Abstract
The Klotho gene displays an extremely shortened life span with loss of function missense mutations leading to premature multiple organ failure, thus resembling human premature aging syndromes. The transmembrane form of Klotho protein functions as an obligatory co-receptor for FGF23. Klotho and FGF23 are crucial components for the regulation of vitamin D metabolism and subsequently blood phosphate levels. The secreted Klotho protein has multiple regulatory functions, including effects on electrolyte homeostasis, on growth factor pathways as well as on oxidative stress, which are currently the object of extensive research. Klotho protein deficiency is observed in many experimental and clinical disease models. Genetic polymorphisms such as the G-395A polymorphism in the promoter region of the Klotho gene have been associated with the development of essential hypertension. The kidneys are the primary site of Klotho production, and renal Klotho is decreased in CKD, followed by a reduction in plasma Klotho. Klotho deficiency has been both associated with progression of CKD as well as with its cardinal systemic manifestations, including cardiovascular disease. Thus, Klotho has been suggested both as a risk biomarker for early detection of CKD and additionally as a potential therapeutic tool in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the ancient Greek mythology, Klotho was the youngest of the Three Fates—including her sisters Lachesis and Atropos. Klotho was responsible for spinning the thread of human life, Lachesis for deciding how much time for life was to be allowed for each person and Atropos for choosing the mechanism of death and ending the life of each mortal by cutting their thread with her “horrifying shears.”
In 1997 a new gene, involved in the suppression of several aging phenotypes was identified and termed Klotho (KL) gene. The Klotho gene was named after the spinner and encodes the Klotho protein [1]. This gene was first identified as a gene mutation in the Klotho mouse, which displays an extremely shortened life span and multiple disorders leading to premature multiple organ failure, thus resembling human premature aging syndromes [1, 2]. Individuals who carry the KL-VS mutation produce higher levels of Klotho enzyme and appear to have an advantage for longevity as well as enhanced cognition.
Klotho and its physiologic functions
Klotho is a transmembrane protein, which is mainly expressed in the renal distal convoluted tubules (DCT) and in the brain choroid plexus [1], but it is also found in other locations, such as in the renal proximal tubules or parathyroid glands [3, 4].
Klotho in the peripheral blood circulation originates either from shedding of the two type I β-glycosidase extracellular domains of Klotho (KL1 and KL2) from the cell surface by proteolysis or a soluble Klotho isoform which can be produced by alternative splicing of the Klotho mRNA. Thus, Klotho protein exists in two forms, respectively, the transmembrane form expressed primarily in renal tubular cells and the secreted circulating form [2, 4]. Membrane-anchored and soluble Klotho proteins have different functional properties. Soluble Klotho functions as an endocrine factor with various effects on the kidneys as well as other tissues, whereas both nuclear and cytoplasmic Klotho forms possess significant beneficial properties including anti-aging and anti-apoptotic functions [5].
Klotho, phosphate and vitamin D
The transmembrane form of Klotho has been shown to form a complex with several receptors of the fibroblast growth factor family (FGFR). FGF are a group of signaling proteins with a conservative core domain of about 120 amino acids. Klotho acts as an obligatory co-receptor for FGF23. The latter is a relatively new endocrine factor secreted from osteocytes in response to high blood phosphate and vitamin D levels, and it is responsible for lowering blood phosphate and vitamin D levels [6, 7]. Thus, after active vitamin D (1,25-dihydroxyvitamin D3) binds to the vitamin D receptor (VDR) in osteocytes, a heterodimer is formed with a nuclear receptor, which activates FGF23 expression by binding to a promoter region of the FGF23 gene. Consequently, FGF23 secreted from the bone exerts its effect the Klotho–FGFR complex in the kidney (the bone–kidney axis) and the parathyroid gland (the bone–parathyroid axis). In the kidney, FGF23 suppresses expression of the gene that encodes 1α-hydroxylase, whereas in the parathyroid gland, FGF23 suppresses expression of PTH. Considering the fact that PTH promotes the expression of 1α-hydroxylase gene, suppression of PTH by FGF23 is associated with reduced 1,25-dihydroxyvitamin D3 serum levels. Thus, Klotho and FGF23 are crucial components for the regulation of vitamin D metabolism and subsequently blood phosphate levels, which is currently considered one of the main culprits responsible for the aging-like phenotypes [8].
