Abstract
Purpose
Patients with chronic kidney disease (CKD) are frail and have high risk of cardiovascular disease. This study was performed to assess the effects of aerobic exercise training in adults with CKD.
Methods
MEDLINE, EMBASE, CENTRAL, Web of Science were searched up to December 2018 to identify eligible randomized controlled trials (RCTs) that studied aerobic exercise in adults with CKD. Primary outcomes include oxygen consumption at peak exercise (VO2 peak), exercise capacity, blood pressure, heart rate, and health-related quality of life (HRQoL). Statistical analysis was performed using Review Manager 5.2.1 software.
Results
Thirty-one trials, containing 1305 adults with CKD, were included. The most used aerobic exercise program was characterized as moderate intensity (15/31), 3 times/week frequency (22/31), 30 min duration (9/31) and 3 months follow-up (12/31). Significant improvement was observed in cardiorespiratory function (VO2 peak) (P < 0.0001), exercise duration (P < 0.0001), HDL-C (P = 0.03) and pain (P = 0.007), physical role (P = 0.03), general health (P = 0.007) of HRQoL after aerobic exercise in patients with CKD. A marginal difference was observed in HR max (P = 0.07). However, no statistical difference was noticed in exercise capacity, blood pressure, resting heart rate, serum lipid and serum creatinine between aerobic training group and control. No subgroup differences were altered in all outcomes when studies were divided based on intensity of exercise training, the treatment of dialysis or the length of intervention.
Conclusions
Aerobic exercise training could benefit adult CKD patients in increasing cardiorespiratory function, exercise duration, HDL-C level and improve health quality of life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Incidence and prevalence of chronic kidney disease (CKD) is increasing world widely. Patients with CKD have an obvious loss of aerobic power and functional capacity compared with healthy individuals of the same age group [1], which caused by several factors, including renal anemia, malnutrition, uremic toxins, acidosis, vitamin D deficiency and altered potassium metabolism [2]. As a result, CKD patients are more susceptible to poor health outcomes, including disability, hospitalization and high mortality [3]. Although exercise training maybe a promising solution, the effect of aerobic exercise training in CKD patients has not yet been completely clarified. Previous studies have shown that aerobic exercise could effectively improve cardiopulmonary capacities, health-related quality of life (HRQL), blood pressure (BP) in CKD patients [4]. However, other clinical studies showed no significant benefit [5]. Therefore, a meta-analysis was performed to clarify the effect of aerobic exercise training on CKD patients.
Materials and methods
Search
Two reviewers independently and systematically searched for studies evaluated aerobic exercise in CKD patients published in English up to December 2018. The following databases were searched: MEDLINE, EMBASE, The Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, Web of Science. The search terms used were: [(aerobic exercise) OR (exertion) OR (exercise therapy) OR (physical education and training) OR (physical fitness) OR (exercise training)] AND [(Chronic Kidney Failure) OR (Kidney Transplantation) OR (hemodialysis) OR (haemodialysis) OR (dialysis) OR (renal disease) OR (kidney disease) OR (CKD or CKF or CRD or CRF or ESKD or ESRD or ESKF or ESRF)] AND [(random*) OR (randomized controlled trial*) OR (RCT)]. The reference lists of review articles and studies included were hand searched for other potentially eligible studies.
Selection
Two independent reviewers selected articles according to the inclusion and exclusion criteria. Disagreements were resolved in consultation with a third reviewer. The inclusion criteria were as follows: (a) adults (≥ 18 years) with CKD (2–5 days stage) were enrolled; (b) the intervention was aerobic exercise training versus non-exercise control OR aerobic exercise training plus co-intervention versus co-intervention; (c) exercise program should include: intensity, frequency and duration (> 2 months); (d) primary outcomes—oxygen consumption at peak exercise (VO2 peak), exercise duration, muscular endurance (sit-to-stand-to-sit test), serum creatinine, health-related quality of life (HRQoL); secondary outcomes—walking capacity (6-min walk), systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate, serum albumin, pre-albumin, triglycerides, total cholesterol, low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C); (e) randomized controlled trial (RCT) and quasi-RCT. The exclusion criteria were as follows: studies in acute kidney injury (AKI) or kidney transplant patients, children, without detailed training plan. Full texts of selected articles were carefully read to determine whether they were eligible. Studies with unobtainable and unusable data were also excluded.
