Abstract
Purpose
To develop a novel application evaluating the effect of tumor volume (TV) and percentage of high-grade tumor volume (%HGTV) on long-term biochemical recurrence-free survival rate (BCRFS) after radical prostatectomy (RP) in patients with pT2 PCa.
Methods
Retrospective analysis of 903 men with pT2 PCa between 1992 and 2004 at a single European tertiary care center was performed. Cox regression models identified risk factors for BCR. A nomogram was developed to predict the BCRFS at 5, 10 and 15 years after RP. Decision curve analyses were performed to identify the net increase in cases identified by the full model.
Results
BCR-free survival rates at 5, 10 and 15 years were 94, 90 and 86 %. In Cox regression analyses, TV, %HGTV and positive surgical margin status (SM) were independent predictors of BCR. Predictive accuracies (PA) at 5, 10 and 15 years of the base model (PSA, Gleason score, SM) were 76.8 % (95 % CI 67.9–78.2 %), 70.5 % (95 % CI 64.9–75.0 %) and 68.1 % (95 % CI 60.6–73.5 %). The full model, including TV and %HGTV, achieved 76.9, 72.4 and 70.7 %. These PA differences were statistically significant at 10 and 15 years (p < 0.001).
Conclusions
TV and %HGTV could potentially serve as valuable measures to stratify patients at high risk of BCR. The use of our nomogram should be considered to counsel patients with pT2 disease and SM and to design appropriate follow-up or treatment regimens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is the commonest non-cutaneous malignancy in men [1]. Radical prostatectomy (RP) is a standard first-line treatment for eligible patients in localized disease and the majority of patients will harbor pT2 PCa in the final histopathological specimen [2–4]. While most of these patients have excellent biochemical recurrence-free survival (BCRFS) rates from 73 to 99 % over a 10-year period [2, 4–6], some will eventually recur. Previous studies have identified variables such as PSA, pathological substages, Gleason score (GS) and/or surgical margin (SM), to be predictive of BCRFS–failure [2–7]. Additionally, several studies have reported the predictive value of pathologic tumor volume (TV) and/or percentage of high-grade tumor volume (%HGTV) in prediction of BCR [7–12]. TV and %HGTV were shown to be independent predictors of BCR, and their inclusion in a multivariable model increased predictive accuracy (PA) [12]. However, other studies have questioned these findings [13, 14].
Clinically, the management of patients with adverse pathologic features and localized disease remains challenging. While adjuvant therapies might be considered in these patients, the risk of overtreatment is inherent. On the basis of these considerations, we assessed long-term BCRFS rates after RP in patients with pathological pT2 PC and aimed to discern the clinical value of TV and %HGTV in this setting. We specifically sought to provide a clinical decision tool to identify patients who would benefit from adjuvant measures.
Patients and methods
Study population
Institutional data of 2095 men who underwent RP between 1992 and 2005 for pT2 PCa were collected. For the current study, we only considered 1181 men with available detailed computer planimetric-assisted quantification of TV and %HGTV. To ensure homogeneity of the cohort, 278 patients were excluded from analyses due to neo-adjuvant or adjuvant androgen-deprivation therapy, lymph node involvement, or missing information on clinical/pathological variables and/or follow-up. This resulted in 903 men eligible for statistical analysis.
Variables
Pathological stage was defined according to the 2002 TNM staging system [15]. Until March 2001, decision for nerve sparing technique (NS) was made on surgeon`s expertise and thereafter based on a tree regression model [16]. RP specimens were processed according to the 3-mm whole-mount Stanford protocol [17]. Contact of tumor cells at the inked margin of the prostate was considered to represent a positive SM. In all prostate tissues, detailed pathological computer planimetric-assisted quantification of TV and %HGTV was performed, as previously reported [18]. For further analysis, TV was subdivided as ≤2 and >2 ml based on the overall median value. HGTV was defined as the volume in the specimen consisting of Gleason score ≥4. During follow-up, patients had PSA value measurements quarterly in the first, twice a year in the second and once a year from the third year after RP. BCR was defined as a PSA level of ≥0.2 ng/ml and rising after an initially undetectable PSA level after RP [7]. Men without BCR or who died from causes other than prostate cancer were censored at the date of their last undetectable serum PSA level. The primary outcome of interest was defined as the overall BCR. Occurrence of metastases and cancer-specific survival were reported as secondary outcomes.
