Abstract
Purpose
Stage pT1 urothelial bladder cancer (UBC) is characterized as a challenging subentity of urothelial carcinoma with an unforeseeable clinical course. In addition to more or less established clinical and histopathological features, we evaluated the role of epithelial–mesenchymal transition (EMT) marker E-cadherin, shown to be of prognostic value in muscle-invasive disease, regarding the prognosis of stage pT1 high-grade (hg) UBC.
Methods
Tissue of 226 stage pT1 hg UBC patients from transurethral resection could be immunostained for E-cadherin. Kaplan–Meier analysis and univariate and multivariate Cox regression analyses regarding progression-free (PFS) and cancer-specific survival (CSS) were performed.
Results
Aberrant expression of E-cadherin was recognized in 74% of patients. Kaplan–Meier analysis showed that aberrant E-cadherin expression was associated with worse 10-year PFS (62 vs. 90%, p = 0.045). In univariate analysis, aberrant E-cadherin staining, associated carcinoma in situ, grading 3 after WHO classification 1973 and infiltrative growth pattern at the invasion front were the statistically significant predictive factors for worse PFS, only infiltrative growth pattern for CSS. With regard to progression, grading 3 after WHO classification of 1973 (HR 6.49; CI 1.54–27.28, p = 0.011) and infiltrative tumor invasion pattern (HR 2.06; CI 1.10–3.86, p = 0.024) revealed as independent factors for PFS, and there was a trend also for E-cadherin expression (HR 0.45; CI 0.19–1.06; p = 0.068). Regarding CSS, infiltrative tumor growth pattern (HR 3.79; CI 1.67–8.60, p = 0.001) was the only statistically significantly independent predictive factor in multivariate Cox regression analysis.
Conclusions
Beside invasion growth pattern and WHO grading 1973 that achieved to be independent prognostic factors, there was a trend for the parameter E-cadherin expression to be of predictive value for PFS in stage pT1 hg urothelial bladder carcinoma after organ-sparing approach. Further studies on genetic level are warranted to define the distinct role of EMT in early-invasive UBC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epithelial–mesenchymal transition (EMT) and mesenchymal–epithelial transition (MET) are essential mechanisms in embryogenesis. Loss of different proteins important for the cell adhesive junctions in epithelial cell layers leads to the disappearance of typical epithelial characteristics such as tissue integrity [1]. Epithelial cells then become able to migrate and build mesenchymal structures. In this way, EMT plays a vital role in the organogenesis of different organisms. Similar but disordered mechanisms were shown for the carcinogenesis of several neoplasms in the transition from early to more invasive stages [2,3,4].
The association between tumor progression and EMT was shown first in colorectal cancer by increased expression of mesenchymal markers and decreased epithelial marker expression [5, 6]. One essential mechanism for the integrity of epithelial cell layers is undisturbed communication between cytoskeleton and cell membrane by different types of catenin binding between the cytoplasmatic domain of cadherins and F-actin, a cytoskeletal protein. Spaderna et al. [6] postulated that the loss of E-cadherin and ß-catenin, visible through a reduced immunohistochemical expression, leads to a more permeable basal membrane due to weakened cell–cell adhesion. This seems to be the initial step of EMT. Even before establishing EMT theory for the progression of epithelial tumors, a prognostic role of E-cadherin for urothelial bladder carcinoma (UBC) was described by De Medina et al. [7]. Muscle-invasive and high-grade tumors showed loss of E-cadherin expression in a higher percentage than non-muscle-invasive as well as low and moderate graded tumors. The immunohistochemical testing of 77 patients after radical cystectomy for UBC reported an association of E-cadherin with cancer-specific survival [8]. Baumgart et al. [9] were able to confirm these findings in a tissue micro-array series of 572 patients of various stages and grades of UBC. Although none of the tested EMT markers was associated with prognosis on multivariate analysis, univariate analysis showed a prognostic value of low-level ß-catenin as well as plakoglobin for cancer-specific survival in a subset of cystectomied patients.
