Abstract
Purpose
Index tumor volume (ITV) measured on radical prostatectomy (RP) specimens has been shown to be associated with adverse pathologic and oncologic outcomes. We evaluate the value of ITV calculated from prostate multiparametric MRI (mpMRI) in predicting adverse clinical and pathologic outcomes.
Materials and methods
Data from a prospectively maintained, single-institution database were analyzed for patients who underwent mpMRI prior to RP (2007–2016). Index tumor was defined as a T2-visible lesion with the longest diameter. Adverse pathologic outcomes were extraprostatic extension (EPE), lymph node invasion (LNI), seminal vesicle invasion (SVI), and positive margins (PM). Logistic and Cox proportional hazard regression were used to assess associations with adverse pathology and biochemical recurrence (BCR), respectively.
Results
Of the 455 patients included, EPE, LNI, SVI and PM were present in 23.5%, 6.2%, 5.5% and 15.7% patients, respectively. Patients with adverse pathologic outcomes had larger median ITV. ITV was found to be an independent predictor of EPE (OR 1.22, p = 0.010), LNI (OR 1.39, p = 0.001), and SVI (OR 1.28, p = 0.009), but not PM (OR 1.03, p = 0.522). Combination of ITV and PSA was found to have predictive ability comparable to that of modified Partin tables (EPE:ITV + PSAAUC = 0.71 vs. PartinAUC = 0.71; LNI:ITV + PSAAUC = 0.92 vs. PartinAUC = 0.90, SVI:ITV + PSAAUC = 0.78 vs. PartinAUC = 0.82). 5 year BCR-free survival (median follow-up 24.9 months) was higher for patients with ITV < 2 cc (84.1% vs. 58.5%, p = 0.001). However, ITV was not found to be an independent predictor of BCR (HR 1.69, p = 0.130).
Conclusions
We demonstrate that ITV measured on mpMRI is a predictor of adverse pathologic and clinical outcomes and can aid in preoperative risk assessment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Index lesion tumor volume (ITV), measured on radical prostatectomy (RP) whole-mount specimen, has been demonstrated to correlate with adverse clinical and pathologic outcomes. A study by Knoedler et al. found that ITV was significantly associated with systemic progression, prostate cancer (PCa)-specific mortality, and all-cause mortality and that the incorporation of ITV into their model significantly increased its predictive ability of all-cause mortality [1].
However, ITV is difficult to measure on pathology accurately, and since there is currently no preoperative surrogate, it cannot be used for preoperative decision making.
Recent advances in MRI technology have rendered it a powerful tool in directing prostate biopsies, with a sensitivity and specificity of 90% and 88% for clinically significant cancers [2]. Prior research has already established that tumor volume (TV) can be measured on MRI [3, 4]. We hypothesized that ITV measured on preoperative MRI could play a role in the prediction of extraprostatic extension (EPE), seminal vesicle invasion (SVI), lymph node invasion (LNI), positive surgical margins (PM), and biochemical recurrence (BCR).
In this study, we analyze the novel approach of measuring ITV on preoperative multiparametric magnetic resonance imaging (mpMRI) for use as a predictive variable.
Materials and methods
Patient selection
Patients undergoing RP for biopsy-proven PCa under various Institutional Review Board-approved protocols were included in the study. Between May’ 07 and Jan’ 16, 499 patients received RP for localized PCa at the National Cancer Institute following mpMRI. Of these, 22 patients were excluded due to lack of MRI-visible lesions on preoperative mpMRI, and an additional 22 were excluded due to prior radiotherapy or androgen deprivation therapy.
Imaging protocol
All patients underwent a diagnostic mpMRIs of the prostate on a 3.0 T (Achieva, Philips Healthcare) scanner, as previously described [5]. Sequences obtained included triplanar T2-weighted, axial dynamic contrast-enhanced (DCE), axial diffusion weighted imaging (DWI) with apparent diffusion coefficient (ADC) mapping with or without MRI spectroscopy. Lesions assigned internal NIH MRI suspicion scores of low, moderate, or high PIRADS scores were not analyzed, as they were not in use throughout our study period, and a large proportion of our patients did not have PIRADS score calculated.