Statistical analysis
Descriptive statistics focused on frequencies and proportions for categorical variables. Means, medians and interquartile ranges (IQRs) were reported for continuously coded variables. Chi square and independent-sample t tests were used to compare the statistical significance of differences in respectively proportions and means. Kaplan–Meier curves assessed actuarial BCRFS probabilities at 5, 10 and 15 years. Uni- and multivariable Cox logistic regression models (Cox LRMs) were used to test several clinical and pathological parameters to predict BCR at 5, 10 and 15 years after RP. Specifically, the base models included PSA, Gleason score and SM. The extended models additionally included TV and %HGTV. For the lack of standardized %HGTV thresholds, we tested several cutoff points predicting BCR using the minimal p value approach as previously described [19]. This assesses whether the predictive ability of continuously coded variables may be improved by dichotomization and was found to be 20 %HGTV.
The impact of each predictor variable on BCRFS rates at 5, 10 and 15 years were graphically depicted by a nomogram. To test the discrimination ability of the base model versus the extended model, we assessed Harrell’s c index [20]. The c index ranges from 0.5 (chance) to 1.0 (perfect discrimination) and describes the probability in a randomly chosen pair of patients that the one with the event (e.g., BCR) has a higher predicted probability of experiencing the event.
Two hundred bootstrap resamples were used for internal validation [21]. Discriminative ability estimates were compared using the Mantel–Haenszel test. Finally, to identify the net increase in the proportion of cases identified by the full model, we calculated decision curve analyses at 5, 10 and 15 years as introduced by Vickers and Elkin [22]. All tests were two-sided with a statistical significance set at p < 0.05. Analyses were conducted using the statistical package for R (the R foundation for Statistical Computing, Vienna, Austria, version 3.1.2).
Results
Descriptive characteristics of patients with pathological pT2 PCa are shown in Table 1. Median patient age was 63 years (IQR 59–66 years) with median pre-treatment PSA level of 6.4 ng/ml (IQR 4.6–9.0 ng/ml), respectively. Follow-up time was 133 (11.1) months (years) on median (IQR 97–157 months [8.1–13.1 years]). Among cancer survivors alone, the median follow-up time was 11.1 years (IQR 9–13.1). The majority of patients had an unsuspicious digital rectal examination (n = 644, 71 %) and a Gleason score of ≤3 + 3 at biopsy (79 %). In 731 men (81 %), nerve sparing was performed during RP. The majority of patients had pathological Gleason score 3 + 3 (n = 578, 64 %), whereas 325 men (36 %) also harbored less favorable Gleason patterns. Positive surgical margins were observed in 13.1 % of patients (n = 118).
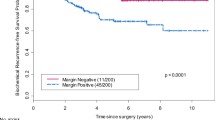
Overall, 137 men (15.2 %) developed BCR and this was proportionally higher in patients with SM (13.4 vs. 27.1 %). BCRFS rates at 5, 10 and 15 years were 91.7, 87.0 and 85.9 %, respectively. Metastatic disease occurred in 1.7 % and cancer-specific/overall mortality in 1.4/12.4 %, respectively. Median survival time was 20.0 years for men without BCR, 15.0 years for men with BCR and 18.3 years overall. Stratified Kaplan–Meier estimators are depicted in Fig. 1 and show significantly different BCRFS rates for (b) tumor volume (TV ≤2 vs. >2 ml), (c) percentage of high-grade tumor volume (%HGTV; ≤20 vs. >20 %), and (d) combination of surgical margin status (SM) and TV (SM− & TV ≤ 2 vs. SM− & TV > 2 ml vs. SM+ & TV ≤ 2 ml vs. SM+ & TV > 2 ml), respectively (all log-rank p < 0.001).
a Kaplan–Meier analyses depicting BCRFS after radical prostatectomy for the entire patient population (n = 903). Additional Kaplan−Meier analyses have been performed b after stratification according to tumor volume (TV; ≤2 vs. >2 ml), c percentage of high-grade tumor volume (%HGTV; ≤20 vs. >20 %), and d combination of surgical margin status (SM) and TV (SM− and TV ≤2 ml vs. SM− and TV >2 ml vs. SM+ and TV ≤2 ml vs. SM+ and TV >2 ml), respectively (all log-rank p < 0.001)
In multivariable Cox LRMs, patients with a Gleason score ≥3 + 4 were more likely to develop BCR relative to patients with a Gleason score ≤3 + 3 (HR 1.8, p = 0.004). Additionally, SM (HR 2.5; p < 0.001), TV > 2 ml (HR 2.0; p < 0.001) and %HGTV > 20 % (HR 1.74, p = 0.02) were independent predictors of BCR after RP (Table 2).
