions observed in all BC patients, HC and follow-up patients. Of the 105 specimens, 39 had miR-136-3p expression, 100 had miR-210-3p expression, 65 had miR-139-5p expression, and 55 had miR-19b1-5p expression (Table 2).
We observed significant differences in the percentages of miR-136-3p, miR-210-3p, mir-139-5p, mir19b1-5p expressing samples or in the expression levels of samples between BC, HC, and follow-up patients or between risk groups and controls or between primary tumor, recurrent tumor and HC (Table S3–8, Figure S1–6).
A total of 55 BC, 34 HC, and 10 follow-up patients were examined by ELISA. It was determined that urinary Ape1/Ref1, BLCA4, Crk, and VIM concentrations between groups differ significantly and there was no significant difference in urinary NMP22 concentrations between samples. The diagnostic power of urinary protein concentrations for BC, investigated by ROC analyses (Figure S7–10).
Diagnostic and prognostic biomarker panel for bladder cancer
Logistic regression analysis of urine exosomal miRNA expression/presence and urinary protein concentrations was performed for miRNAs/proteins that were significantly different between the groups. The logistic regression model was examined with Hosmer and Lemeshow test (p = 0.9879), the result was greater than 0.05, indicating that the fit between model and data was adequate [23]. All variables included in logistic regression analysis contributed significantly to the panel (p < 0.05). The coefficient, standard error, odds ratio and p values are summarized in the table S9. The resulting logistic regression model is formulated below:
ROC curve analysis of logistic regression results showed that the panel obtained had AUC = 0.899 (95% confidence interval (CI) 0.822–0.950) for discriminating BC patients from non-cancerous ones. The sensitivity and specificity of the panel for the threshold value > 0.571 were 80.0% and 86.4%, respectively. The ROC curve and dot diagram for the cut off value of 0.571 are shown in the Fig. 1a, b. When follow-up patients excluded, panel discriminated BC patients from HC with 80% sensitivity and 88.2 specificity (Fig. 1c, d).
a ROC analysis of logistic regression model for bladder cancer patients and non-cancerous samples. b Dot diagram of the panel for bladder cancer patients and non-cancerous samples. c ROC analysis of logistic regression model for bladder cancer patients and healthy controls. d Dot diagram of the panel for bladder cancer patients and healthy controls
Logistic regression model analyzed to distinguish LR patients from HC. ROC revealed that AUC = 0.976 (95% confidence interval (CI) 0.866–0.999), 93.33 sensitivity, and 97.06% specifity for threshold value of 0.63383 (Fig. 2a, b). If threshold value is adjusted to 0.571 (the main cut-off value of the panel), sensitivity and specificity of the panel shifts to 93.3% and 88.2%, respectively.
Panel could discriminate LR BC patients from non-cancerous samples (HC and follow-up patients), indicating that panel is not affected by residual effects of BC in follow-up patients (Fig. 3a, b). (AUC = 0.976, sensitivity = 93.3% and specificity = 95.5%; cut-off = 0.6976).
Discussion
Urinary biomarkers are of great importance for the diagnosis and follow-up of BC [3, 5, 9, 24, 25]. We used urine exosomal miRNAs, since miRNAs in urinary exosomes are more conserved than physiological conditions, and exosomes are not random cellular vesicles, they are both affected by various cellular events and directing various cellular processes such as intercellular communication [5, 22, 24, 26, 27].
In a single study that examined urinary miR-136-3p expression, Weber et al. found that miR-136-3p was expressed in only BC not in HC, but our study refuted this finding [6]. 44% of BC samples, 23% of HC and 42% of follow-up patients expressed miR-136-3p. In 8 of 34 HC, miR-136-3p expression was detected. According to logistic regression analysis, miR-136-3p contributed significantly to the diagnostic panel with an odds ratio of 3.6657 (p = 0.0363). (Table 2, S3, S4, S5, S6, Figure S1).
Several studies have reported that miR-139-5p is downregulated in BC tissues [10, 11]. Furthermore, the MMP11, a direct target of miR-139-5p, associated with shorter overall survival [11]. We found that, miR-139-5p expression in the group of metastatic patients was lower than the other patient groups followed by HC. Interestingly, the follow-up patients had the highest expression. The expression of miR-139-5p increased more than twofold in the low-intermediate and HR groups compared to the muscle-invasive metastatic group, miR-139-5p presence contributed significantly to diagnostic panel (p = 0.0389). (Figure S3, S4, Table S8).