Less is known on the impact of EMT markers for the prognosis of non-muscle-invasive UBC. Muramaki et al. [10] showed a statistically significant higher percentage of recurrences for negative E-cadherin staining in an immunohistochemical analysis of 115 patients. Two research groups have undertaken an investigation of pT1 UBC subset. Clairotte et al. [11] confirmed other findings of pathologic E-cadherin/ß-catenin expression in a group of 71 patients, but only γ-catenin was found to be associated with progression-free survival upon multivariate analysis. Moyano Calvo et al. [12] analyzed a cohort of 88 stage pT1 patients for EMT markers, showing that the loss of β-catenin was statistically significantly associated with recurrence.
However, more studies on EMT and other markers, especially for early-invasive UBC, would be of great value because of the unpredictable course of the disease, which ranges widely from no further disease to death of UBC [13]. We analyzed the immunohistochemical staining of E-cadherin and its value for progression and survival in a large cohort of 295 patients with stage T1 high-grade (hg) UBC. Furthermore, various clinical and histopathological parameters were evaluated regarding these endpoints.
Methods
Patient characteristics
After patients’ informed consent, we retrospectively examined tumor tissues from 263 patients primary diagnosed with stage pT1 by transurethral resection of the bladder (TURB) and reresection 4–6 weeks after in every case and bladder-sparing therapeutic approach between 1989 and 2009. TURB and further treatment were carried out in a single-center urology department and in outpatient urologists. Stage pT1 recurrence or progression to muscle-invasive disease resulted in an advice for deferred CX.
We assessed patients’ histopathological and clinical data retrospectively and collected information regarding tumor recurrence, disease progression and cancer-specific survival. Median follow-up was 44 months (interquartile range 27–76 months).
Histopathological assessment
All bladder tumors were diagnosed according to the TNM classification. We also performed a multireviewer assessment involving experienced uropathologists (SB, AH and FH) in order to assure the diagnosis of pT1 and to define the grading to the WHO classifications of 1973 and 2004. Also, other histopathological and clinicopathological factors like growth pattern, invasion pattern, associate CIS, focality and tumor size were assessed by the same reviewers.
Immunohistochemical assessment and analysis
Four-µm sections were cut from formalin-fixed and paraffin-embedded tissue blocks and mounted on poly-l-lysine-coated glass slides. Immunohistochemistry was carried out in a BenchMark IHC Full System immunostainer (Roche Diagnostics, Mannheim, Germany) using the avidin–biotin–peroxidase method with diaminobenzidine as chromatogen according to the manufacturer’s instructions, as primary antibody mouse monoclonal E-cadherin antibody, Dako Deutschland GmbH, Hamburg, Germany, dilution 1:100, was used.
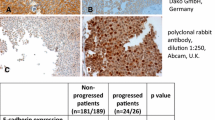
Expression of E-cadherin was visualized with a Primo Star microscope (Carl Zeiss Microimaging, Jena, Germany) under 100-fold magnification. Evaluation of the immunostained slides was performed by two independent reviewers without knowledge of clinical and follow-up data by reporting semiquantitative staining in steps of 10% from negative to >90% positive staining of urothelial carcinoma areas. Deviations in their results led to a consensus decision between the two assessors. In accordance with previous studies, normal expression of E-cadherin was defined as staining of ≥90%, and staining <90% was defined as aberrant [14,15,16]. Examples are shown in Fig. 1.
Statistical analysis
Statistical analysis was performed using SPSS version 23.0 (IBM Deutschland GmbH, Ehningen, Germany). Progression-free (PFS) and cancer-specific survival (CSS) rates were calculated by Kaplan–Meier analysis and log-rank test. Multivariate Cox regression analyses were used to assess the value of E-cadherin expression and histopathological parameters for progression and cancer-specific survival. p values < 0.05 were considered statistically significant.
