Abstract
Purpose
To prove that phosphodiesterase type-4 inhibitors could potentially treat obesity-associated overactive bladder through modulation of the systemic inflammatory response.
Methods
In this 12-week study, 90 female Sprague–Dawley rats were divided into three groups: (1) vehicle-treated normal diet (ND)-fed rats; (2) vehicle-treated high-fat diet (HFD)-fed rats; and (3) roflumilast-treated HFD-fed rats. Oral roflumilast (5 mg/kg/day) was administered during the last 4 weeks of HFD feeding in the test group. At 12 weeks, a urodynamic study was performed in ten rats of each group. Bladder tissue was extracted, the bladder mucosa was separated under microscopy, and bladder detrusor smooth muscle (DSM) expression of TNF-α, interleukin (IL)-6, IL-1β, and nuclear factor kappa B (NF-κB) were analyzed using Western blotting and quantitative reverse transcription-polymerase chain reaction (qRT-PCR).
Results
Bodyweights of the HFD-fed rats significantly increased and were not ameliorated by roflumilast treatment. Cystometry evidenced augmented frequency and non-void contractions in obese rats that were also prevented by roflumilast. These alterations were accompanied by a markedly increased expression of TNF-α, IL-6, IL-1β, and NF-κB in DSM of obese rats. Furthermore, roflumilast decreased expression of inflammatory factors in DSM.
Conclusions
Oral treatment with roflumilast in rats fed an HFD restores normal bladder function and downregulates expression of inflammatory factors in the bladder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Overactive bladder (OAB) is characterized by urgency, with or without urgency urinary incontinence, usually associated with increased daytime frequency and nocturia. Usually, symptoms and a diagnosis of urodynamically demonstrable detrusor overactivity (DO) are combined in OAB [1]. OAB significantly affects quality of life and mental health in affected patients and is mainly manifested as increased nocturia causing sleep disorders [2]. It is well known that the potential etiology and contributing factors of OAB are complex and diverse. In recent years, OAB incidence among elderly patients has shown an upward trend, and the prevalence of OAB has increased with age. Obese people with body mass index (BMI) above 25 are more likely to develop OAB. Therefore, obesity is considered a specific cause of OAB [2, 3].
Obesity has been established as a common risk factor for lower urinary tract symptoms (LUTS), including bladder hyperactivity and urinary incontinence [4]. Moreover, obesity is associated with a chronic inflammatory response characterized by a proinflammatory state with oxidative stress, increased acute-phase reactants, and activation of inflammatory signaling pathways. The increase in concentrations of interleukin (IL)-1, IL-6, and tumor necrosis factor alpha (TNF-α) in detrusor smooth muscle (DSM), associated with obesity, may cause bladder detrusor dysfunction, including DO [5,6,7]. Epidemiological research has shown that increased BMI is positively correlated with LUTS and obese individuals are more likely to develop prominent symptoms [8]. Female rats were fed a high-fat diet (HFD) to induce overweight, high insulin, high glucose blood concentrations, and DO. Female Sprague–Dawley (SD) rats fed a hyperlipidemic diet gained more weight and showed increased voiding and non-voiding bladder contractility on cystometry, suggesting bladder overactivity [9, 10]. Thus, the establishment of this disease model is the premise of research on related OAB symptoms in obese rats.
In the clinical setting, conventional anticholinergic drugs find widespread use in OAB treatment, but have certain side effects, such as xerostomia, constipation, headache, and blurred vision, that limit their application [11]. Phosphodiesterase type-4 (PDE4) plays a key role in the degradation of cyclic adenosine monophosphate (cAMP) in inflammatory cells as well as in vascular endothelial cells, smooth muscle cells, and related inflammatory keratinocytes [12]. PDE4 inhibitors can improve cAMP levels in airway smooth muscle to facilitate airway smooth muscle relaxation in the treatment of chronic obstructive pulmonary disease (COPD) [13]. In the physiological state, PDE4 inhibitors can induce relaxation and reduce the frequency of contractions in the DSM in guinea pigs, rats, and non-human primates [14,15,16]. Therefore, we hypothesized that PDE4 inhibitors could treat obesity-associated OAB through modulation of a systemic chronic inflammatory response. In this work, the obesity-associated OAB model was established using a HFD feeding to detect interventional effects of the PDE4 inhibitor roflumilast through Western blotting and quantitative reverse transcription-polymerase chain reaction (qRT-PCR) in OAB treatment.