There are different NaPi co-transporters which play a significant role in the regulation of phosphate homeostasis by the kidneys, which are variously expressed in the kidney proximal tubules [9]. A new mechanism for the regulation of this class of transporters has been introduced via the soluble Klotho protein, which directly inhibits the activity of the NaPi-2a co-transporter in the proximal tubule independently of FGF23 and without affecting the amount of the co-transporter expressed [3].
Klotho and calcium homeostasis
Klotho maintains calcium homeostasis through its suppression of PTH and 1,25 VitD3. However, there is a novel aspect introduced, which involves the direct effect of Klotho on the reabsorption of calcium by the kidney. Chang et al. [10] demonstrated that soluble Klotho stimulates transient receptor potential cation channel subfamily 5 (TRPV5), which is associated with regulation of calcium excretion by the kidneys. Thus, Klotho exerts its effect by stabilizing TRPV5 on the cell surface. The extracellular domain of Klotho protein acts as a potent activator of the TRPV5 and TRPV6 which are calcium channels expressed predominantly in renal tubular cells and duodenal epithelial cells and are responsible for Ca2+ absorption from the kidney and intestine [10, 11].
Klotho and potassium
One of the major mediators of urinary K+ reabsorption is the ROMK1 channels. Accordingly, it was shown by Cha et al. [12] that acute Klotho infusion augments the expression of apical membrane ROMK1 channels, thus causing diminished kaliuresis, and this mechanism of action resembles that of TRPV5. Klotho removes terminal sialic acids from N-glycan of ROMK1 and exposes underlying disaccharide galactose-N-acetyl glucosamine, which serves as a ligand for a ubiquitous galectin-1. Binding to galectin-1 at the cell surface prevents clathrin-mediated endocytosis of ROMK1, with ensuing accumulation of functional ROMK1 on the plasma membrane. Currently, the actions of Klotho as a hormone affecting potassium balance remain to be clarified as there are not observed alterations of the plasma potassium levels in mice which overexpress Klotho or are Klotho deficient. Regarding the potential clinical significance of the anti-kaliuretic effects of Klotho, most probably hyperkalemia may not be observed in pathological processes associated with Klotho deficiency, including AKI and CKD. On the other hand, it should be kept in mind that therapeutic administration of Klotho, which might cause hyperkalemia [5].
Klotho and anti-aging properties
The permanent and irreversible growth arrest of the aging cells is the key event in the pathophysiological process of aging. Thus, although aging cells remain viable, they lose their capacity for cell multiplication and cell repair. Furthermore, gene transcription mechanisms are affected, which translates into altered protein processing, including growth factors [13]. There have been suggested several and potentially overlapping mechanisms of premature aging and cellular senescence, including DNA and mitochondrial instability, decreased signaling of anabolic hormones such as IGF1, inflammation, free radical excess, telomere shortening, and recently phosphate toxicity, and systemic Klotho deficiency [13].
Nevertheless, the underlying mechanisms which would explain the reason why a lack of Klotho is associated with aging-like phenotypes remain to be clarified. It should be noted that precise laboratory assays which measure circulating Klotho levels are not available at present [14]. Thus, it is unclear whether the aging phenotype associated with Klotho deficiency in the tissues and more so in the kidneys should be attributed to regional effects or the operating mechanism involves a diffuse interplay of factors affecting the organism as a whole. Preliminary data suggest that reduced systemic levels of Klotho promote cellular senescence in all tissues, whereas its role in mineral homeostasis is only the tip of the iceberg discovered so far [15]. Accordingly, experimental data have suggested various endocrine functions of circulating Klotho which prevent cellular senescence via intricate pathways. Thus, induction of the transcription factors FOXO which are important regulators of the cellular oxidative stress response and thus increasing the expression of antioxidant proteins such as superoxide dismutase [16], anti-inflammatory effects [17], endothelial protection from senescence [18], anti-fibrotic properties [19] and prevention of vascular calcifications are some of possible mechanisms which have been put forward regarding this issue [20].
On the other hand, the role of Klotho in the regulation of mineral homeostasis might be as well significant regarding its anti-aging properties [21]. This has been suggested from experimental data which have shown that the aging phenotype of Klotho knockout mice is prevented by administration of a phosphate or 1,25-dihydroxyvitamin D restricted diet or via generation of Klotho and 1alpha-hydroxylase double-knockout mice [22–24] (Table 1).