Results
Histopathological patient characteristics
After excluding five patients with low-grade disease and 32 patients due to loss for immunohistochemical analysis, the median age of the 226 stage pT1 high-grade patients included in the study was 72 years (interquartile range 65–79 years). Seventy-seven percentage of the patients were male. According to the WHO grading classification 1973, tumor grade was G2 in 30% and G3 in 70%. Solid tumors appeared in 11%, patients showed an associated CIS in 23%, multifocal tumors were present in 18% of patients and tumor size was ≥3 cm in 56%. Invasion patterns were infiltrative in 24% and non-infiltrative in 76%. Please refer to Table 1.
Treatment and course of patients
After TURB and reresection, the included patients underwent bladder-sparing approach. Recurrent disease occurred in 31 and 18% showing progressive disease (stage ≥ pT2). A further 14% of patients were radically cystectomied due to recurrence or progression in the follow-up period. Ten patients of the studied patients died of UBC and 33% died of causes other than UBC.
Immunohistochemical E-cadherin expression and association with clinicopathological factors
E-cadherin expression was analyzable in 226 patients with 74% of patients showing aberrant expression. Female gender (p = 0.049) and unifocal tumors (p = 0.012) were statistically significantly associated with aberrant expression of E-cadherin. All other clinicopathological parameters, including performance of instillation therapy, showed no statistically significant correlation with E-cadherin staining.
Kaplan–Meier analysis of E-cadherin staining and univariate regression analysis of E-cadherin staining and the studied clinical and histopathological parameters for PFS and CSS
Although failing to reach statistical significance, there was a trend for patients with aberrant E-cadherin expression for worse 10-year CSS (81 vs. 90%, p = 0.230), but even a statistical significant for worse 10-year PFS (62 vs. 90%, p = 0.045). Please refer to Fig. 2.
Regarding progression, grading 2 after WHO classification of 1973, non-infiltrative growth pattern at the invasion front, associated CIS, normal E-cadherin staining were the statistically significantly predictive factors for better prognosis in univariate regression analysis with statistical significance for grading (p = 0.002), invasion pattern (p = 0.002), associated CIS (p = 0.014) and E-cadherin staining (p = 0.045). Regarding cancer-specific survival in univariate regression analysis, statistically significant results were reached only for tumor invasion pattern (p = 0.001). Please refer to Table 2.
Multivariate Cox regression analysis of the five best clinical, histopathological and immunohistochemical parameters regarding PFS and CSS
We performed a multivariate Cox regression analysis regarding PFS and CSS of the respective statistically significantly predictive markers in univariate analysis for both endpoints. With regard to progression, grading 3 after WHO classification of 1973 (HR 6.49; CI 1.54–27.28, p = 0.011) and infiltrative tumor invasion pattern (HR 2.06; CI 1.10–3.86, p = 0.024) revealed as independent factors for PFS. Normal E-cadherin staining slightly failed to be of independent prognostic association for better PFS (HR 0.45; CI 0.19–1.06; p = 0.068). Regarding CSS, infiltrative tumor growth pattern (HR 3.79; CI 1.67–8.60, p = 0.001) was the only statistically significantly independent prognostic factor in multivariate Cox regression analysis. Please refer to Table 3.
Discussion
Stage pT1 represents the most challenging subentity of urothelial bladder carcinoma (UBC). This is due to an unforeseeable prognosis, which results in ambiguous clinical courses. While a relevant group of patients, particularly those with high-grade tumors, progress to muscle-invasive stages or even develop metastasis, other groups of stage pT1 UBC patients never even experience recurrence. Shahin et al. [13] showed in a series of 153 stage pT1G3 UBC patients that approximately one-third of patients can be included in each of the following groups: patients with never recurring tumors, patients with non-muscle-invasive recurrence and patients with progression requiring deferred cystectomy and having a high risk of dying from metastatic disease.
One of the problems with different factors proven to be of prognostical value in the past, e.g., histopathological factors like associated carcinoma in situ (CIS), multifocality, tumor size, pT1 substaging or infiltrative growth pattern, is their high interobserver variation and a tendency toward contradictory findings [17,18,19,20,21,22,23,24]. This meant that investigations into more reliable and valid techniques other than histopathological assessment needed to be undertaken. Immunohistochemistry would appear to be just such a method, with at least semiquantitative character. Using this tool, nearly every known or assumed pathway of carcinogenesis can be assessed for its prognostic impact.