Materials and methods
Animals
Ninety adult female SD rats (average weight 205.7 ± 6.6 g; China Medical University, Shenyang, PRC) were used in this study. All experimental procedures were approved by the Institutional Animal Care and Use Committee of China Medical University.
Diet-induced obesity and study treatment
For 12 weeks, study animals were housed three per cage on a 12-h light–dark cycle, and either normal diet (ND) (fat: 5%; protein: 20%; carbohydrate: 75%) or HFD (fat: 30%; protein: 14%; carbohydrate: 56%) that induces obesity as previously described [17, 18]. Study animals were divided into three groups (N = 30 in each group): (1) vehicle-treated ND-fed (ND + vehicle) rats (normal diet for 8 weeks before receiving the vehicle); (2) vehicle-treated HFD-fed (HFD + vehicle) rats (HFD for 8 weeks before receiving the vehicle); and (3) roflumilast-treated HFD-fed (HFD + roflumilast) rats (HFD for 8 weeks before receiving roflumilast). Roflumilast (5 mg/kg/day; MedChemexpress, USA) or vehicle (sterile water used as solvent for roflumilast) was administered orally by gavage during the last 4 weeks of HFD or ND feeding. All rats were weighed at 12 weeks, and urodynamic studies were conducted in ten rats of each group. Study animals were then killed in a carbon dioxide tank prior to collection of bladder specimens; the bladder mucosa was separated under microscopy, and the DSM tissue was preserved in liquid nitrogen.
Cystometry
Based on previously described experimental methods, the anaesthetized cystometry was performed and general anesthesia was induced with 5% isoflurane/O2 gas inspiration using a facial mask [19, 20]. A catheter was inserted into the bladder dome after surgically exposing the bladder and was connected to a physiological pressure transducer and an injection pump (Dantec Menuet, Denmark). Cystometry was performed by infusing warm saline (37–38 °C) into the bladder at a flow rate of 12 mL/h. Three voiding events were recorded for each rat to assess: maximum voiding pressure (the maximum pressure during voiding), bladder capacity (the volume of saline infused to induce the voiding), voiding volume (the volume of micturition), voiding interval (the interval between voids), and the number of NVCs during 1 voiding event. NVCs were defined as spontaneous contractions (>4 cmH2O from the baseline bladder pressure) that did not result in a void. Bladders assessed by cystometry were not used in other experiments.
qRT-PCR
Total RNA was isolated from SD rat DSM strips using RNA isolater Extraction Reagent (Vazyme Biotech Co., Ltd, China). Reverse transcription of total RNA was undertaken using the HIScriptII One Step qRT-PCR SYBR Green Kit (Vazyme Biotech Co., Ltd) according to the manufacturer’s instructions. Real-time PCR was then performed using the synthesized mRNA on an ABI PRISM 7500 sequence detection system with SYBR GREEN PCR Master Mix. Real-time PCR was carried out to analyze the mRNA expression of TNF-α, IL-6, IL-1β, NF-κB, and GAPDH using specific primers (Table 1). PCR conditions were 50 °C for 5 min followed by 95 °C for 30 s and then 58 °C for 40 s for a total of 40 cycles. All reactions were run thrice and were normalized to GAPDH. Melt curves were utilized to analyze and assess the accuracy of the PCR results. Gene expression was evaluated by 2−△△Ct values. The relative mRNA expression of each target gene was normalized to that of GAPDH.
Western blotting
Briefly, protein from the SD rat DSM strips was isolated and homogenized in a homogenizer with RIPA buffer (50 mM Tris, 150 mM NaCl, 1% Triton X-100, 0.1% SDS, and 1% sodium deoxycholate) with a protease inhibitor cocktail (Beyotime Biotechnology, China). Protein concentrations were measured using the BCA Protein Assay kit (Beyotime Biotechnology). Approximately 10–20 µg protein samples were separated in a denaturing 10 or 12% SDS-PAGE gel and transferred to a nitrocellulose membrane. The membranes were washed, blocked (5% bovine serum albumin [BSA] in Tris-buffered saline with Tween 20 [TBS-T]), and incubated in primary rabbit antibody of TNF-α (1:500; Abcam), IL-1β (1:1000; Abcam), NF-κB (1:1000; Abcam), β-actin (1:2000; Cell Signaling Technology), or mouse antibody of IL-6 (1:1000; Abcam) overnight at 4 °C. Secondary antibodies were conjugated with horseradish peroxidase. Visualization was done with ECL Western blotting detection reagents (Beyotime Biotechnology). The relative density of target protein to β-actin was evaluated using Gel-Pro32 software.