ITV assessment on MRI
Index lesion was defined as the largest lesion visible on T2W MRI. Volume was calculated using the ellipsoid formula with the longest diameter as the length, perpendicular diameter as the width, and slice count multiplied by 0.3 mm/slice as the depth of the lesion.
Data collection
Demographic, clinical, and pathologic data were obtained from a prospectively maintained database. All RP procedures were performed by a single surgeon (P.A.P.) using a robotic-assisted laparoscopic technique with concomitant lymphadenectomy. All surgical specimens were processed using the whole-mount technique for EPE, SVI, LNI and PM.
Predicted probabilities of PM, EPE, and LNI were obtained using modified Partin tables [6]. Patients were monitored post-RP with periodic PSA testing. BCR was defined as two consecutive PSA values ≥ 0.2 ng/ml, a single PSA value ≥ 0.4 ng/ml, or receipt of salvage therapy [7].
Statistical analysis
Statistical analysis was performed using SPSS ver21 (Chicago, IL, USA). Pearson Chi-square and Mann–Whitney tests were used to compare the differences between categorical and continuous variables, respectively. Logistic regression analysis was used to assess associations of clinical, imaging, and histopathological variables with adverse pathologic features. Multicollinearity among the predictor variables was evaluated by calculating the variance inflation factors. Significant collinearity among the predictor variables was ruled out if the variance inflation factor of individual predictors was below 10. Receiver-operating characteristic (ROC) curves were used to characterize and compare the predictive ability of ITV with that of Partin tables for PM, EPE, and LN. On BCR analysis, additional two patients were excluded due to persistently elevated PSA after RP, and three were excluded due to distant metastasis at the time of RP. For BCR analysis, ITV was analyzed as a binary variable (< 2.0 cc and ≥ 2.0 cc), as analyzed by prior studies [8, 9]. Kaplan–Meier curves were created to determine BCR-free survival, and the log-rank test was used to compare survival between cohorts. Cox proportional hazard analysis was used to analyze predictors of BCR.
Results
Patient demographics
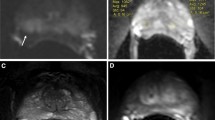
Among the patients considered for eligibility, 455 patients met inclusion criteria. Patient demographics, clinical, imaging and biopsy data are depicted in Table 1. In our cohort, median age and PSA were 60 years (IQR 10) and 6.2 ng/ml (IQR 5.7), and 23.3% were “high-risk” (Gleason 8–10) on biopsy. Median ITV was 0.836 cc (IQR 1.308). Example is in Fig. 1.
57 y/o male with PSA 11.47 ng/ml, Gleason 4 + 4 on biopsy, NIH MRI score = high, and radical prostatectomy on 6/14/2013. On pathology, the patient had Gleason 4 + 4, positive margins. Index tumor volume was calculated from T2W MRI by the ellipsoid formula, using slice count × 0.3 mm/slice as an estimation for tumor depth
EPE analysis
One hundred and two (22.8%) of 455 patients had EPE on pathology. Median ITV was significantly larger in the EPE cohort vs non-EPE: (1.302 cc vs 0.754 cc). In addition, patients with EPE had higher median PSA (8.9 ng/ml vs 5.7 ng/ml, p < 0.001), and a higher proportion of these patients were clinical stage > T1c (19.0% vs 6.8%, p < 0.001) and biopsy Gleason Score (bGS) ≥ 8 (45.1% vs 16.8%, p < 0.001). Multivariate analysis showed that ITV (Odds Ratio (OR) 1.22, p = 0.010), PSA (p = 0.003), clinical stage > T1c (p = 0.001), and bGS (p = 0.001) were independent predictors of EPE (Table 2).
ROC curves were drawn using the predicted probabilities of ITV and Partin tables for EPE (Fig. 2). The area under the curves (AUCs) for ITV (0.66, p = 0.142), ITV and PSA combined (0.71, p = 0.912) and modified Partin tables (0.71) in predicting EPE were comparable.