Data extraction and quality assessment
Data extraction was carried out independently by two reviewers using standard data extraction forms. Quality of the studies were assessed using the Tool to Assess Risk of Bias in Randomized Controlled Trials (Contributed by the CLARITY Group at McMaster University) [6], in which each assessed item received a “Definitely Yes (low risk of bias)”, “Definitely No (high risk of bias)”, “Probably No”, or “Probably Yes”. All disagreements were resolved by discussion or by a third researcher.
Statistical analysis
Review Manager (5.2.1) was used to estimate the effect of the outcomes. All outcomes were analyzed using fixed or random effects model. Heterogeneity across studies was analyzed using I2 statistic method. When there was no heterogeneity (I2 ≤ 50%, P > 0.05), fixed effect meta-analyses were performed. Otherwise random-effect model was applied. Funnel plots were used to examine the potential publication bias. Subgroup analysis was performed for the length of exercise intervention (< 6 months, ≥ 6 months), the treatment of dialysis and intensity of exercise training (low, moderate, high) when there were sufficient data.
Results
Search results
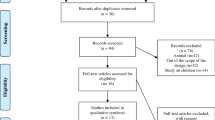
A total of 1863 reports were screened and 161 studies were read in detail. 130 studies were excluded according to exclusion criteria: duplication (n = 6); abstract (n = 27); other exercise (n = 23); wrong control group (n = 30); not RCT (n = 2); no adequate data (n = 34); protocol (n = 8). Finally, 31 trials [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37] and 1305 patients were included in the current study (Fig. 1).
Characteristics of included studies
The main characteristics of included studies are shown in Tables 1 and 2; 22 studies were single-center studies, five were multicenter studies, the other four studies did not provide this information. The total number of patients included was 1305, ranging from 11 to 227 patients in each study. Mean age of study participants ranged from 37 to 72.5 years. Most of the participants in CKD 5d stage included were treated with hemodialysis. The mostly used aerobic exercise program was moderate intensity (15/31), 3 times/week frequency (22/31), 30 min duration (9/31) with 3 months follow-up (12/31).
Quality of included studies
In general, only six studies reported the method of randomization (Headley 2014, Koufaki 2002, Matsufuji 2015, Mustata 2011, Tang 2017, Wu 2014), 4 of 45 studies used adequate allocation concealment (Craenenbroeck 2014, Matsufuji 2014, Mustata 2011, Toussaint 2008), 1 study had blinded participants (Parsons 2004). The risk of bias assessments of the included studies is summarized in Fig. 2.
Meta-analysis
Exercise capacity
Aerobic capacity
Aerobic capacity was measured using VO2 peak in 17 studies (464 patients). Meta-analysis showed that aerobic exercise training could significantly improve aerobic capacity (VO2 peak) in CKD patients (Table 3; MD 2.08, P < 0.0001; I2 = 25%). Between the two subgroups, the length of intervention < 6 months and ≥ 6 months, the difference in post–pre change in VO2 peak were 2.60 (P = 0.0002) and 1.54 (P = 0.03), respectively. When studies were divided based on intensity of exercise training, the same results were shown on this outcome. VO2 peak was significantly better in patients treated with dialysis compared with non-dialysis (MD 3.07, P < 0.0001 vs MD 0.77, P = 0.31).
Exercise duration and muscular endurance
Exercise duration was analyzed in six trials (177 participants). Results showed that aerobic exercise could remarkably improve exercise duration in CKD patients (Table 3; MD 155.57, P < 0.0001; I2 = 23%). Subgroup analysis based on the length of exercise and the treatment of dialysis also reached similar result (< 6 months, MD 146.17, P = 0.003 vs ≥ 6 months, MD 165.61, P = 0.001) and (dialysis, MD 235.19, P < 0.0001 vs non-dialysis, MD 114.88, P = 0.009). Muscular endurance was evaluated using the Sit-to-Stand-to-Sit-60 (STS60) score in five studies (445 patients). Meta-analysis indicated that aerobic exercise could not improve muscular endurance. Subgroup analysis showed no difference when studies were divided based on intensity of exercise training or the length of intervention (Table 3).