Statistical analysis
Data were further analyzed with GraphPad Prism 5.0 (GraphPad software, San Diego, CA, USA) and are expressed as mean ± SEM (n = the number of strips and N = the number of rats). Statistical significance was tested using one-way ANOVA, followed by Newman–Keuls comparison test; P < 0.05 was considered an indication of statistical significance.
Results
Body weight changes
The body weight of obese rats increased significantly as compared to rats in the ND-fed group (HFD + vehicle 651.4 ± 8.4 g, N = 30; ND + vehicle 370.4 ± 9.1 g, N = 30; P < 0.05). However, bladder weight did not significantly differ between normal and obese groups (ND + vehicle 96.9 ± 3.2 mg, N = 30; HFD + vehicle 97.6 ± 4.1 mg, N = 30; P > 0.05). Oral roflumilast treatment for 4 weeks did not have any effect on the body weight (646.8 ± 10.5 g, N = 30; P > 0.05) and bladder weight (94.8 ± 5.7 mg, N = 30; P > 0.05) compared to the parameters of HFD-fed rats receiving the vehicle.
The changes in bladder function in obese rats
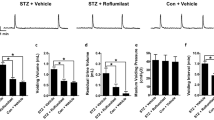
During cystometry, rats in the ND + vehicle group showed regular storage cycles with rare NVCs (Fig. 1a). Obese rats exhibited the reduced bladder capacity (0.3 ± 0.08 ml, N = 10) and voiding volume (0.26 ± 0.09 ml, N = 10) compared to the parameters (0.64 ± 0.06 ml and 0.61 ± 0.07 ml, N = 10; P < 0.05; Fig. 1a–c) of normal rats. Maximum voiding pressure did not significantly differ between normal (42.5 ± 6.3 cm H2O, N = 10) and obese (40.6 ± 7.1 cm H2O, N = 10; P > 0.05; Fig. 1a, d) rats. Moreover, there was a decrease in the voiding interval (ND + vehicle 3.5 ± 0.3 min, N = 10; HFD + vehicle 1.6 ± 0.4 min, N = 10; P < 0.05; Fig. 1a, e) and more frequent NVCs were detected (ND + vehicle 0.41 ± 0.2, N = 10; HFD + vehicle 3.6 ± 0.5, N = 10; P < 0.05; Fig. 1a, f), indicating that DO was already induced.
The changes and effects of roflumilast on healing bladder function in obese rats. a Representative cystometrogram illustrating that the changes and effect of roflumilast on bladder function in obese rats. Meanwhile, the decreased bladder capacity (b), reduced voiding volume (c), shorter voiding interval (e), and more frequent NVCs (f) were observed in obese rats (N = 10) compared with normal animals (N = 10; *P < 0.05 HFD + vehicle versus ND + vehicle). Moreover, roflumilast improved the changed urodynamic parameters in obese rats (N = 10; # P < 0.05 HFD + roflumilast versus HFD + vehicle). However, there is no significant difference in maximum voiding pressure among the three groups. The arrow indicates the number of voiding
Effect of roflumilast on healing bladder dysfunction in obese rats
Oral roflumilast treatment for 4 weeks improved the bladder function, such as bladder capacity (0.54 ± 0.08 ml; N = 10), voiding volume (0.52 ± 0.08 ml; N = 10), voiding interval (2.8 ± 0.4 min; N = 10), and the frequency of NVCs (0.7 ± 0.4; N = 10) in obese rats, compared to rats from HFD + vehicle groups (N = 10; P < 0.05; Fig. 1). In contrast, maximum voiding pressure remained similar for roflumilast-treated HFD-fed rats (41.5 ± 6.8 cm H2O, N = 10; P > 0.05; Fig. 1a, d). Furthermore, the improved cystometric parameters detected after roflumilast treatment in obese rats were similar to the cystometric parameters in ND + vehicle rats (N = 10; P > 0.05; Fig. 1). Our present results indicated roflumilast induced an improvement in bladder function and voiding efficiency in obese rats.