LNI analysis
Twenty-five (5.5%) patients had LNI, with a median ITV over three times higher than that of the non-LNI cohort (19.5 ng/ml vs 5.9 ng/ml, p < 0.001). As expected, PSA, > cT1c, and bGS ≥ 8 were significantly higher in the LNI cohort (p < 0.001, p = 0.001, and p < 0.001, respectively). On multivariate logistic regression analysis, ITV (OR 1.39, p = 0.001) and PSA p = 0.001) and clinical stage > T1c (p = 0.009) were found to be independent predictors of LNI (Table 2).
As with EPE, the AUC of ITV (0.81, p = 0.151) and ITV with PSA (0.92, p = 0.614) were comparable to that of Partin tables (0.90).
SVI analysis
Patients with SVI had more than double the median ITV of patients without SVI (1.902 cc vs 0.801 cc, p < 0.001). In the case of SVI, only ITV (OR 1.28, p = 0.009) and bGS ≥ 8 (p < 0.001) were independent predictors on multivariate analysis (Table 2).
Again, Partin tables had comparable AUC (0.82) to both ITV (0.75, p = 0.283) and ITV combined with PSA (0.78, p = 0.419).
PM analysis
Seventy-one patients (15.7%) in our analysis had PM, of whom 45.7% had EPE. Patients with PM had slightly higher median ITV. (0.977 cc vs 0.807 cc, p = 0.046). However, unlike with the other adverse pathologic parameters, ITV was not found to be an independent predictor of PM in our cohort (Table 2).
BCR analysis
In total, 49 patients (10.9%) experienced BCR during the median follow-up time of 24.9 months (IQR 28.6). Patients with ITV ≥ 2 cc had a higher incidence of BCR (18.9% vs 8.7%, p = 0.005). Estimated 5-year BCR-free survival of the cohort was 78.3% (84.1% for ITV < 2.0 cc, 58.5% for ITV ≥ 2.0 cc, log-rank = 0.001) (Fig. 3). On Cox regression, age (p < 0.001), PSA (p = 0.002), > cT1c (p = 0.049), and Gleason 8–10 on biopsy (p < 0.001) were found to be independent predictors of BCR (Table 3). Although ITV was predictive on univariate analysis (HR 2.70, p = 0.001), it was not found to be an independent predictor (HR 1.69, p = 0.130).
Discussion
The relationship between ITV, measured on post-prostatectomy pathology, and adverse pathologic outcomes has been well established. To the best of our knowledge, this is the first study to evaluate the predictive capacity of preoperative ITV assessment using prostate mpMRI. Although there are several algorithms in use for PCa risk-stratification, there is still a great deal of uncertainty in the prediction of patient outcomes [10]. This is particularly true in intermediate-risk cancers, which often vary widely in rates of adverse pathology and cancer recurrence [11]. We believe that ITV on MRI has the potential to help differentiate patients at higher clinical and pathologic risk.
EPE is a relatively common adverse outcome, with rates of EPE ranging from 10 to 15% in low-risk cancers to as high as 45–50% in higher risk cancers [12]. The ability to predict EPE is valuable in preoperative planning, as the presence or absence of EPE may influence patient selection for nerve-sparing procedures. Gleason score on biopsy, PSA, and clinical stage have all been established as independent predictors of EPE, which is consistent with our results [13, 14]. We found that ITV on MRI was an independent predictor of EPE. We also found that ITV and PSA combined was a comparable predictor of EPE when juxtaposed with Partin tables, one of the most frequently used tools to predict pathologic outcomes. The prognostic value of ITV was previously demonstrated by Chun et al., who showed that both pathologic total TV and percentage of high-grade TV were predictors of EPE [15]. As index lesions tend to be higher grade tumors, our results are aligned with their study. EPE itself can also be measured on MRI; however, its detection continues to present a challenge, and a study by Kongnyuy et al. found the sensitivity and specificity of mpMRI for EPE to be only 56% and 72%, respectively [16]. This is because EPE can be quite subtle and is often a microscopic diagnosis. Therefore, a surrogate indicator of EPE may be needed. ITV thus represents an additional, more-easily-measured preoperative predictor of EPE than direct visualization on MRI.