Walking capacity
Walking capacity was determined by 6-min walk test (m) in eight studies with 496 participants. Results suggested no difference between aerobic exercise group and control group in CKD patients. There was no significant difference in subgroup analysis based on intensity of exercise training or the length of intervention. Patients untreated with hemodialysis had a greater effect on walking capacity during aerobic training, but there were only two small-scale RCTs include non-dialysis CKD patients (two trials, 113 patients, MD 0.58, P = 0.003).
Resting blood pressure and heart rate
There was no significant difference in either resting SBP or DBP in aerobic exercise training group (Table 3, 12 studies, 514 participants). Subgroup analysis on SBP and DBP did not report significant difference. It was also found that aerobic exercise could not significantly affect the resting heart rate when compared with control (Table 3). However, increased maximum heart rate (max HR) could be observed in aerobic exercise training group. Nevertheless, the difference was just marginal (Table 3, MD 5.69, P = 0.07). The length of exercise and the treatment of dialysis during aerobic training did not show obvious effect on resting and max heart rate in CKD patients (Table 3).
Lab indexes
No significant changes were observed in serum triglycerides, total cholesterol, LDL-C, albumin, pre-albumin levels between aerobic exercise group and control group (Table 3). However, significantly increased HDL-C level was found in aerobic exercise group when compared with control group (Table 3, MD 3.54, P = 0.03). Serum creatinine level had not a significantly change between exercise and control groups. Subgroup analysis suggested the change of blood lipid was not significant in patients treated and untreated with dialysis (Table 3).
Health-related quality of life
Seven of the 31 included trials reported a validated HRQoL measure with 36-item Short Form [7, 9, 13, 19, 21, 30, 31]. Meta-analysis showed that aerobic exercise could remarkably relieve pain (P = 0.007, I2 = 49%) and improve quality of life on physical role, general health with significant heterogeneity (Table 3). Physical function, social function, and mental health were increased in aerobic exercise training group, however, the difference was just marginal (Table 3; P = 0.09, 0.08 and 0.08, respectively). Other items were not pooled for insufficient studies.
Assessment of publication bias
An assessment of publication bias was conducted for the VO2 peak and HRQoL of physical role that contained enough study data (Fig. 3). Funnel plots were visually assessed as reasonably symmetrical, indicating little publication or small study bias.
Discussion
Chronic kidney disease (CKD) is a worldwide public health problem. The physical fitness in adults with CKD is progressively reduced and the main causes are renal anemia and skeletal muscle disorder. Frailty is associated with the risk of adverse prognosis and the quality of life [2]. Aerobic exercise training is the most widely used program, however, the great difference in experimental design and outcome indicators make its effect in CKD patients is unclear at present. Our research updates and evaluates the effects of aerobic exercise training in adults with CKD.
Regular exercise improves cardiorespiratory function, strength and physical function, and health-related quality of life in CKD patients [38]. VO2 peak is one of the most commonly studied parameters in cardiorespiratory function. When compared to control group, we found that aerobic exercise improved VO2 peak and exercise duration, while there were no significant differences in walking capacity and muscular endurance. The intensity of aerobic exercise program and the length of intervention did not alter results. These findings are in accordance with the previous Cochrane review [2]; Aerobic exercise training will improve VO2 peak (2.08 mL/kg/min) and physical fitness with clinically significant [39]. VO2 peak was significantly greater in patients treated with dialysis than non-dialysis (MD 3.07, P < 0.0001 vs MD 0.77, P = 0.31), but the RCTs including patients untreated with dialysis were small-scale and not statistically significant (six trials, 167 patients, P = 0.31). Due to the relatively short intervention period, a beneficial effect in walking capacity and muscular endurance were not expected. Hence, our study supports the application of aerobic exercise in adult CKD patients, especially those undertaking hemodialysis. The recommend aerobic exercise program should be moderate intensity of training lasting 30–60 min per time, 3 times/week and last for 3–6 months.