Increased expression of TNF-α, IL-6, IL-1β, and NF-κB in DSM of obese rats
Obesity induces changes in systemic hormone secretion that modulate the systemic inflammatory response, which involves production and release of inflammatory mediators, including TNF-α, IL-6, IL-1β, and NF-κB [21]. Therefore, we determined whether there were changes in the inflammatory medium in the smooth muscle layer of the bladder in lean and obese rats. Protein expression of NF-κB (ND + vehicle 0.64 ± 0.05; HFD + vehicle 0.92 ± 0.07), TNF-α (ND + vehicle 0.35 ± 0.04; HFD + vehicle 0.67 ± 0.06), IL-6 (ND + vehicle 0.31 ± 0.07; HFD + vehicle 0.84 ± 0.1), and IL-1β (ND + vehicle 0.34 ± 0.09; HFD + vehicle 0.68 ± 0.08) was enhanced in DSM from obese rats (n = 16) as compared to that in normal rats (n = 16; P < 0.05; Fig. 2).
Oral roflumilast treatment attenuated increased expression of inflammatory factor protein in obese rat bladder. Comparison of protein expression levels of NF-κB (a and b), TNF-α (c and d), IL-6 (e and f), and IL-1β (g and h) in the DSM. The data are the ratio of target protein with reference. The protein levels of vehicle-treated HFD-fed rats (n = 6) were significantly higher than those of the ND + vehicle group (n = 6; *P < 0.05 HFD + vehicle versus ND + vehicle). The protein levels of roflumilast-treated HFD-fed rats were significantly reduced compared to vehicle-treated HFD-fed rats (n = 6; # P < 0.05 HFD + roflumilast versus HFD + vehicle). Data are shown as relative protein expression normalized to β-actin
To observe molecular events proceeding from a chronic HFD, some genes were evaluated by RT-PCR. The results demonstrated that expression of NF-κB/p65 mRNA was significantly increased in smooth muscle from rats on the HFD (0.89 ± 0.09; n = 16) compared to those fed normal chow (0.61 ± 0.07; n = 16; P < 0.05; Fig. 3a). Accordingly, genes for TNF-α (ND + vehicle 0.34 ± 0.09; HFD + vehicle 0.63 ± 0.07), IL-6 (ND + vehicle 0.26 ± 0.08; HFD + vehicle 0.81 ± 0.1), and IL-1β (ND + vehicle 0.39 ± 0.09; HFD + vehicle 0.66 ± 0.08), which are modulated directly by NF-κB, were also upregulated (n = 16; P < 0.05; Fig. 3b–d).
Enhanced mRNA expression of inflammatory factor was reduced after oral roflumilast treatment in obese rat bladder. qRT-PCR analyses showed increased mRNA expression of NF-κB (a), TNF-α (b), IL-6 (c), and IL-1β (d) in vehicle-treated HFD-fed rats, compared with normal chow-fed rats (n = 16; *P < 0.05 HFD + vehicle versus ND + vehicle). The group of roflumilast-treated HFD-fed rats showed significantly decreased mRNA expression in the DSM (n = 16) compared with DSM in vehicle-treated HFD-fed rats (n = 16; # P < 0.05 HFD + roflumilast versus HFD + vehicle). Data are shown as relative mRNA expression normalized to GAPDH
Roflumilast inhibits inflammatory response in obese rats
To assess whether this PDE4 inhibitor is involved in the inhibition of the inflammatory response and consequent DO in obese rats, study animals were treated with oral roflumilast. Obese rats treated with roflumilast showed decreased expression of inflammatory cytokines (NF-κB 0.68 ± 0.06, TNF-α 0.41 ± 0.06, IL-6 0.39 ± 0.09, and IL-1β 0.36 ± 0.09) in bladder smooth muscle when compared to vehicle-treated obese rats, as demonstrated by the gray level (n = 16; P < 0.05; Fig. 2). Similarly, a qRT-PCR test confirmed that roflumilast treatment reduced the expression of inflammatory factor genes in obese rats (NF-κB 0.64 ± 0.08, TNF-α 0.39 ± 0.08, IL-6 0.37 ± 0.09, and IL-1β 0.41 ± 0.09; n = 16; P < 0.05; Fig. 3). Moreover, the reduced expression of inflammatory factor genes and proteins after roflumilast treatment in obese rats was similar to the expression profile in ND + vehicle rats (n = 16; P > 0.05; Figs. 2, 3). Therefore, PDE4 inhibitors may play a primary role in inhibiting the release of inflammatory mediators and the activation of immune cells [22, 23].