The discovery of the Klotho–FGF-23 endocrine axis apart from providing new insight into the regulating mechanisms of phosphate homeostasis has also suggested a significant association of phosphate with the relatively new concept of vascular aging. Elevated blood phosphate levels are currently considered as a significant risk factor for cardiovascular disease, including left ventricular hypertrophy, not only in patients with end-stage CKD or patients with overt cardiovascular disease, but also in the general population as well [25–29].
Furthermore, the influence of Klotho on growth factors and specifically its suppressive effects on insulin/IGF-1 signaling has also been suggested as a putative mechanism for Klotho’s anti-aging properties. Attenuation of GH/IGF-1 or insulin signaling in experimental models including invertebrates and mammals has been associated with longevity [8, 30]. Klotho-deficient mice are hypoglycemic and very sensitive to insulin [31, 32], whereas on the other hand, Klotho overexpressing transgenic mice display moderate resistance to insulin and IGF-1, although they maintain normal fasting blood glucose levels and are not diabetic [32]. Thus, one of the properties of secreted Klotho protein is inhibition of insulin- and IGF-1-induced auto-phosphorylation of the insulin receptor and IGF-1 receptor in cultured cells [32, 33]. Although the mechanism for this activity has not been elucidated until now, it is possible that secreted Klotho protein may modify glycans of the insulin/IGF-1 receptors and as a consequence inhibits their activity or diminishes their quantity on the cell surface [32, 33].
Finally, recent data suggest that the secreted Klotho protein binds to multiple Wnt ligands, thus preventing Wnt ligand binding to its respective cell surface receptor and subsequently inhibited Wnt signal transduction pathways [34]. Although Wnt signaling controls stem cell proliferation and differentiation as well as tissue regeneration, its continuous activation may be associated with deleterious effects on cells rapid exhaustion and depletion of cells [34]. A relevant example is that the skin of Klotho-deficient mice has a reduced number of epidermal stem cells within the hair follicles [34].
Thus, the aging phenotype associated with Klotho deficiency should be attributed to both the intrinsic anti-aging properties of Klotho as well as secondary effects in mineral metabolism. It is noteworthy that all the proposed mechanisms of premature aging and cellular senescence, such as DNA and mitochondrial instability, inflammation, free radical excess, telomere shortening, phosphate-related toxicity and systemic Klotho deficiency, seem to be present in the uremic environment.
Klotho and salt sensitivity
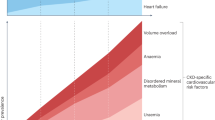
Aging is a complex process characterized by gradual decline of one’s health status. On the other hand, arterial hypertension is a common characteristic related to aging. The prevalence of arterial hypertension increases with age, being more than double in the elderly compared to the young population and more than two-thirds of individuals over age 65 years suffer from hypertension [35] (Fig. 1).
Salt intake is one of the main environmental factors contributing to the development of hypertension and several previous studies have reported an increase in salt sensitivity with advancing age. Thus, as salt sensitivity is more prevalent in the old rather than the young population, likewise the association of age and salt sensitivity is more marked in hypertensive compared to normotensive individuals [36, 37]. Xiao et al. [38] reported that Klotho levels in the serum decline with age in humans after 40 years of age, in a screened population with age range from 0 to 91 years. Although the Klotho level decreases and salt sensitivity increases with age, it is not clear whether Klotho deficiency by itself is associated with enhanced salt sensitivity (Fig. 2).