Epithelial–mesenchymal transition (EMT) was detected as a possible way of progression initiation toward metastasis in various human carcinomas [5, 6]. Essential for the development of human tissue during embryogenesis, EMT seems to regain importance for the progression of epithelial tumors. Typical EMT markers are proteins that play a role in cell adhesion like E-cadherin that is linked to actin cytoskeleton by a panel of catenins. In the past changes of E-cadherin and ß-catenin, expression in some analysis could be associated with prognosis of different human tumors in advanced stages [6, 9, 25].
In a relatively small collective of 55 UBC patients of various stages, Kashibuchi et al. [26] described expression patterns of EMT markers E-cadherin, α-catenin, β-catenin and γ-catenin. Loss of expression or heterogeneous expression within the tumor tissue was defined as “aberrant” staining. While aberrant expression of all markers was statistically significantly associated with advancing T stage, only E-cadherin showed such value for grading after the WHO classification of 1973. Aberrant staining of all studied EMT markers was associated statistically significantly with CSS of UBC patients. Multivariate analysis only revealed a prognostic value for α-catenin, not for E-cadherin and other EMT markers. There was no differentiation within the group of non-muscle-invasive tumors in this analysis. In a larger series of 153 UBC patients, Bryan et al. [27] compared expression of E-cadherin and β-catenin of stage Ta, T1 and ≥ T2 tumors. They showed a statistically significantly loss of both E-cadherin and β-catenin expression for advancing stage and grade. These markers had no statistically significant effect on CSS. Hu et al. [16] confirmed these findings with 72 UBC patients for increasing aberrant staining of E-cadherin and β-catenin regarding WHO grading 2004 and T stage (<T2 and ≥T2) but not multifocality and tumor size. For abnormal E-cadherin, Shi et al. [28] showed similar findings with additional statistical significance for recurrent UBC. In a comparable study on 80 UBC patients, Jang et al. [15] proved a decrease in positive staining of E-cadherin and an increase in positive staining of nuclear β-catenin in advanced stages and grades. There was an association for nuclear β-catenin but not for E-cadherin with recurrence. Similar findings were revealed by Baumgart et al. [9] with increasing low EMT marker staining from non-muscle-invasive to muscle-invasive stages in a cohort of 572 UBC patients. CSS was analyzed only for cystectomied patients showing worse survival for low expression of ß-catenin and plakoglobin.
Only sparse data exist on the value of EMT for stage pT1 UBC. Clairotte et al. [11] confirmed other findings of aberrant E-cadherin and ß-catenin expression in a subset group of 71 patients regarding grading, but these markers were not found to be associated with progression-free survival within stage pT1 UBC. Moyano Calvo et al. [12] analyzed a cohort of 88 stage pT1 patients for EMT markers. Regarding multifocality, but not WHO grading 1973 and tumor size ≥3 cm, increasing share of tumors with negative staining of E-cadherin and β-catenin showed statistical significance. Only aberrant staining of β-catenin was significantly statistically associated with recurrence.
In our series, aberrant staining of E-cadherin in a large stage pT1 UBC cohort of 226 patients appeared in 74%. This finding was not associated with histopathological parameters but with female gender and unifocal tumors. We could show that there was a predictive value regarding worse PFS for patients with aberrant E-cadherin staining and a trend toward worse cancer-specific survival in Kaplan–Meier analysis and univariate regression analysis. As we previously showed in another stage pT1 UBC series [29], the WHO grading classification 1973 was again of statistical significant predictability for progression and cancer-specific survival in univariate analysis, as did infiltrative pattern at the submucous invasion front and associated CIS. Regarding survival, infiltrative growth pattern at the tumor invasion front stayed to be the only independent predictive feature in multivariate Cox regression analysis. This finding underlined the results of a study of Denzinger et al. [20] dealing with the impact of tumor growth pattern for stage pT1 UBC.