Discussion
In fact, obesity is a chronic systemic inflammatory response, and inflammation and immune response constitute important aspects of it [24]. Following administration of an HFD, rats in the obese group had increased inflammatory media in the bladder smooth muscle, which showed that systemic chronic inflammatory reaction also affects bladder smooth muscle.
Obesity is recognized as a low-intensity inflammatory response, mainly mediated by the high level of serum C-reactive protein (CRP) and TNF-α, IL-6, IL-1β, and other inflammatory factors in metabolism and tissue dysfunction, which plays a major role in the body [6, 21, 25]. To verify that PDE can provide a potential novel treatment option for obesity-associated OAB, we selected SD rats, according to recommendations in the literature, to build the model [17], and the urodynamically demonstrable DO can help objectively diagnose OAB [26]. Simultaneously, through urodynamic study, the obese group was compared with the normal group; the number of NVCs was significantly increased, the duration of voiding interval was shorter, and bladder capacity was significantly decreased in obese rats. In the literature [18, 27], experimental results have shown that the OAB model is successful for research purposes. Moreover, experimental results show that the smooth muscle of obese rats has significantly higher expression of TNF-α, IL-6, IL-1β, and NF-κB than that in rats belonging to the normal group. Results showed that both inflammatory factor gene and protein expressions were increased with the degree of obesity, and obesity was one of the causes of excessive bladder activity.
Furthermore, repeated nerve stimulation by inflammatory factors can lead to permanent changes or sensitization [28]. Inflammatory cytokines promote smooth muscle cell DNA and mitochondrial damage as well as induce smooth muscle cell proliferation and fibrosis, resulting in impairment of bladder function [6, 29]. Experiments show that inflammatory mediators induce high expression of protein and mRNA in bladder smooth muscle of obese rats, which leads to excessive activity of bladder detrusor, with resultant symptoms of OAB. Therefore, it chronic inflammation is associated with OAB.
Acetylcholine is the main neurotransmitter in DSM, and antimuscarinic agents can competitively inhibit acetylcholine in DSM cells and bladder wall receptors to reduce nerve sensitivity, thereby playing a role in the treatment of detrusor disorders [30, 31]. However, conventional antimuscarinic pharmacotherapy causes side effects such as dry mouth, constipation, headache, and cardiac that limit the application of these drugs. It has been shown that [14, 32] PDE4 inhibitors can induce bladder smooth muscle relaxation and reduce bladder smooth muscle contraction frequency in rats and of non-human primates. Therefore, PDE4 inhibitors may potentially have application as novel treatment for obesity-associated OAB. Of these, roflumilast is an FDA-approved oral PDE4 inhibitor that plays a major role in inflammatory cells to induce PDE4-specific effects in various cells and reduce the cAMP degradation, thereby blocking proinflammatory signaling, and also has anti-inflammatory activity [33]. Moreover, roflumilast inhibits inflammatory reaction induced by lipopolysaccharide [22, 34]. In addition [15], PDE4 inhibitors can regulate smooth muscle contractions in normal or pathological bladder, through a mechanism that inhibits smooth muscle contraction through mediation of protein kinase A and protein kinase G, increasing calcium recovery, and thereby promoting relaxation of bladder smooth muscle. Experimental results show that, following administration of an oral phosphodiesterase inhibitor, symptoms of urinary irritation in the treatment group were relieved significantly compared to that in the obese control group. Further, protein and mRNA expression of inflammatory factors in bladder smooth muscle in the treatment group were decreased after 4 weeks of oral roflumilast. According to Oba et al. [35], during the course of treatment with oral roflumilast, gastrointestinal reactions, neurological symptoms, and other symptoms were mild or moderate, and occurred in the early stage of treatment and disappeared gradually during the course of treatment. The PDE4 inhibitor significantly inhibited the release of inflammatory cytokines such as TNF-α, IL-1β, and IL-6 by monocytes, macrophages, eosinophils, mast cells, and T cells [23]. Therefore, PDE4 inhibitors may prove to be a new treatment for obesity-associated OAB.
In conclusion, this experiment relied on serological and cystometric data to construct an OAB model and evaluate the symptoms of rats in each group, specifically to demonstrate the therapeutic effect of PDE4 inhibitors on OAB, through the expression of inflammatory factor proteins and genes, in DSM cells. However, research on excitability and contractility of bladder smooth muscle was not further developed. Future experiments will aim to further study bladder smooth muscle to seek more favorable and definitive evidence of PDE4 inhibitors in OAB.