A recent study demonstrated that Klotho deficiency caused salt-sensitive hypertension and renal damage through chemokine mediated inflammation. Accordingly, Zhou et al. [39] investigated whether Klotho deficiency affects BP and salt sensitivity using Klotho mutant heterozygous mice and wild-type mice. They observed that systolic blood pressure in heterozygous mice began to increase at the age of 15 weeks, reached a peak level at the age of 17 weeks and remained elevated thereafter. On the other hand, systolic blood pressure remained stable in wild-type mice. Additionally, increased salt intake caused further augmentation of blood pressure in heterozygous mice, but it had no influence on the blood pressure levels of wild-type mice. Systolic blood pressure was also remarkably increased in heterozygous mice after being administered 2 % saline diet. On the other hand, salt-induced increase in blood pressure in heterozygous mice was abolished by administration of a specific antagonist to chemokine receptor 2, which is associated with monocyte chemotaxis. It was also shown that salt administration was significantly associated with enhanced expression of monocyte chemotactic protein-1 and the infiltration of macrophages and T-cells in kidneys of heterozygous mice. Treatment with the chemokine antagonist abolished these effects as well as attenuated structural renal damage and functional renal impairment induced by salt loading [39]. These findings suggest that a decrease in Klotho levels may be an etiologic factor for the enhanced salt sensitivity and salt-sensitive hypertension observed in the elderly population. Furthermore, an interplay between the inflammatory pathways including chemokine receptor 2-mediated inflammation may be part of the pathophysiologic mechanism involved in Klotho deficiency-induced salt-sensitive hypertension [39] (Fig. 3).
Klotho spinning the thread of human life. TRPV5 transient receptor potential cation channel subfamily V member 5, TRPV6 transient receptor potential cation channel subfamily V member 6, ROMK1 ROMK inward-rectifier K1 channels, NaPi-1&2 sodium/phosphate transporter 1&2, MCP-1 monocyte chemotactic protein-1, CC2 chemokine receptor 2, PTH parathormone
In vitro and in vivo studies have shown that blockade of the renin–angiotensin system (RAS) increases soluble Klotho levels. It has been shown in experimental models that Klotho exerts its kidney protection via targeted inhibition of RAS [40]. The effect of RAS blockade on soluble Klotho in patients with diabetic kidney disease was evaluated by Karalliede et al. [41]. Thus, 76 patients with type 2 diabetes and diabetic nephropathy, who were randomized to valsartan plus hydrochlorothiazide or amlodipine treatment, were evaluated at baseline, and 24 weeks after randomization, aortic pulse wave velocity and albuminuria were also measured at baseline and in 24 weeks. Valsartan plus hydrochlorothiazide treatment significantly increased mean soluble Klotho levels compared with amlodipine. Additionally, there was a significant difference observed between treatment groups regarding soluble Klotho and serum phosphate levels with valsartan plus hydrochlorothiazide treatment (P = 0.03 and P = 0.04, respectively). The levels of blood pressure achieved were similar between the two groups, whereas levels of soluble Klotho were not associated with aortic pulse wave velocity and albuminuria, which decreased significantly in patients who received valsartan/hydrochlorothiazide [41]. Furthermore, a recently published experimental study demonstrated that Klotho deficiency promoted arterial stiffening and hypertension induced by a high fat diet [42].
On the other hand, it was suggested that the aforementioned FGF23 plays a significant role as a key regulator of renal sodium reabsorption and plasma volume and may explain the association of FGF23 with cardiovascular risk in chronic kidney disease patients. Andrukhova et al. [43] have shown that FGF23 directly regulates the membrane expression of the Na/Cl co-transporter in distal renal tubules by a signaling mechanism involving the FGF receptor/aKlotho complex. Renal sodium reabsorption and distal tubular membrane expression of the sodium channel are reduced in mouse models of FGF23 and aKlotho deficiency. Conversely, gain of FGF23 function by injection of wild-type mice with recombinant FGF23 or by elevated circulating levels of endogenous FGF23 in Hyp mice increases distal tubular sodium uptake and membrane abundance of the sodium channel, causing volume expansion, hypertension and heart hypertrophy in a aKlotho and dietary salt dependent fashion. Thiazide diuretics, which inhibit the sodium channel, abolished FGF23-induced volume expansion and heart hypertrophy. These findings suggest that FGF23 with aKlotho complex play a significant role as key regulators of renal sodium reabsorption and plasma volume, thus providing reasonable evidence regarding the link of FGF23 with cardiovascular risk in chronic kidney disease patients [43].
Klotho and hypertension
More than 10 mutations or single-nucleotide polymorphisms (SNPs) have been described in the human Klotho gene, with some of them being associated with control of systolic blood pressure and fasting blood glucose, atherosclerotic coronary artery disease, cardio-embolic stroke as well as the human life span [44–48].