It was found that miR-19b1-5p, an oncogenic miRNA that targets PTEN gene [12], expression levels in urine supernatants [14] and tissue samples of BC patients were higher compared to controls [13]. In the muscle-invasive metastatic patient group, the expression of miR-19b1-5p was lower than the LIR group and the HR group (p = 0.05 and p = 0.013, respectively). When evaluated according to sub-risk groups, we determined that muscle-invasive group had lower expression than HC, LR, and HR group patients. (p = 0.013; p = 0.016 and p = 0.002, respectively). miR-19b1-5p expression of HC was about 4.3-folds of invasive patients and the expression of NMIBC samples was 14.8-folds of invasive BC samples. miR-19b1-5p contributed significantly to the panel with an odds ratio of 4.3690 (p = 0.0366), ROC analysis revealed that AUC shifts from 0.890 to 0.871 when miR-19b1-5p excluded from the panel (Figure S5, S6).
miR-16-5p, miR-21 expressions did not differ between sample groups. Therefore, we did not incorporate to logistic regression.
It has been reported that miR-210-3p expression is increased in urine of BC patients compared to HC and even superior to urine cytology in diagnosis [28]. However, miR-210-3p has not been previously studied in urinary exosomes of BC patients. We found that bladder cancer patients and high-risk bladder cancer patients had 3.6-fold expression of healthy controls (p = 0.049 and 0.035, respectively).(Table S7, Figure S2). However, miR-210-3p expression or presence did not contribute significantly to logistic regression analysis.
Urinary Ape1/Ref1 concentrations in the BC samples were about two times higher than the HC and follow-up samples (p < 0.00001 and p = 0.00148). It was previously confirmed that Ape1/Ref1 increased in urine samples of BC patients compared with controls [16]. However, no information was provided regarding the normalization of urinary protein concentrations in this study. According to our findings, Ape1/Ref1 for creatinine normalized threshold expression value of 6.27, sensitivity and specificity of 78.2% and 84%, while AUC = 0.820 and p < 0.0001 (Figure S7). In addition, when the LR patient group and HC were compared, AUC = 0.924 for the expression value of 7.35, sensitivity was 93.33% and specificity was 91.18% (p < 0.0001) (figure S9, 10). Ape1/Ref1 concentration significantly contributed to diagnostic panel with an odds ratio of 1.7765 (p = 0.0084).
The cumulative diagnostic value of BLCA-4 was determined by compiling various studies (93% sensitivity, 97% specificity AUC = 0.9607) [29]. Our findings are also in the same direction, we found that BLCA-4 concentration in urine samples of BC patients was approximately twofold HC and follow-up patients (p < 0.000009 and p = 0.002). ROC analysis revealed that AUC = 0.794 for BC and AUC = 0.925 for low-grade BC and significantly contributed to the logistic regression model with an odds ratio of 1.1141 (p = 0.0184).
There are no studies in the literature examining CRK expression in BC. However, CRK has been shown to induce EMT and metastasis through the HGF/c-Met pathway in BC cells [18]. We aimed to examine whether a protein that takes place in EMT and metastasis is present in urine, and if so, how important it is from the diagnostic point of view. CRK concentration was significantly increased in BC samples compared to HC and follow-up patients (p = 0.002 and p = 0.043). In the logistic regression analysis, the contribution to the model was found to be significant with an odds ratio of 0.4904 (p = 0.0043).
Upregulation of VIM and fibronectin in MIBC has been associated with resistance to treatment and poor prognosis [20]. VIM concentration of BC patients was almost twofold of HC and follow-up patients; urinary VIM concentration is a very strong marker for the diagnosis of BC in the early stage (AUC = 0.941), but it is not strong enough for discriminating BC from HC (AUC = 775) or contributing to the logistic regression model (p > 0.05).
Urinary NMP22 is studied extensively in BC samples and FDA-approved 2 tests have been developed for BC diagnosis, but the results in the literature are quite contradictory, the sensitivity of urinary NMP22 tests ranges between 56 and 81% [19, 30]. Although we examined NMP22 with the idea that it might increase the power of the study, we found no significant difference in urinary NMP22 concentrations between sample groups.
In this study, urinary protein and exosomal miRNAs with the most significant differences in the diagnosis of BC were examined by logistic regression analysis. Logistic regression model was able to diagnose BC with 80% sensitivity and 88% specificity (AUC = 0.903). The same model was differentiated the LR patients from HC with 93% sensitivity, 97% specificity (AUC = 0.976). In LR early-stage patients, the panel is more sensitive, suggesting that changes in expression and concentration of molecules in the panel are early events in BC. Further culture studies clarify this idea. Nonetheless, our panel can prevent unnecessary cystoscopies, increase the patient comfort and reduce the economic burden of LR patients.