References
Drake MJ (2014) Do we need a new definition of the overactive bladder syndrome? ICI-RS 2013. Neurourol Urodyn 33(5):622–624
Gulur DM, Drake MJ (2004) Management of overactive bladder. Dis Manage Health Outcomes 350(2):786–799
Wen JG, Li JS, Wang ZM, Huang CX, Shang XP, Su ZQ, Lu YT, Suo ZH, Wang Y, Qin GJ (2014) The prevalence and risk factors of OAB in middle-aged and old people in China. Neurourol Urodyn 33(4):387–391
Laven BA, Orsini N, Andersson SO, Johansson JE, Gerber GS, Wolk A (2008) Birth weight, abdominal obesity and the risk of lower urinary tract symptoms in a population based study of Swedish men. J Urol 179(5):1895–1896
Wellen KE, Gs H (2003) Obesity-induced inflammatory changes in adipose tissue. J Clin Investig 112(12):1785–1788
Haldar S, Dru C, Choudhury D, Mishra R, Fernandez A, Biondi S, Liu Z, Shimada K, Arditi M, Bhowmick NA (2015) Inflammation and pyroptosis mediate muscle expansion in an interleukin-1beta (IL-1beta)-dependent manner. J Biol Chem 290(10):6574–6583. doi:10.1074/jbc.M114.617886
Bouchelouche K, Alvarez S, Horn T, Nordling J, Bouchelouche P (2006) Human detrusor smooth muscle cells release interleukin-6, interleukin-8, and RANTES in response to proinflammatory cytokines interleukin-1β and tumor necrosis factor-α. Urology 67(1):214–219
Rohrmann S, Smit E, Giovannucci E, Platz EA (2005) Association between markers of the metabolic syndrome and lower urinary tract symptoms in the Third National Health and Nutrition Examination Survey (NHANES III). Prostate 67(15):1693–1698
Rahman NU, Phonsombat S, Bochinski D, Carrion RE, Nunes L, Lue TF (2007) An animal model to study lower urinary tract symptoms and erectile dysfunction: the hyperlipidaemic rat. BJU Int 100(3):658–663
Leiria LO, Sollon C, Calixto MC, Lintomen L, Mónica FZ, Anhê GF, De NG, Zanesco A, Grant AD, Antunes E (2012) Role of PKC and CaV1.2 in detrusor overactivity in a model of obesity associated with insulin resistance in mice. PLoS ONE 7(11):e48507–e48507
Staskin DR, Robinson D (2009) Oxybutynin chloride topical gel: a new formulation of an established antimuscarinic therapy for overactive bladder. Expert Opin Pharmacother 10(18):3103–3111
Schafer PH, Truzzi F, Parton A, Wu L, Kosek J, Zhang LH, Horan G, Saltari A, Quadri M, Lotti R, Marconi A, Pincelli C (2016) Phosphodiesterase 4 in inflammatory diseases: effects of apremilast in psoriatic blood and in dermal myofibroblasts through the PDE4/CD271 complex. Cell Signal 28(7):753–763. doi:10.1016/j.cellsig.2016.01.007
Sturton G, Fitzgerald M (2002) Phosphodiesterase 4 inhibitors for the treatment of COPD. Chest 121(5 Suppl):192S–196S
Oger S, Behr-Roussel D, Gorny D, Denys P, Lebret T, Alexandre L, Giuliano F (2007) Relaxation of phasic contractile activity of human detrusor strips by cyclic nucleotide phosphodiesterase type 4 inhibition. Eur Urol 51(3):772–780; discussion 780–781
Longhurst PA, Briscoe JA, Rosenberg DJ, Leggett RE (1997) The role of cyclic nucleotides in guinea-pig bladder contractility. Br J Pharmacol 121(8):1665–1672
Xin W, Li N, Cheng Q, Petkov GV (2014) BK channel-mediated relaxation of urinary bladder smooth muscle: a novel paradigm for phosphodiesterase type 4 regulation of bladder function. J Pharmacol Exp Ther 349(1):56
Hansen MJ, Jovanovska V, Morris MJ (2004) Adaptive responses in hypothalamic neuropeptide Y in the face of prolonged high-fat feeding in the rat. J Neurochem 88(4):909–916
Fan EW, Chen LJ, Cheng JT, Tong YC (2014) Changes of urinary bladder contractility in high-fat diet-fed mice: the role of tumor necrosis factor-alpha. Int J Urol 21(8):831–835. doi:10.1111/iju.12428