Shimoyama et al. [49] in order to determine the association of Klotho gene SNPs with clinical or laboratory data including systolic blood pressure, lipid profile, glucose metabolism, bone mineral density in 476 healthy Japanese individuals, genotyped 2 common single-nucleotide polymorphisms in human Klotho gene, the G-395A in the promoter region and the C1818T in exon 4. It was shown that men who were A allele carriers of G-395A compared with GG had a high body fat ratio and low HDL cholesterol level. On the other hand, as far as women were concerned, A allele carriers of G-395A compared with GG, and T allele carriers of C1818T compared with CC, had higher blood glucose levels. With respect to age, body mass index, body fat ratio and waist circumference, they were higher in men younger than 60 years old who were A allele carriers of G-395A compared to GG. Likewise in women younger than 60 years, bone mineral density was higher in A allele carriers of G-395A compared with GG, whereas systolic blood pressure and blood glucose were higher in T carriers of C1818T compared with CC. These data suggest that Klotho gene single-nucleotide polymorphisms have an impact on lipid metabolism and obesity in men [49]. Finally regarding blood pressure control, the study results suggest that the 2395A variant of the G-395A single-nucleotide polymorphism might be protective against the development of essential hypertension by upregulating the Klotho gene expression, since the A allele was shown to possess higher promoter activity in vitro [49].
Wang et al. [50] investigated whether the G-395A polymorphism of Klotho gene is associated with essential hypertension in a sample which included 215 patients with essential hypertension and 220 non-hypertensive subjects. Differences in genotype distributions of the G-395A polymorphism between the hypertensive and non-hypertension groups were statistically significant, and moreover, the G-395A polymorphism was demonstrated to be significantly associated with essential hypertension in subjects over 60 years old, in females and in non-smokers. Subsequently, the authors of this study suggest that since the G-395A polymorphism of the human Klotho gene is associated with essential hypertension it might serve as a potential regulatory site in the future, thus affecting Klotho enzymatic activity [50].
Another study by Wang et al. [51] examined whether Klotho gene delivery attenuates the progression of spontaneous hypertension and inhibits renal damage in spontaneous hypertensive rats. Data from the study revealed that Klotho expression and production were markedly suppressed in spontaneous hypertensive rats. Klotho gene delivery mediated by an adeno-associated virus carrying mouse Klotho full-length cDNA, halted further elevation of blood pressure in spontaneous hypertensive rats, an effect which was maintained during the total study length. However, Klotho gene delivery did not decrease the BP of spontaneous hypertensive rats to the control level; thus, it remains to be clarified by future research whether Klotho plays a part in the initiation of spontaneous hypertension [51].
Vascular endothelial cells use reactive oxygen species, such as superoxide and hydrogen peroxide, as signaling molecules under physiological conditions; however, excessive production of reactive oxygen species in disease states involving the heart and blood vessels may overcome the antioxidant defense mechanisms of cells, resulting in stimulation of oxidative stress, damage to the vessel wall and, ultimately, development of atherosclerosis [52, 53]. Saito et al. [54] demonstrated in a study using rats with multiple atherosclerosis risk factors such as hypertension, obesity, severe hyperglycemia and hypertriglyceridemia that adenovirus-mediated Klotho gene delivery can ameliorate vascular endothelial dysfunction, increase nitric oxide production, reduce elevated blood pressure and prevent medial hypertrophy together with perivascular fibrosis. Subsequently, they suggested that since Klotho gene delivery had a beneficial impact on endothelial function most probably via stimulation of nitric oxide signaling, therapeutic interventions based on Klotho might play an important part in the modulation of arterial hypertension and vascular remodeling [54].
The primary source of reactive oxygen species in the vasculature is NADPH oxidase and it is considered to play a significant part in mediating vascular inflammation and endothelial injury in many models of cardiovascular disease [52, 55]. Additionally, it has a key role in the hemodynamic responses to angiotensin II and subsequently in the pathogenesis of hypertension. Notably, Klotho gene delivery increased the level of the circulating Klotho, suggesting a direct action of Klotho in the endothelial vascular system via an unknown until now receptor [51]. Additionally, it was shown that Klotho gene delivery in the spontaneous hypertensive rats, increased plasma Interleukin 10 levels [51]. Interelukin 10 has been associated with inhibition of vascular smooth muscle cell and T cell proliferation as well as macrophage activation and as a result suppression of inflammation [56].