Prediction of LNI and SVI is also important in preoperative planning, as patients with LNI and SVI tend to have poorer outcomes than their counterparts [17]. The sensitivity of preoperative mpMRI for detecting SVI is approximately 40%, and it is even less effective for the detection of LNI, particularly in nodes < 5 mm [17, 18]. mpMRI is limited to detection of enlarged lymph nodes which often does not occur in PCa. In particular, detection of LNI is applicable when determining the extent of pelvic lymph node dissection (PLND) required, which is recommended in the majority of patients who undergo RP, based on nomogram-calculated preoperative risk of LNI. In our analysis, ITV on MRI was an independent predictor of LNI and SVI and produced results comparable to Partin tables. This is consistent with data from Knoedler et al. which found that ITV on pathology was an independent predictor of both LNI and SVI [1]. Of note, there were no patients with Gleason 6 disease who were found to have LNI on pathology, which may contribute to the argument against LN dissection in a lower risk population. As the current predictive capacity for SVI and LNI is still evolving, ITV on MRI may provide another preoperative metric by which to determine the risk of LNI and SVI and, therefore, aid in preoperative decision making.
Of particular interest is the relationship between ITV and PM. The presence of positive surgical margins is an established independent predictor of BCR [19]. In some studies, it has also been associated with metastatic progression [20] and PCa-specific mortality [21]. The location and size of prostate tumors on MRI have been increasingly incorporated into operative decision making, allowing surgeons to balance preservation of the bladder neck and neurovascular bundles with adequate cancer control. However, even with the superior imaging, the rate of PM can be as high as 30–50% in high-stage cancers, PM EPE [22]. In our analysis, preoperative PSA was the only independent predictor of PM. This is consistent with a meta-analysis by Novara et al., which demonstrated that although PSA and pathology GS were generally found to be predictive of PM, bGS was not a predictor [23]. Several analyses have also demonstrated an association between TV measured on pathology and PM following RP, although we did not find this to be the case [1, 15]. Chun et al. suggested that total TV, rather than high-grade TV, was predictive of PM [15]. They found that total pathologic tumor volume significantly increased the predictive accuracy of their PM model, while high-grade tumor volume had no effect. As this study, in measuring index lesion, focuses on higher grade tumors, it is possible that future studies will find total TV measured on MRI to be an independent predictor of PM.
Biochemical recurrence is a commonly accepted intermediate metric for the success or failure of PCa treatment. Standard preoperative nomograms use PSA, clinical stage, and bGS to predict the likelihood of BCR. Recently, the use of mpMRI has been considered as an additional predictor of BCR [24]. As an easily measured variable, ITV would be a helpful addition to predictive models of BCR. Numerous studies have determined a correlation between TV on pathology and BCR. A meta-analysis by Meng et al. found that both TV and % TV were independent predictors of BCR [25]. However, in our analysis, we did not find ITV on MRI to be an independent predictor of BCR, although patients with ITV < 2 cc had a significantly longer BCR-free survival compared to those with larger ITVs. Due to a small number of events, our BCR analysis may be underpowered to identify ITV as an independent predictor. It is possible that future studies analyzing a greater number of patients with BCR will reveal an association with ITV.
The additional prognostic information obtained from ITV on MRI may also provide valuable insight while planning prostate interventions. ITV in combination with other clinical predictors can help in risk-stratifying patients with intermediate and high-risk cancer to determine which patients would be suitable candidates for focal therapy and RP, respectively, thus achieving more accurate selection of focal candidates. However, to utilize ITV in this fashion, larger studies will be needed to validate this preoperative variable and to find ITV cutoffs for appropriately risk-stratifying PCa patients.