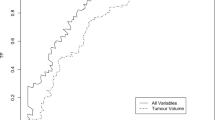
The regression coefficients-based nomogram (Fig. 2a) to predict BCRFS consisting of PSA, Gleason score and surgical margin showed c-indices of 76.8 % (95 % CI 67.9–78.2 %), 70.5 % (95 % CI 64.9–75.0 %) and 68.1 % (95 % CI 60.6–73.5 %) at 5, 10 and 15 years after RP, respectively. Inclusion of TV and %HGTV increased the c-indices to 76.9 % (95 % CI 71.2–81.1 %), 72.4 (95 % CI 68.5–78.3 %) and 70.7 % (95 % CI 63.2–75.8 %) at 5, 10 and 15 years, respectively. These differences in c-indices were statistically significant at 10 and 15 years (both p < 0.001). Decision curve analysis demonstrated a net clinical benefit of the full model across a range of clinically relevant threshold probabilities for BCR at all determined time points (Fig. 2b).
a Nomogram predicting biochemical recurrence-free survival (BCRFS) at 5, 10 and 15 years after radical prostatectomy in patients with pathologically organ confined prostate cancer. To obtain nomogram-predicted probability of BCRFS, locate patient values at each axis. Draw a vertical line to the “Point” axis to determine how many points are attributed for each variable value and sum the points for all variables. Locate the sum on the “Total Points” line to be able to assess the individual probability of BCRFS on the “Predicted value” line for each time point (5 and 10 years, respectively). b In decision curve analysis (DCA), the developed nomogram appears to confer an advantage in identifying patients with BCRFS. For example, applying a nomogram-derived probability threshold of 15 % at 10 years after RP, use of the nomogram including TV and %HGTV (Model 1) would incur a net benefit of 20 over the base model (Model 2). The net benefit at a threshold probability 15 % can be interpreted as using the full model leads to the equivalent of a net 20 true-positive results per 100 patients without an increase in the number of false-positive results. PSA, prostate specific antigen; SM−, negative surgical margin; SM+, positive surgical margin; c index, predictive accuracy
Discussion
Men with pT2 PCa after RP generally present excellent BCRFS. However, some patients fail to remain BCR-free [2, 4, 5]. Disease characteristics, such as PSA, GS, pathological stage or SM, have been described as predictive factors of BCR after RP [2–7, 23]. Additionally, some authors have also investigated the predictive role of TV and HGTV on BCR [7–11]. Pathologists do not regularly provide TV assessments due to higher outlays and the complexities of the protocol. However, these tumor characteristics are promising markers to classify patients at risk of BCR. In order to clarify the cost–benefit ratio of these tumor characteristics on long-term BCRFS rates in men with pT2 PCa, we developed a novel predictive model based on TV and %HGTV and evaluated its discriminative accuracy.