Importantly, Klotho gene delivery in spontaneous hypertensive rats was shown to inhibit the development of pathologic features of end-stage kidney tissue, such as tubular atrophy and dilation and tubular deposition of proteinaceous material due to failed reabsorption of low molecular weight proteins by the renal tubular system. It should be noted that Klotho gene delivery in spontaneously hypertensive rats attenuated glomerular collapse and interstitial collagen deposition, events which are associated with loss of glomerular filtration [51].
Klotho and chronic kidney disease
Both acute kidney injury and CKD are associated with Klotho deficiency; nevertheless, Klotho deficiency in CKD is continuous. Thus, Klotho deficiency has been shown to increase the kidney’s susceptibility to acute injury, to delay subsequent kidney regeneration and to induce development of renal fibrosis.
Considering the anti-apoptotic properties of Klotho, its deficiency significantly accentuates apoptosis in cultured kidney and endothelial cells [18, 57]. Moreover, it is suggested that Klotho prevents renal fibrosis primarily through inhibiting TGF-β1 signaling [58] which is considered a key factor in renal fibrogenesis [59]. Although the suppressive effects of Klotho on the insulin-like growth factor and on the Wnt signal pathway may be as well associated with Klotho’s anti-fibrotic properties, so far this concept remains to be clarified by further evidence. Recent evidence has shown that Klotho inhibits Wnt pathway-associated β-catenin activation, thus improving renal fibrosis [40]. Additionally, recent experimental data have shown that Klotho may also attenuate the development of myocardial hypertrophy caused by uremic toxins in mice with CKD [60].
Although increased basic fibroblast growth factor-2 (FGF2) and reduced Klotho levels are both considered to have a tight link with the pathogenesis of renal fibrosis, the relationship between the two is still elusive. In a recent study, Guan et al. [61] demonstrated that FGF2 induced the transition of cultured proximal kidney tubular epithelial cells to mesenchymal ones, accompanied by a reduction in Klotho expression, whereas recombinant Klotho prevented the action of FGF2. Klotho was shown to suppress the activation of protein kinase ½ which is necessary for the FGF2 activity. Additionally, FGF2-induced fibroblast proliferation and activation was also hindered by Klotho. The inhibitory effect of Klotho on the activity of FGF2 should probably be ascribed to its competing with FGF2 for binding to FGF receptor 1. Thus, there appears to a feedback pathway connecting Klotho depletion and FGF2 activation in the setting of renal fibrosis. Moreover, with regard to the above findings, it may be presumed that inhibition of FGF2 signaling might be one of the mechanisms via which Klotho attenuates renal fibrosis [61].
In CKD, renal Klotho is decreased, followed by a reduction in plasma Klotho. The downregulation of Klotho increases FGF23 production via unknown mechanisms, and subsequently 1, 25 VitD3 production in the kidney. It remains to be clarified whether the event of the parathyroid gland becoming resistant to the suppressive effect of FGF23 on PTH production is mediated by low plasma Klotho levels. Decreased levels of FGF23 receptor and attenuated Klotho expression as occurs in the uremic milieu could cause resistance of the parathyroid glands to FGF23 action, thus inducing secondary hyperparathyroidism. [5]. In addition to the classical traditional risk factors, FGF23 and Klotho are considered as novel contributors to the development of ectopic calcification in soft tissues including large vessels such as the aorta [20]. Increasing Klotho blood levels by genetic manipulation inhibits vascular calcifications in animals with CKD [20]. The suppressive influence of Klotho on vascular calcifications in CKD is multi-factorial including effects on several of the pathways described above, such as decreasing plasma Pi and inhibiting Pi-induced Pit-1 and Pit-2 activation in the vasculature, suppressing cell senescence, apoptosis and death in vascular endothelial cells as well as smooth muscle cells, induced by a variety of insults including Pi, and serving as an anti-inflammatory modulator [62, 63]. This information is coupled with the results of a recent study which showed that decreased levels of circulating soluble Klotho in CKD might be an independent etiologic factor of uremic cardiomyopathy, apart from associated FGF23 and phosphate effects [64].
One of the main characteristics of CKD is diminished Klotho tissue expression which occurs from the early stages of the disease. Thus, Klotho might serve as a relevant biomarker for risk exposure in patients with CKD. At present, the exact mechanism via which CKD causes diminished Klotho levels is not clear. Downregulation of Klotho gene expression by the uremic toxins [65] may be one of the possible mechanisms. Moreover, Klotho expression is hindered by inflammation, thus promoting accelerated organ aging in the setting of the inflammatory environment of CKD [13, 66].