Of all urothelial bladder carcinoma, pT1-staged patients represent the most serious challenge for urologists. While a relevant group of patients, especially of high-grade tumors, develop progress to muscle invasion or even metastasis, another part of stage pT1 UBC patients never even experience recurrence. There are many ongoing attempts to establish additional markers for prognosis in this field. Epithelial–mesenchymal transition was detected as a possible way of progression initiation toward metastazation in muscle-invasive carcinoma. We could show statistically significant differences for aberrant staining of EMT marker E-cadherin on protein level regarding progression and a trend for cancer-specific survival speaking for this hypothesis. Further studies especially including more reliable and valid methods on genetic level are highly appreciated to define the role of EMT in early-invasive UBC. Up to this point, taking into account the WHO grading classification of 1973 and growth pattern at the subcutaneous invasion front seems to be the most valuable step to predict patients’ prognosis best. Stage pT1 UBC with grade 3 tumors and infiltrative growth pattern should be considered for early cystectomy, especially in case of associated CIS.
References
Baum B, Settleman J, Quinlan MP (2008) Transitions between epithelial and mesenchymal states in development and disease. Semin Cell Dev Biol 19:294–308
Shook D, Keller R (2003) Mechanisms, mechanics and function of epithelial–mesenchymal transitions in early development. Mech Dev 120:1351–1383
Tarin D (2005) The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res 65:5996–6001
McConkey DJ, Lee S, Choi W et al (2010) Molecular genetics of bladder cancer: emerging mechanisms of tumour initiation and progression. Urol Oncol Semin Invest 28:429–440
Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T (2005) Opinion: migrating cancer stem cells—an integrated concept of malignant tumour progression. Nat Rev Cancer 5(9):744–749
Spaderna S, Schmalhofer O, Hlubek F et al (2006) A transient, EMT-linked loss of basement membranes indicates metastasis and poor survival in colorectal cancer. Gastroenterology 131(3):830–840
De Medina SG, Popov Z, Chopin DK et al (2008) Relationship between E-cadherin and fibroblast growth factor receptor 2b expression in bladder carcinomas. Oncogene 18:5722–5726
Byrne RR, Shariat SF, Brown R et al (2001) E-cadherin immunostaining of bladder transitional cell carcinoma, carcinoma in situ and lymph node metastases with long-term followup. J Urol 165(5):1473–1479
Baumgart E, Cohen MS, Silva Neto B et al (2007) Identification and prognostic significance of an epithelial-mesenchymal transition expression profile in human bladder tumors. Clin Cancer Res 13(6):1685–1694
Muramaki M, Miyake H, Terakawa T, Kumano M, Sakai I, Fujisawa M (2012) Expression profile of E-cadherin and N-cadherin in non-muscle-invasive bladder cancer as a novel predictor of intravesical recurrence following transurethral resection. Urol Oncol 30(2):161–166
Clairotte A, Lascombe I, Fauconnet S, Kantelip B et al (2006) Expression of E-cadherin and alpha-, beta-, gamma-catenins in patients with bladder cancer: identification of gamma-catenin as a new prognostic marker of neoplastic progression in T1 superficial urothelial tumors. Am J Clin Pathol 125(1):119–126
Moyano Calvo JL, Blanco Palenciano E, Beato Moreno A et al (2006) Prognostic value of E-cadherina, beta catenin, Ki-67 antigen and p53 protein in the superficial bladder tumors. Actas Urol Esp 30(9):871–878
Shahin O, Thalmann GN, Rentsch C, Mazzucchelli L, Studer UE (2003) A retrospective analysis of 153 patients treated with or without intravesical bacillus Calmette-Guerin for primary stage T1 grade 3 bladder cancer: recurrence, progression and survival. J Urol 169(1):96–100 discussion 100
San Miguel Fraile P, Gómez de María C, Donis Quintairos L, Carrera Vazquez A, Iglesias Martínez P, Barreiro Barbosa MJ (2007) [Expression of E-cadherin and catenins in urothelial carcinomas]. Actas Urol Esp 31(4):355–360