Several of our findings are noteworthy. First, we report excellent long-term outcomes of patients with pT2 disease. The majority (85.9 %) of our patients were disease-free after 15 years. While BCR is rare in pT2 disease, it is significantly more pronounced in patients with SM. In our current cohort, 27 % of men with SM developed a BCR, which was significantly higher than in patients without SM. Pettenati et al. [24] corroborated these findings when they found a 33 % BCR after PSM in an institutional cohort of 630 patients. Although SM constitutes an adverse pathologic feature, there is an ongoing debate about the need for adjuvant therapies in patients with SM in pT2 disease. In light of evidence for favorable outcomes in locally advanced (≥pT3) tumors [25, 26], adjuvant radiotherapy might be considered, but has to be weighted against worse toxicity profiles. Thus, current guidelines advocate either adjuvant radiotherapy or a PSA-based follow-up with concurrent salvage radiotherapy as viable treatment options [27]. As SM falls short as a lone determinant of adjuvant therapies, it might rather be considered a stratification tool for the definition of high-risk disease in conjunction with TV and %HGTV as central determinants of BCR [11, 12, 28, 29]. Similar to Rampersaud et al. we used different categories for TV and %HGTV to distinguish between low and high risk of BCR. Patients with higher absolute TV expectably showed the highest risk of BCR. Likewise, %HGTV was highly associated with BCR, which is in accordance with the previous reports [9, 30]. The inclusion of TV and %HGTV in our nomogram resulted in significant improvements of PA at 10 and 15 years (p < 0.001). Contrary to previous reports on the weak discriminative accuracy of TV and %HGTV [9], our study provides strong evidence for the inclusion of these variables by utilizing a larger cohort study and substantially longer follow-up time. It might further be speculated that a more tailored patient sample selection as well as newer grading methods and improved statistical analyses have contributed to the different findings between earlier studies and ours. In line with our results, the addition of TV and %HGTV to the panel of reported pathological results in patients with positive surgical margin holds potential for the optimization of (long-term) risk prediction of BCR. Therefore, our newly developed nomogram is a promising tool to predict BCRFS in these patients and guide adjuvant treatment decisions. On the other hand, the predictions from our nomogram can enable clinicians to individually tailor a more stringent, PSA-based follow-up to patients with high BCR risk; further, patients with pathologically insignificant PCa could undergo a less rigorous follow-up, e.g., PSA test every second year or less after 10 years of inconspicuous follow-up. The initially higher costs of obtaining TV and %HGTV could be balanced by foregoing unnecessary and costly adjuvant therapies as well as unnecessary follow-up PSA readings.
Despite its strength, our study has some limitations. First, all patients were operated in a single European tertiary referral center; therefore, rates of pathological organ confined PCa may be affected by selection bias and differ from other geographical regions and institutions. In detail, it has to be acknowledged that 278 men were excluded because of unavailability of data or additional therapy regimens. Although this ascertained homogeneity of the study cohort, sampling bias might have subsequently increased. Secondly, our study comprised a majority of Gleason 3 + 3 pattern scores at final pathology and initial biopsy. According to the latest guidelines, these patients would currently constitute candidates for active surveillance. However, our study comprises a historical cohort when such recommendations were not readily available. The influence of tumor volume on BCR in current cohorts, with higher ratios of intermediate- and high-risk patients, needs to be further evaluated. In the same sense, our current model needs external validation. Furthermore, computer planimetric evaluation of tumor volume as performed on the specimens in our study, is itself prone to bias. First, it was first described over 20 years ago with a potential weakness in assessing tumor volume in case of unusual tumor shapes. Second, in patients with positive surgical margin status, it might have underestimated the true tumor volume. However, our cohort provides essential and valuable information in conjunction with an extended follow-up cohort. Finally, it has to be acknowledged that statistical models such as nomograms depend on their development cohort. Our nomogram should be validated with an external patient cohort to truly assess accuracy and calibration of the model. While the active use of nomograms has been questioned, advances in the routine applicability of these predictive tools have been made, and their use should be further encouraged [31, 32].
Taken together, our findings show good BCR-free survival rates after 5, 10 and 15 years in pT2 PCa patients after RP. The developed nomogram for prediction of BCRFS showed a high predictive accuracy after internal validation. TV and %HGTV could potentially serve as valuable measures to stratify patients at high risk of BCR. The use of our nomogram should be considered to counsel patients with pT2 disease and SM and to design appropriate follow-up or treatment regimens.