Conclusions
The clinical utility of Klotho might cover two fields, diagnosis and therapy, respectively. Klotho may be used as a sensitive biomarker for early CKD detection. On the other hand, Klotho therapy via either exogenous administration or induction of endogenous Klotho production may prove beneficial in patients with AKI and CKD, due to enhancement of kidney recovery mechanisms as well as effects on the systemic manifestations of kidney disease. As we are prone to use Klotho for diagnostic and therapeutic purposes in CKD, additional research is required to shed light on the intricate and overlapping pathways of the Klotho protein actions so as to add specificity within particular disease models.
References
Kuro-o M, Matsumura Y, Aizawa H et al (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390:45–51
Shiraki-Iida T, Aizawa H, Matsumura Y et al (1998) Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett 424:6–10
Hu MC, Shi M, Zhang J et al (2010) Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J 24:3438–3450
Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y (1988) Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun 26:626–630
Hu MC, Kuro-o M, Moe OW (2013) Renal and extrarenal actions of Klotho. Semin Nephrol 33:118–129
Kuro-o M (2006) Klotho as a regulator of fibroblast growth factor signaling and phosphate/calcium metabolism. Curr Opin Nephrol Hypertens 15:437–441
Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP et al (2006) Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 281:6120–6123
Kuro-o M (2009) Klotho and aging. Biochim Biophys Acta 1790:1049–1058
Moe OW (2009) PiT-2 coming out of the pits. Am J Physiol Renal Physiol 296:F689–F690
Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG (2005) The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science 310(5747):490–493
Cha SK, Ortega B, Kurosu H, Rosenblatt KP, Kuro O, Huang CL (2008) Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci USA 105(28):9805–9810
Cha SK, Hu MC, Kurosu H, Kuro-o M, Moe O, Huang CL (2009) Regulation of renal outer medullary potassium channel and renal K(+) excretion by Klotho. Mol Pharmacol 76:38–46
Tosato M, Zamboni V, Ferrini A, Cesari M (2007) The aging process and potential interventions to extend life expectancy. Clin Interv Aging 2:401–412
Sugiura H, Tsuchiya K, Nitta K (2011) Circulating levels of soluble alpha-Klotho in patients with chronic kidney disease. Clin Exp Nephrol 15:795–796
Stenvinkel P, Larsson TE (2013) Chronic kidney disease: a clinical model of premature aging. Am J Kidney Dis 62:339–351
Yamamoto M, Clark JD, Pastor JV et al (2005) Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem 11(280):38029–38034
Maekawa Y, Ishikawa K, Yasuda O et al (2009) Klotho suppresses TNF-alpha-induced expression of adhesion molecules in the endothelium and attenuates NF-kappaB activation. Endocrine 35:341–346
Maekawa Y, Ohishi M, Ikushima M et al (2011) Klotho protein diminishes endothelial apoptosis and senescence via a mitogen-activated kinase pathway. Geriatr Gerontol Int 11:510–516
Sugiura H, Yoshida T, Shiohira S et al (2012) Reduced Klotho expression level in kidney aggravates renal interstitial fibrosis. Am J Physiol Renal Physiol 15(302):F1252–F1264
Hu MC, Shi M, Zhang J et al (2011) Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 22(1):124–136
Olauson H, Lindberg K, Amin R et al (2012) Targeted deletion of Klotho in kidney distal tubule disrupts mineral metabolism. J Am Soc Nephrol 23:1641–1651
Sitara D, Kim S, Razzaque MS et al (2008) Genetic evidence of serum phosphate-independent functions of FGF-23 on bone. PLoS Genet 4(8):e1000154
Ohnishi M, Nakatani T, Lanske B, Razzaque MS (2009) Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1alpha-hydroxylase. Kidney Int 75:1166–1172
Ohnishi M, Razzaque MS (2010) Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging. FASEB J 24:3562–3571
Block GA, Hulbert-Shearon TE, Levin NW, Port FK (1998) Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 31:607–617