Cardiovascular disease is a major cause of mortality in CKD patients. Blood pressure and heart rate play important roles in the progression of CVD. In both normotensive and hypertensive subjects, it is reported that aerobic training could reduce blood pressure at rest, especially in hypertensive subjects [40]. However, in line with the latest review in CKD stages 3–4 [41], our result did not indicate difference in diastolic or systolic blood pressure after aerobic training in adults CKD patients. The possible explanation might be a generally well controlled BP at baseline and the use of antihypertensive drugs. It is well known that regular exercise training lowers resting HR in healthy people. Nevertheless, our meta-analysis and the previous Cochrane review [2] showed that aerobic training could decrease the rest HR in CKD patients (1.75 bpm), but the difference could not reach the statistical significance (P = 0.16). Relatively small sample size which may mask the effect of aerobic training. The association between aerobic exercise and max HR remains a heated debate. Studies in healthy people reported that aerobic training could affect the max HR by the following mechanism: plasma volume expansion, enhanced baroreflex function, alteration of the electrophysiology of the sinoatrial node and decreased ß-adrenergic receptor number. As a result, max HR may be reduced during aerobic exercise [42, 43]. However, both this meta-analysis and the Cochrane review [2] could only find a marginal increase of max HR after aerobic training in CKD patients (5.69 bpm, P = 0.07). Absence of adequate procedural details of measuring max HR or the short duration of exercise may be the reason of this negative finding. Subgroup analysis also had non-significant effects on blood pressure, resting HR and max HR. More research focusing on blood pressure, resting HR and max HR in CKD patients are needed before conclusions can be drawn.
Dyslipidemia is a common comorbidity in patients with CKD, and is a major risk factor for CVD. Low HDL levels were shown to be a significant risk factor for coronary heart disease [44]. In the current study, we found that aerobic training could increase the serum HDL-C concentrations obviously (P = 0.03), while not affecting the triglycerides, total cholesterol, LDL-C levels in adult CKD patients. The comparison based on patients treated and untreated with dialysis did not alter results. The result is, however, based on a relatively small sample size and further research is needed. It was reported that aerobic training might play a role in the maintenance of kidney function and improve estimated glomerular filtration rate (eGFR) in patients with CKD [41]. However, our meta-analysis showed that aerobic exercise training could not decrease the serum creatinine level in non-dialysis patients CKD patients (CKD 2–5 stage). Further high quality and multicenter research is needed to clarify this effect.
Health-related quality of life (HRQoL) was apparently decreased in CKD patients [45]. Our study suggested that aerobic training could improve the HRQoL of CKD patients in physical role, general health and pain management significantly when compared with control. Therefore, it is plausible that aerobic exercise have beneficial effect on emotional and behavioral aspects including increased social interaction, decreased anxiety and improved attitude toward self.
This study had several limitations. First, most included trials were short term (3–6 months); second, intention-to-treat (ITT) analysis was not used in most studies, and there were deficiencies in the reporting of methodological and results information, such as method of randomization, blindly, dropout rate, adverse events, and compliance. These might have inflated the apparent results; third, some outcomes had been measured with different methods, which complicates the pooling of results. Finally, the effects of aerobic exercise training between males and females or in CKD patients without hypertension and/or diabetes remained unknown for insufficient original data from RCTs. Further studies with large-scale, multicenter, longer exercise interventions and more sensitive indicators would provide more scientific information on the effects of aerobic exercise in adults with CKD.
Conclusion
Aerobic exercise training will benefit adults with CKD and help them in their maximal oxygen consumption, exercise duration, HDL-C and health quality of life. Although differences in muscular endurance, blood pressure, HR, blood lipid, kidney function did not reach statistical significance between aerobic exercise and usual care group, it is speculated that exercise training in CKD patients may still have potential benefits. Future long-term studies focusing on multidisciplinary programs, such as Tai Chi, Yoga, dancing, and patient-relevant outcomes are warranted.