References
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics. CA Cancer J Clin 65(1):5–29. doi:10.3322/caac.21254
Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC (2003) Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol 169(2):517–523. doi:10.1097/01.ju.0000045749.90353.c7
Kordan Y, Salem S, Chang SS, Clark PE, Cookson MS, Davis R, Herrell SD, Baumgartner R, Phillips S, Smith JA Jr, Barocas DA (2009) Impact of positive apical surgical margins on likelihood of biochemical recurrence after radical prostatectomy. J Urol 182(6):2695–2701. doi:10.1016/j.juro.2009.08.054
Eggener SE, Scardino PT, Walsh PC, Han M, Partin AW, Trock BJ, Feng Z, Wood DP, Eastham JA, Yossepowitch O, Rabah DM, Kattan MW, Yu C, Klein EA, Stephenson AJ (2011) Predicting 15-year prostate cancer specific mortality after radical prostatectomy. J Urol 185(3):869–875. doi:10.1016/j.juro.2010.10.057
Kane CJ, Im R, Amling CL, Presti JC Jr, Aronson WJ, Terris MK, Freedland SJ (2010) Outcomes after radical prostatectomy among men who are candidates for active surveillance: results from the SEARCH database. Urology 76(3):695–700. doi:10.1016/j.urology.2009.12.073
Caso JR, Tsivian M, Mouraviev V, Polascik TJ, Moul JW (2010) Pathological T2 sub-divisions as a prognostic factor in the biochemical recurrence of prostate cancer. BJU Int 106(11):1623–1627. doi:10.1111/j.1464-410X.2010.09439.x
Ahyai SA, Zacharias M, Isbarn H, Steuber T, Eichelberg C, Kollermann J, Fisch M, Karakiewicz PI, Huland H, Graefen M, Chun FK (2010) Prognostic significance of a positive surgical margin in pathologically organ-confined prostate cancer. BJU Int 106(4):478–483. doi:10.1111/j.1464-410X.2009.09162.x
Ramos CG, Roehl KA, Antenor JA, Humphrey PA, Catalona WJ (2004) Percent carcinoma in prostatectomy specimen is associated with risk of recurrence after radical prostatectomy in patients with pathologically organ confined prostate cancer. J Urol 172(1):137–140. doi:10.1097/01.ju.0000132139.40964.75
Palisaar RJ, Graefen M, Karakiewicz PI, Hammerer PG, Huland E, Haese A, Fernandez S, Erbersdobler A, Henke RP, Huland H (2002) Assessment of clinical and pathologic characteristics predisposing to disease recurrence following radical prostatectomy in men with pathologically organ-confined prostate cancer. Eur Urol 41(2):155–161
Merrill MM, Lane BR, Reuther AM, Zhou M, Magi-Galluzzi C, Klein EA (2007) Tumor volume does not predict for biochemical recurrence after radical prostatectomy in patients with surgical Gleason score 6 or less prostate cancer. Urology 70(2):294–298. doi:10.1016/j.urology.2007.03.062
Song C, Kang T, Yoo S, Jeong IG, Ro JY, Hong JH, Kim CS, Ahn H (2011) Tumor volume, surgical margin, and the risk of biochemical recurrence in men with organ-confined prostate cancer. Urol Oncol. doi:10.1016/j.urolonc.2010.11.005
Chun FK, Briganti A, Jeldres C, Gallina A, Erbersdobler A, Schlomm T, Walz J, Eichelberg C, Salomon G, Haese A, Currlin E, Ahyai SA, Benard F, Huland H, Graefen M, Karakiewicz PI (2007) Tumour volume and high grade tumour volume are the best predictors of pathologic stage and biochemical recurrence after radical prostatectomy. Eur J Cancer 43(3):536–543. doi:10.1016/j.ejca.2006.10.018
Kikuchi E, Scardino PT, Wheeler TM, Slawin KM, Ohori M (2004) Is tumor volume an independent prognostic factor in clinically localized prostate cancer? J Urol 172(2):508–511. doi:10.1097/01.ju.0000130481.04082.1a
Salomon L, Levrel O, Anastasiadis AG, Irani J, De La Taille A, Saint F, Vordos D, Cicco A, Hoznek A, Chopin D, Abbou CC (2003) Prognostic significance of tumor volume after radical prostatectomy: a multivariate analysis of pathological prognostic factors. Eur Urol 43(1):39–44
Greene FPD, Fleming I et al (2002) American Joint Committee on cancer staging manual, 6th edn. Springer, New York
Graefen M, Haese A, Pichlmeier U, Hammerer PG, Noldus J, Butz K, Erbersdobler A, Henke RP, Michl U, Fernandez S, Huland H (2001) A validated strategy for side specific prediction of organ confined prostate cancer: a tool to select for nerve sparing radical prostatectomy. J Urol 165(3):857–863
Srigley JR, Humphrey PA, Amin MB, Chang SS, Egevad L, Epstein JI, Grignon DJ, McKiernan JM, Montironi R, Renshaw AA, Reuter VE, Wheeler TM (2009) Protocol for the examination of specimens from patients with carcinoma of the prostate gland. Arch Pathol Lab Med 133(10):1568–1576. doi:10.1043/1543-2165-133.10.1568