Using the same markers, it is highly advantageous in terms of labor, time and cost to distinguish both LR patients and BC from HC. Moreover, the inclusion of miRNAs in the model, not in terms of expression level, but in terms of the presence or absence of expression, will minimize the individual errors, since it will remove the normalization and quantitation steps of the experiments. The sensitivity and specificity of the panel developed were superior to urine cytology ranging from 30% and 86% to 83% and 43% in various studies, respectively.
The sensitivity and specificity of the panel we obtained is superior to urine cytology; using the same molecules to differentiate BC from HC and to differentiate LR BC from HC; Although it is more sensitive and specific than FDA-approved urine biomarkers, it should be analyzed in larger cohorts. Nevertheless, urine exosomal miR-19b1-5p, 136-3p, 139-5p expression and urinary APE1/Ref1, BLCA-4, CRK concentrations are promising candidates in terms of bladder cancer diagnosis.
Abbreviations
- BC:
-
Bladder cancer
- NMICB:
-
Non-muscle-invasive bladder cancer
- MIBC:
-
Muscle-invasive bladder cancer
- LR:
-
Low risk
- HR:
-
High risk
- IR:
-
Intermediate risk
- LIR:
-
Low-intermediate risk
- FDA:
-
Food and drug administration
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
NICE (2017) Bladder cancer: diagnosis and management of bladder cancer. BJU Int 120(6):755–765. https://doi.org/10.1111/bju.14045
Tan WS, Tan WP, Tan MY et al (2018) Novel urinary biomarkers for the detection of bladder cancer: a systematic review. Cancer Treat Rev 69:39–52. https://doi.org/10.1016/j.ctrv.2018.05.012
Chakraborty A, Dasari S, Long W, Mohan C (2019) Urine protein biomarkers for the detection, surveillance, and treatment response prediction of bladder cancer. Am J Cancer Res 9(6):1104
Liu X, Liu X, Wu Y et al (2017) MicroRNAs in biofluids are novel tools for bladder cancer screening. Oncotarget 8(19):32370–32379. https://doi.org/10.18632/oncotarget.16026
Weber JA, Baxter DH, Zhang S et al (2010) The MicroRNA spectrum in 12 body fluids. Clin Chem 56(11):1733–1741. https://doi.org/10.1373/clinchem.2010.147405
Eissa S, Matboli M, Essawy NOE, Kotb YM (2015) Integrative functional genetic-epigenetic approach for selecting genes as urine biomarkers for bladder cancer diagnosis. Tumor Biol 36(12):9545–9552. https://doi.org/10.1007/s13277-015-3722-6
Armstrong DA, Green BB, Seigne JD, Schned AR, Marsit CJ (2015) MicroRNA molecular profiling from matched tumor and bio-fluids in bladder cancer. Mol Cancer 14(1):194. https://doi.org/10.1186/s12943-015-0466-2
Sapre N, Macintyre G, Clarkson M et al (2016) A urinary microRNA signature can predict the presence of bladder urothelial carcinoma in patients undergoing surveillance. Br J Cancer 114(4):454–462. https://doi.org/10.1038/bjc.2015.472
Ratert N, Meyer HA, Jung M et al (2013) MiRNA profiling identifies candidate mirnas for bladder cancer diagnosis and clinical outcome. J Mol Diagn 15(5):695–705. https://doi.org/10.1016/j.jmoldx.2013.05.008
Yonemori M, Seki N, Yoshino H et al (2016) Dual tumor-suppressors miR-139-5p and miR-139-3p targeting matrix metalloprotease 11 in bladder cancer. Cancer Sci 107(9):1233–1242. https://doi.org/10.1111/cas.13002
Enokida H, Yoshino H, Matsushita R, Nakagawa M (2016) The role of microRNAs in bladder cancer. Investig Clin Urol 57(Suppl 1):S60. https://doi.org/10.4111/icu.2016.57.S1.S60
Han Y, Chen J, Zhao X et al (2011) MicroRNA expression signatures of bladder cancer revealed by deep sequencing. PLoS One 6(3):e18286. https://doi.org/10.1371/journal.pone.0018286(Wölfl S, ed)
Pazourkova E, Pospisilova S, Svobodova I et al (2016) Comparison of microRNA content in plasma and urine indicates the existence of a transrenal passage of selected microRNAs. Adv Exp Med Biol 924:97–100. https://doi.org/10.1007/978-3-319-42044-8_18