Jang TJ, Cha WH, Lee KS (2010) Reciprocal correlation between the expression of cyclooxygenase-2 and E-cadherin in human bladder transitional cell carcinomas. Virchows Arch 457(3):319–328
Hu X, Ruan Y, Cheng F, Yu W, Zhang X, Larré S (2011) p130Cas, E-cadherin and β-catenin in human transitional cell carcinoma of the bladder: expression and clinicopathological significance. Int J Urol 18(9):630–637
Hurle R, Losa A, Manzetti A, Lembo A (1999) Intravesical bacillus Calmette-Guerin in stage T1, grade 3 bladder cancer therapy: a 7-year follow-up. Urology 54:258–263
Lebret T, Gaudez F, Herve JM, Barre P, Lugagne PM, Botto H (1998) Low-dose BCG instillations in the treatment of Stage T1 Grade 3 bladder tumours: recurrence, progression and success. Eur Urol 34:67–72
Orsola A, Trias I, Raventós CX et al (2005) Initial high-grade T1 urothelial cell carcinoma: feasibility and prognostic significance of lamina propria invasion microstaging (T1a/b/c) in BCG-treated and BCG-non-treated patients. Eur Urol 48:231–238
Denzinger S, Burger M, Fritsche HM et al (2009) Prognostic value of histopathological tumour growth patterns at the invasion front of T1G3 urothelial carcinoma of the bladder. Scand J Urol Nephrol 43(4):282–287
Brake M, Loertzer H, Horsch R, Keller H (2000) Recurrence and progression of stage T1, grade 3 transitional cell carcinoma of the bladder following intravesical immunotherapy with bacillus Calmette-Guerin. J Urol 163:1697–1705
Pansadoro V, Emiliozzi P, de Paula F, Scarpone P, Pansadoro A, Sternberg CN (2002) Long-term follow-up of G3T1 transitional cell carcinoma of the bladder treated with intravesical bacille Calmette-Guerin: 18-year experience. Urology 59:227–231
Bertz S, Denzinger S, Otto W et al (2011) Histomorphological parameters can improve risk stratification in pT1 urothelial bladder cancer—evaluation of a large (hospital-based) single-centre series. Histopathology 59(4):722–732
van Rhijn BW, van der Kwast TH, Alkhateeb SS et al (2012) A new and highly prognostic system to discern t1 bladder cancer substage. Eur Urol 61(2):378–384
Bringuier PP, Umbas R, Schaafsma HE, Karthaus HF, Debruyne FM, Schalken JA (1993) Decreased E-cadherin immunoreactivity correlates with poor survival in patients with bladder tumors. Cancer Res 53(14):3241–3245
Kashibuchi K, Tomita K, Schalken JA, Kume H, Takeuchi T, Kitamura T (2007) The prognostic value of E-cadherin, alpha-, beta- and gamma-catenin in bladder cancer patients who underwent radical cystectomy. Int J Urol 14(9):789–794
Bryan RT, Atherfold PA, Yeo Y et al (2008) Cadherin switching dictates the biology of transitional cell carcinoma of the bladder: ex vivo and in vitro studies. J Pathol 215(2):184–194
Shi B, Laudon V, Yu S, Dong D, Zhu Y, Xu Z (2008) E-cadherin tissue expression and urinary soluble forms of E-cadherin in patients with bladder transitional cell carcinoma. Urol Int 81(3):320–324
Otto W, Denzinger S, Fritsche HM et al (2011) The WHO classification of 1973 is more suitable than the WHO classification of 2004 for predicting survival in pT1 urothelial bladder cancer. BJU Int 107(3):404–408
Acknowledgements
The authors thank Ms. Nina Nießl and Mrs. Stefanie Götz for their excellent support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to report.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Wolfgang Otto and Johannes Breyer contributed equally to this work.
Rights and permissions
About this article
Cite this article
Otto, W., Breyer, J., Herdegen, S. et al. WHO 1973 grade 3 and infiltrative growth pattern proved, aberrant E-cadherin expression tends to be of predictive value for progression in a series of stage T1 high-grade bladder cancer after organ-sparing approach. Int Urol Nephrol 49, 431–437 (2017). https://doi.org/10.1007/s11255-016-1491-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-016-1491-9