Henke RP, Kruger E, Ayhan N, Hubner D, Hammerer P, Huland H (1994) Immunohistochemical detection of p53 protein in human prostatic cancer. J Urol 152(4):1297–1301
Mazumdar M, Glassman JR (2000) Categorizing a prognostic variable: review of methods, code for easy implementation and applications to decision-making about cancer treatments. Stat Med 19(1):113–132
Harrell FE Jr, Lee KL, Mark DB (1996) Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15(4):361–387. doi:10.1002/(SICI)1097-0258(19960229)15:4<361:AID-SIM168>3.0.CO;2-4
Steyerberg EW, Harrell FE Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD (2001) Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol 54(8):774–781
Vickers AJ, Elkin EB (2006) Decision curve analysis: a novel method for evaluating prediction models. Med Decis Mak: Internat J Soc Med Decis Mak 26(6):565–574. doi:10.1177/0272989x06295361
Loeb S, Hernandez DJ, Mangold LA, Humphreys EB, Agro M, Walsh PC, Partin AW, Han M (2008) Progression after radical prostatectomy for men in their thirties compared to older men. BJU Int 101(12):1503–1506. doi:10.1111/j.1464-410X.2008.07500.x
Pettenati C, Neuzillet Y, Radulescu C, Herve JM, Molinie V, Lebret T (2015) Positive surgical margins after radical prostatectomy: What should we care about? World J Urol. doi:10.1007/s00345-015-1580-x
Thompson IM Jr, Tangen CM, Paradelo J, Lucia MS, Miller G, Troyer D, Messing E, Forman J, Chin J, Swanson G, Canby-Hagino E, Crawford ED (2006) Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA J Am Med Assoc 296(19):2329–2335. doi:10.1001/jama.296.19.2329
Van der Kwast TH, Bolla M, Van Poppel H, Van Cangh P, Vekemans K, Da Pozzo L, Bosset JF, Kurth KH, Schroder FH, Collette L (2007) Identification of patients with prostate cancer who benefit from immediate postoperative radiotherapy: EORTC 22911. J Clin Oncol: Off J Am Soc Clin Oncol 25(27):4178–4186. doi:10.1200/JCO.2006.10.4067
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, Mottet N (2014) EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 65(1):124–137. doi:10.1016/j.eururo.2013.09.046
Chung BI, Tarin TV, Ferrari M, Brooks JD (2011) Comparison of prostate cancer tumor volume and percent cancer in prediction of biochemical recurrence and cancer specific survival. Urol Oncol 29(3):314–318. doi:10.1016/j.urolonc.2009.06.017
Rampersaud EN, Sun L, Moul JW, Madden J, Freedland SJ (2008) Percent tumor involvement and risk of biochemical progression after radical prostatectomy. J Urol 180(2):571–576. doi:10.1016/j.juro.2008.04.017 (discussion 576)
Stamey TA, McNeal JE, Yemoto CM, Sigal BM, Johnstone IM (1999) Biological determinants of cancer progression in men with prostate cancer. JAMA, J Am Med Assoc 281(15):1395–1400
Nguyen CT, Kattan MW (2009) Development of a prostate cancer metagram: a solution to the dilemma of which prediction tool to use in patient counseling. Cancer 115(13 Suppl):3039–3045. doi:10.1002/cncr.24355
Capitanio U, Jeldres C, Shariat SF, Karakiewicz P (2008) Clinicians are most familiar with nomograms and rate their clinical usefulness highest, look-up tables are second best. Eur Urol 54(4):958–959. doi:10.1016/j.eururo.2008.04.082
Author contributions
Christian P. Meyer and Jens Hansen were involved in data collection and management, analyzed the data, and wrote and edited the manuscript. Katharina Boehm, Firas Abdollah analyzed the data and edited the manuscript. Derya Tilki, Guido Sauter, Hartwig Huland and Markus Graefen were involved in data collection and edited the manuscript. Margit Fisch and Quoc-Dien Trinh edited the manuscript. Felix K.H. Chun was involved in project development, data collection and management, and edited the manuscript. Sascha A. Ahyai was involved in project development, data collection and management, and wrote and edited the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. For this type of study, formal consent is not required. This article does not contain any studies with animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Meyer, C.P., Hansen, J., Boehm, K. et al. Tumor volume improves the long-term prediction of biochemical recurrence-free survival after radical prostatectomy for localized prostate cancer with positive surgical margins. World J Urol 35, 199–206 (2017). https://doi.org/10.1007/s00345-016-1861-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-016-1861-z