References
Moinuddin I, Leehey DJ et al (2008) A comparison of aerobic exercise and resistance training in patients with and without chronic kidney disease. Adv Chronic Kidney Dis 15(1):83–96
Heiwe S, Jacobson SH (2011) Exercise training for adults with chronic kidney disease. Cochrane Database Syst Rev 5(10):1–395 (Review)
Ortiz A, Sanchez-Niño MD (2019) Sarcopenia in CKD: a roadmap from basic pathogenetic mechanisms to clinical trials. Clin Kidney J 12(1):110–112
Roshanravan B, Gamboa J et al (2017) Exercise and CKD: skeletal muscle dysfunction and practical application of exercise to prevent and treat physical impairments in CKD. Am J Kidney Dis 69(6):837–852
Heiwe S, Jacobson SH et al (2014) Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis 64(3):383–393
Akl EA, Sun X et al (2012) Specific instructions for estimating unclearly reported blinding status in randomized trials were reliable and valid. J Clin Epidemiol 65(3):262–267
Wu Y, He Q et al (2014) Effect of individualized exercise during maintenance haemodialysis on exercise capacity and health-related quality of life in patients with uraemia. J Int Med Res 42(3):718–727
Takashi Aoike D, Baria F et al (2015) Impact of home-based aerobic exercise on the physical capacity of overweight patients with chronic kidney disease. Int Urol Nephrol 47(2):359–367
Van Craenenbroeck AH, Van Craenenbroeck EM et al (2015) Effect of moderate aerobic exercise training on endothelial function and arterial stiffness in CKD stages 3–4: a randomized controlled trial. Am J Kidney Dis 66(2):285–296
Frey S, Mir AR et al (1999) Visceral protein status and caloric intake in exercising versus nonexercising individuals with end-stage renal disease. J Ren Nutr 9(2):71–77
Groussard C, Rouchon-Isnard M et al (2015) Beneficial effects of an intradialytic cycling training program in patients with end-stage kidney disease. Appl Physiol Nutr Metab 40(6):550–556
Headley S, Germain M et al (2012) Exercise training improves HR responses and VO2peak in predialysis kidney patients. Med Sci Sports Exerc 44(12):2392–2399
Headley S, Germain M et al (2014) Short-term aerobic exercise and vascular function in CKD stage 3: a randomized controlled trial. Am J Kidney Dis 64(2):222–229
Hristea D, Deschamps T et al (2016) Combining intra-dialytic exercise and nutritional supplementation in malnourished older haemodialysis patients: towards better quality of life and autonomy. Nephrology (Carlton) 21(9):785–790
Kosmadakis GC, John SG et al (2012) Benefits of regular walking exercise in advanced pre-dialysis chronic kidney disease. Nephrol Dial Transplant 27(3):997–1004
Koufaki P, Mercer TH et al (2002) Effects of exercise training on aerobic and functional capacity of end-stage renal disease patients. Clin Physiol Funct Imaging 22(2):115–124
Kouidi E, Iacovides A et al (1997) Exercise renal rehabilitation program: psychosocial effects. Nephron 77(2):152–158
Leehey DJ, Moinuddin I et al (2009) Aerobic exercise in obese diabetic patients with chronic kidney disease: a randomized and controlled pilot study. Cardiovasc Diabetol 8:62
Manfredini F, Mallamaci F et al (2017) Exercise in patients on dialysis: a multicenter, randomized clinical trial. J Am Soc Nephrol 28(4):1259–1268
Matsufuji S, Shoji T et al (2015) Effect of chair stand exercise on activity of daily living: a randomized controlled trial in hemodialysis patients. J Ren Nutr 25(1):17–24
Mustata S, Groeneveld S et al (2011) Effects of exercise training on physical impairment, arterial stiffness and health-related quality of life in patients with chronic kidney disease: a pilot study. Int Urol Nephrol 43(4):1133–1141
Pomidori L, Lamberti N et al (2016) Respiratory muscle impairment in dialysis patients: can minimal dose of exercise limit the damage? A Preliminary study in a sample of patients enrolled in the EXCITE trial. J Nephrol 29(6):863–869
Reboredo MM, Neder JA et al (2011) Constant work-rate test to assess the effects of intradialytic aerobic training in mildly impaired patients with end-stage renal disease: a randomized controlled trial. Arch Phys Med Rehabil 92(12):2018–2024