We recognize the limitations inherent to retrospective design study. All results are from a single institution with a higher risk patient population (26.4% D’Amico high risk), compared to 4–15% in other contemporary cohorts [26, 27]. Therefore, the results cannot necessarily be generalized to populations with a larger percentage of lower risk cancers, although as non-operative interventions such as active surveillance gain traction [28], there may be a trend towards higher risk cancer at the time of prostatectomy. The other limitation is the lack of PIRADS scores for the majority of our patients, and our inability to analyze this more standardized MRI metric. There were also a relatively small number of patients with poor outcomes. This might have reduced the power of the study and may have rendered it more difficult to determine statistical associations. In addition, as our study focused on the tumor volume of the index lesion specifically, we cannot comment on the role that secondary tumor volumes play in the prediction of outcomes. As PCa has a tendency to be multifocal in nature, it is possible that the sum of TV from all lesions could have additional predictive ability.
There is also a concern that MRI falls short for the precise estimation of prostate TV. Although a recent study by Turkbey et al. indicated that MRI may be adequate for accurate estimation of ITV specifically [3]. Finally, as we did not have a second dataset available on which to perform an external validation of the predictive ability of ITV and ITV + PSA, it will be necessary to validate this model in other large cohorts.
We demonstrate that ITV measured on preoperative MRI is a novel factor that has comparable predictive ability for pathologic outcomes when compared to pathologic ITV. We believe that the strength of the association between ITV and EPE, SVI, and LNI merits further investigation as a potential predictive factor for consideration prior to surgery and radiation therapy. If validated, ITV on MRI may provide an easily obtained biomarker for nomogram prediction of PCa staging and surgical planning.
References
Knoedler JJ, Karnes RJ, Thompson RH et al (2014) The association of tumor volume with mortality following radical prostatectomy. Prostate Cancer Prostatic Dis 17:144–148
Wysock JS, Mendhiratta N, Zattoni F et al (2016) Predictive value of negative 3T multiparametric magnetic resonance imaging of the prostate on 12-core biopsy results. BJU Int 118:515–520
Turkbey B, Mani H, Aras O et al (2012) Correlation of magnetic resonance imaging tumor volume with histopathology. J Urol 188:1157–1163
Villers A, Puech P, Mouton D et al (2006) Dynamic contrast enhanced, pelvic phased array magnetic resonance imaging of localized prostate cancer for predicting tumor volume: correlation with radical prostatectomy findings. J Urol 176:2432–2437
Turkbey B, Pinto PA, Mani H et al (2010) Prostate cancer: value of multiparametric MR imaging at 3 T for detection–histopathologic correlation. Radiology 255:89–99
Institute JH-JBBU (2012) Partin tables. https://www.hopkinsmedicine.org/brady-urology-institute/specialties/conditions-and-treatments/prostate-cancer/fighting-prostate-cancer/partin-table.html. Accessed 23 Mar 2012
Cookson MS, Aus G, Burnett AL et al (2007) Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol 177:540–545
Meyer CP, Hansen J, Boehm K et al (2016) Tumor volume improves the long-term prediction of biochemical recurrence-free survival after radical prostatectomy for localized prostate cancer with positive surgical margins. World J Urol 35(2):199–206
Merrill MM, Lane BR, Reuther AM et al (2007) Tumor volume does not predict for biochemical recurrence after radical prostatectomy in patients with surgical Gleason score 6 or less prostate cancer. Urology 70:294–298
Jung JW, Lee JK, Hong SK et al (2015) Stratification of patients with intermediate-risk prostate cancer. BJU Int 115:907–912
Beauval JB, Ploussard G, Cabarrou B et al (2016) Improved decision making in intermediate-risk prostate cancer: a multicenter study on pathologic and oncologic outcomes after radical prostatectomy. World J Urol 35(8):1191–1197
Somford DM, Hamoen EH, Futterer JJ et al (2013) The predictive value of endorectal 3 Tesla multiparametric magnetic resonance imaging for extraprostatic extension in patients with low, intermediate and high risk prostate cancer. J Urol 190:1728–1734