Zhang DZ, Lau KM, Chan ESY et al (2014) Cell-free urinary MicroRNA-99a and MicroRNA-125b are diagnostic markers for the non-invasive screening of bladder cancer. PLoS One 9(7):e100793. https://doi.org/10.1371/journal.pone.0100793(Dahiya R, ed)
Choi S, Shin JH, Lee YR et al (2016) Urinary APE1/Ref-1: a potential bladder cancer biomarker. Dis Mark 2016:1–8. https://doi.org/10.1155/2016/7276502
Deininger S, Hennenlotter J, Rausch S et al (2018) No influence of smoking status on the performance of urine markers for the detection of bladder cancer. J Cancer Res Clin Oncol 144(7):1367–1373. https://doi.org/10.1007/s00432-018-2639-z
Matsumoto R, Tsuda M, Wang L et al (2015) Adaptor protein CRK induces epithelial-mesenchymal transition and metastasis of bladder cancer cells through HGF/c-Met feedback loop. Cancer Sci 106(6):709–717. https://doi.org/10.1111/cas.12662
Mbeutcha A, Lucca I, Mathieu R, Lotan Y, Shariat SF (2016) Current status of urinary biomarkers for detection and surveillance of bladder cancer. Urol Clin North Am 43(1):47–62. https://doi.org/10.1016/j.ucl.2015.08.005
Knowles MA, Hurst CD (2015) Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer 15(1):25–41. https://doi.org/10.1038/nrc3817
Tang KWA, Toh QC, Teo BW (2015) Normalisation of urinary biomarkers to creatinine for clinical practice and research–when and why. Singapore Med J 56(1):7–10. http://www.ncbi.nlm.nih.gov/pubmed/25640093. Accessed 28 Nov 2018
Zhou H, Yuen PST, Pisitkun T et al (2006) Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int 69(8):1471–1476. https://doi.org/10.1016/j.polymdegradstab.2005.10.005
Çokluk Ö (2010) Lojistik regresyon analizi: kavram ve uygulama. Kuram ve Uygulamada Eğitim Bilimleri 10(3):1357–1407
Andreu Z, Otta Oshiro R, Redruello A et al (2017) Extracellular vesicles as a source for non-invasive biomarkers in bladder cancer progression. Eur J Pharm Sci 98:70–79. https://doi.org/10.1016/j.ejps.2016.10.008
Soria F, Droller MJ, Lotan Y et al (2018) An up-to-date catalog of available urinary biomarkers for the surveillance of non-muscle invasive bladder cancer. World J Urol. https://doi.org/10.1007/s00345-018-2380-x
Crossland RE, Norden J, Bibby LA, Davis J, Dickinson AM (2016) Evaluation of optimal extracellular vesicle small RNA isolation and qRT-PCR normalisation for serum and urine. J Immunol Methods 429:39–49. https://doi.org/10.1016/j.jim.2015.12.011
Soung YH, Nguyen T, Cao H, Lee J, Chung J (2016) Emerging roles of exosomes in cancer invasion and metastasis. BMB Rep 49(1):18–25. https://doi.org/10.5483/bmbrep.2016.49.1.239
Eissa S, Matboli M, Hegazy MGA, Kotb YM, Essawy NOE (2015) Evaluation of urinary microRNA panel in bladder cancer diagnosis: relation to bilharziasis. Transl Res 165(6):731–739. https://doi.org/10.1016/j.trsl.2014.12.008
Cai Q, Wu Y, Guo Z et al (2015) Urine BLCA-4 exerts potential role in detecting patients with bladder cancers: a pooled analysis of individual studies. Oncotarget 6(35):37500–37510. https://doi.org/10.18632/oncotarget.6061
Mowatt G, Zhu S, Kilonzo M et al (2010) Systematic review of the clinical effectiveness and cost-effectiveness of photodynamic diagnosis and urine biomarkers (FISH, ImmunoCyt, NMP22) and cytology for the detection and follow-up of bladder cancer. Health Technol Assess (Rockv) 14(4):1–331. https://doi.org/10.3310/hta14040
Acknowledgements
This study was funded by Marmara University Research Commission BAPKO-SAG-C-DRP-1310160441.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The research was funded by Marmara University Research Commission BAPKO-SAG-C-DRP-1310160441.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committee, reference number 09.2016.416 and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Güllü Amuran, G., Tinay, I., Filinte, D. et al. Urinary micro-RNA expressions and protein concentrations may differentiate bladder cancer patients from healthy controls. Int Urol Nephrol 52, 461–468 (2020). https://doi.org/10.1007/s11255-019-02328-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-019-02328-6