Esteve Simó V, Junqué A et al (2014) Complete low-intensity endurance training programme in haemodialysis patients: improving the care of renal patients. Nephron Clin Pract 128(3–4):387–393
Tang Q, Yang B et al (2017) Effects of individualized exercise program on physical function, psychological dimensions, and health-related quality of life in patients with chronic kidney disease: a randomized controlled trial in China. Int J Nurs Pract 23(2):e12519
Wilund KR, Tomayko EJ et al (2010) Intradialytic exercise training reduces oxidative stress and epicardial fat: a pilot study. Nephrol Dial Transplant 25(8):2695–2701
Goldberg AP, Geltman EM et al (1983) Therapeutic benefits of exercise training for hemodialysis patients. Kidney Int Suppl 16:S303–S309
Carmack Cindy L, Amaral-Melendez Marta et al (1995) Exercise as a component of the physical and psychological rehabilitation of hemodialysis patients. Int J Rehabilit Health 1(1):14–23
Parsons TL, Toffelmire EB et al (2004) The effect of an exercise program during hemodialysis on dialysis efficacy, blood pressure and quality of life in end-stage renal disease (ESRD) patients. ClinNephrol 61(4):261–274
Zhao C, Ma H et al (2016) Long-term bicycle riding ameliorates the depression of the patients undergoing hemodialysis by affecting the levels of interleukin-6 and interleukin-18. Neuropsychiatr Dis Treat 13:91–100
Matsumoto Y, Furuta A et al (2007) The impact of pre-dialytic endurance training on nutritional status and quality of life in stable hemodialysis patients (Sawada study). Ren Fail 29(5):587–593
Deligiannis A, Kouidi E et al (1999) Effects of physical training on heart rate variability in patients on hemodialysis. Am J Cardiol 84(2):197–202
Deligiannis A, Kouidi E et al (1999) Cardiac effects of exercise rehabilitation in hemodialysis patients. Int J Cardiol 70(3):253–266
Eidemak I, Haaber AB et al (1997) Exercise training and the progression of chronic renal failure. Nephron 75(1):36–40
Konstantinidou E, Koukouvou G et al (2002) Exercise training in patients with end-stage renal disease on hemodialysis: comparison of three rehabilitation programs. J Rehabil Med 34(1):40–45
Toussaint ND, Polkinghorne KR et al (2008) Impact of intradialytic exercise on arterial compliance and B-type natriuretic peptide levels in hemodialysis patients. Hemodial Int 12(2):254–263
Tsuyuki K, Kimura Y et al (2003) Oxygen uptake efficiency slope as monitoring tool for physical training in chronic hemodialysis patients. Ther Apher Dial 7(4):461–467
Johansen KL (2005) Exercise and chronic kidney disease: current recommendations. Sports Med 35(6):485–499
Swank AM, Horton J et al (2012) Modest increase in peak VO2 is related to better clinical outcomes in chronic heart failure patients: results from heart failure and a controlled trial to investigate outcomes of exercise training (HF-AC TION). Circulation Heart failure 5(5):579–585
Cornelissen VA, Fagard RH (2005) Effects of endurance training on blood pressure, blood pressure-regulating mechanisms, and cardiovascular risk factors. Hypertension 46:667–675
Vanden Wyngaert K, Van Craenenbroeck AH et al (2018) The effects of aerobic exercise on eGFR, blood pressure and VO2 peak in patients with chronic kidney disease stages 3-4: a systematic review and meta-analysis. PLoS One 13:0203662
Zavorsky GS (2000) Evidence and possible mechanisms of altered maximum heart rate with endurance training and tapering. Sports Med 29(1):13–26
Hellsten Y, Nyberg M (2016) Cardiovascular adaptations to exercise training. Compr Physiol 6:1–32
Hager MR, Narla AD et al (2017) Dyslipidemia in patients with chronic kidney disease. Rev Endocr Metab Disord 18(1):29–40
Suh MR, Jung HH et al (2002) Effects of regular exercise on anxiety, depression, and quality of life in maintenance hemodialysis patients. Ren Fail 24:337–345
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pei, G., Tang, Y., Tan, L. et al. Aerobic exercise in adults with chronic kidney disease (CKD): a meta-analysis. Int Urol Nephrol 51, 1787–1795 (2019). https://doi.org/10.1007/s11255-019-02234-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-019-02234-x