Pelzer AE, Colleselli D, Bektic J et al (2008) Pathological features of Gleason score 6 prostate cancers in the low and intermediate range of prostate-specific antigen level: is there a difference? BJU Int 101:822–825
Nakanishi H, Troncoso P, Babaian RJ (2008) Prediction of extraprostatic extension in men with biopsy Gleason score of 8 or greater. J Urol 180:2441–2445 (discussion 2445-6)
Chun FK, Briganti A, Jeldres C et al (2007) Tumour volume and high grade tumour volume are the best predictors of pathologic stage and biochemical recurrence after radical prostatectomy. Eur J Cancer 43:536–543
Kongnyuy M, Sidana A, George AK et al (2017) Tumor contact with prostate capsule on magnetic resonance imaging: a potential biomarker for staging and prognosis. Urol Oncol 35:30.e1–30.e8
Abdollah F, Abdo A, Sun M et al (2013) Pelvic lymph node dissection for prostate cancer: adherence and accuracy of the recent guidelines. Int J Urol 20:405–410
Briganti A, Gallina A, Nazareno S et al (2013) 374 External validation of the eau guidelines for pelvic lymph node dissection among patients treated with robotic assisted radical prostatectomy. J Urol 189:e151–e152
Yossepowitch O, Briganti A, Eastham JA et al (2014) Positive surgical margins after radical prostatectomy: a systematic review and contemporary update. Eur Urol 65:303–313
Boorjian SA, Karnes RJ, Crispen PL et al (2010) The impact of positive surgical margins on mortality following radical prostatectomy during the prostate specific antigen era. J Urol 183:1003–1009
Chalfin HJ, Dinizo M, Trock BJ et al (2012) Impact of surgical margin status on prostate-cancer-specific mortality. BJU Int 110:1684–1689
Preston MA, Blute ML (2014) Positive surgical margins after radical prostatectomy: does it matter? Eur Urol 65:314–315
Novara G, Ficarra V, Mocellin S et al (2012) Systematic review and meta-analysis of studies reporting oncologic outcome after robot-assisted radical prostatectomy. Eur Urol 62:382–404
Ho R, Siddiqui MM, George AK et al (2016) Preoperative Multiparametric Magnetic Resonance Imaging Predicts Biochemical Recurrence in Prostate Cancer after Radical Prostatectomy. PLoS One 11:e0157313
Meng Y, Li H, Xu P et al (2015) Do tumor volume, percent tumor volume predict biochemical recurrence after radical prostatectomy? A meta-analysis. Int J Clin Exp Med 8:22319–22327
Bhindi B, Mamdani M, Kulkarni GS et al (2015) Impact of the U.S. preventive services task force recommendations against prostate specific antigen screening on prostate biopsy and cancer detection rates. J Urol 193:1519–1524
Banerji JS, Wolff EM, Massman JD et al (2016) Prostate needle biopsy outcomes in the Era of the U.S. preventive services task force recommendation against prostate specific antigen based screening. J Urol 195:66–73
Klotz L, Vesprini D, Sethukavalan P et al (2015) Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 33:272–277
Acknowledgements
Supported by the Intramural Research Program of National Institutes of Health, National Cancer Institute, Center for Cancer Research, Center for Interventional Oncology, and the National Institutes of Health Medical Research Scholars Program, a public–private partnership supported jointly by National Institutes of Health and contributions to the Foundation for National Institutes of Health from Pfizer Inc., The Doris Duke Charitable Foundation, The Alexandria Real Estate Equities Inc., Mr. and Mrs. Joel S. Marcus, the Howard Hughes Medical Institute and other private donors (http://fnih.org/work/education-training-0/medical-research-scholars-program).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no affiliation with any organization with a direct or indirect financial interest in the subject matter discussed in the manuscript. NIH, Philips Healthcare have a cooperative research, NIH and Philips share intellectual property in the field and development agreement.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sugano, D., Sidana, A., Jain, A.L. et al. Index tumor volume on MRI as a predictor of clinical and pathologic outcomes following radical prostatectomy. Int Urol Nephrol 51, 1349–1355 (2019). https://doi.org/10.1007/s11255-019-02168-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-019-02168-4