Li N, He X, Li Z, Liu Y, Wang P (2016) Partial bladder outlet obstruction is associated with decreased expression and function of the small-conductance Ca2+-activated K+ channel in guinea pig detrusor smooth muscle. Int Urol Nephrol 49(1):17–26. doi:10.1007/s11255-016-1455-0
Li N, Ding H, He X, Li Z, Liu Y (2017) Expression and function of the small-conductance Ca2+-activated K+ channel is decreased in urinary bladder smooth muscle cells from female guinea pig with partial bladder outlet obstruction. Int Urol Nephrol. doi:10.1007/s11255-017-1592-0
Bulló M, García-Lorda P, Megias I, Ph.D. JS-SMD (2003) Systemic inflammation, adipose tissue tumor necrosis factor, and leptin expression. Obes Res 11(4):525–531
Conti M, Richter W, Mehats C, Livera G, Park JY, Jin C (2003) Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. J Biol Chem 278(8):5493–5496. doi:10.1074/jbc.R200029200
Essayan DM (1999) Cyclic nucleotide phosphodiesterase (PDE) inhibitors and immunomodulation. Biochem Pharmacol 57(9):965–973
de Heredia FP, Gómezmartínez S, Marcos A (2012) Obesity, inflammation and the immune system. Proc Nutr Soc 71(71):332–338
Lee MK, Yvan-Charvet L, Masters SL, Murphy AJ (2016) The modern interleukin-1 superfamily: divergent roles in obesity. Semin Immunol. doi:10.1016/j.smim.2016.10.001
Fry CH, Sahai A, Vahabi B, Kanai AJ, Birder LA (2014) What is the role for biomarkers for lower urinary tract disorders? ICI-RS 2013. Neurourol Urodyn 33(5):602–605. doi:10.1002/nau.22558
Schäfer W, Abrams P, Liao L, Mattiasson A, Pesce F, Spangberg A, Sterling AM, Zinner NR, Kerrebroeck PV (2002) Good urodynamic practices: uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn 21(3):261–274
Seki S, Sasaki K, Fraser MO, Igawa Y, Nishizawa O, Chancellor MB, de Groat WC, Yoshimura N (2002) Immunoneutralization of nerve growth factor in lumbosacral spinal cord reduces bladder hyperreflexia in spinal cord injured rats. J Urol 168(168):2269–2274
Yoon J, Ryoo S (2013) Arginase inhibition reduces interleukin-1beta-stimulated vascular smooth muscle cell proliferation by increasing nitric oxide synthase-dependent nitric oxide production. Biochem Biophys Res Commun 435(3):428–433. doi:10.1016/j.bbrc.2013.05.002
Abrams P, Andersson KE (2007) Muscarinic receptor antagonists for overactive bladder. BJU Int 100(5):987–1006. doi:10.1111/j.1464-410X.2007.07205.x
Andersson KE (2016) Potential future pharmacological treatment of bladder dysfunction. Basic Clin Pharmacol Toxicol 119(3 Suppl):75–85
Truss MC, Uckert S, Stief CG, Forssmann WG, Jonas U (1996) Cyclic nucleotide phosphodiesterase (PDE) isoenzymes in the human detrusor smooth muscle. I. Effect of various PDE inhibitors on smooth muscle tone and cyclic nucleotide levels in vitro. Urolithiasis 24(3):123–128
Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ (2009) Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet 374(9691):685–694
Houslay MD, Schafer P, Zhang KY (2005) Phosphodiesterase-4 as a therapeutic target. Drug Discov Today 10(22):1503–1519
Oba Y, Lone NA (2012) Efficacy and safety of roflumilast in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Ther Adv Respir Dis 7(1):13–24
Funding
This study was funded by a grant from LNCCC of LNCCC-D16-2015 to Ning Li.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interests with regard to the work reported in this manuscript.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Ding, H., Li, N., He, X. et al. Treatment of obesity-associated overactive bladder by the phosphodiesterase type-4 inhibitor roflumilast. Int Urol Nephrol 49, 1723–1730 (2017). https://doi.org/10.1007/s11255-017-1671-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-017-1671-2