Dhingra R, Sullivan LM, Fox CS et al (2007) Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 14(167):879–885
Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA (2009) Serum phosphate and left ventricular hypertrophy in young adults: the coronary artery risk development in young adults study. Kidney Blood Press Res 32:37–44
Mathew S, Tustison KS, Sugatani T, Chaudhary LR, Rifas L, Hruska KA (2008) The mechanism of phosphorus as a cardiovascular risk factor in CKD. J Am Soc Nephrol 19:1092–1105
Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G (2005) Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 25(112):2627–2633
Tatar M, Bartke A, Antebi A (2003) The endocrine regulation of aging by insulin-like signals. Science 299(5611):1346–1351
Utsugi T, Ohno T, Ohyama Y et al (2000) Decreased insulin production and increased insulin sensitivity in the klotho mutant mouse, a novel animal model for human aging. Metabolism 49:1118–1123
Kurosu H, Yamamoto M, Clark JD et al (2005) Suppression of aging in mice by the hormone Klotho. Science 309(5742):1829–1833
Wolf I, Levanon-Cohen S, Bose S et al (2008) Klotho: a tumor suppressor and a modulator of the IGF-1 and FGF pathways in human breast cancer. Oncogene 27(56):7094–7105
Liu H, Fergusson MM, Castilho RM et al (2007) Augmented Wnt signaling in a mammalian model of accelerated aging. Science 317(5839):803–806
Mancia G, Fagard R, Narkiewicz K et al (2013) 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 31:1281–1357
Osanai T, Kanazawa T, Yokono Y, Uemura T, Okuguchi T, Onodera K (1993) Effect of aging on sensitivity of blood pressure to salt. Nihon Ronen Igakkai Zasshi 30:30–34
Weinberger MH, Fineberg NS (1991) Sodium and volume sensitivity of blood pressure. Age and pressure change over time. Hypertension 18:67–71
Xiao NM, Zhang YM, Zheng Q, Gu J (2004) Klotho is a serum factor related to human aging. Chin Med J (Engl) 117:742–747
Zhou X, Chen K, Lei H, Sun Z (2015) Klotho gene deficiency causes salt-sensitive hypertension via monocyte chemotactic protein-1/CC chemokine receptor 2-mediated inflammation. J Am Soc Nephrol 26:121–132
Zhou L, Mo H, Miao J et al (2015) Klotho ameliorates kidney injury and fibrosis and normalizes blood pressure by targeting the renin-angiotensin system. Am J Pathol 185:3211–3223
Karalliedde J, Maltese G, Hill B, Viberti G, Gnudi L (2013) Effect of renin-angiotensin system blockade on soluble Klotho in patients with type 2 diabetes, systolic hypertension, and albuminuria. Clin J Am Soc Nephrol 8:1899–1905
Lin Y, Chen J, Sun Z (2016) Antiaging gene klotho deficiency promoted high-fat diet-induced arterial stiffening via inactivation of AMP-activated protein kinase. Hypertension 67:564–573
Andrukhova O, Slavic S, Smorodchenko A et al (2014) FGF23 regulates renal sodium handling and blood pressure. EMBO Mol Med 6:744–759
Arking DE, Krebsova A, Macek M et al (2002) Association of human aging with a functional variant of klotho. Proc Natl Acad Sci USA 22(99):856–861
Imamura A, Okumura K, Ogawa Y et al (2006) Klotho gene polymorphism may be a genetic risk factor for atherosclerotic coronary artery disease but not for vasospastic angina in Japanese. Clin Chim Acta 371:66–70
Kim Y, Kim JH, Nam YJ et al (2006) Klotho is a genetic risk factor for ischemic stroke caused by cardioembolism in Korean females. Neurosci Lett 30(407):189–194
Rhee EJ, Oh KW, Yun EJ et al (2006) Relationship between polymorphisms G395A in promoter and C1818T in exon 4 of the KLOTHO gene with glucose metabolism and cardiovascular risk factors in Korean women. J Endocrinol Invest 29:613–618
Rhee EJ, Oh KW, Lee WY et al (2006) The differential effects of age on the association of KLOTHO gene polymorphisms with coronary artery disease. Metabolism 55:1344–1351
Shimoyama Y, Nishio K, Hamajima N, Niwa T (2009) KLOTHO gene polymorphisms G-395A and C1818T are associated with lipid and glucose metabolism, bone mineral density and systolic blood pressure in Japanese healthy subjects. Clin Chim Acta 406:134–138
Wang HL, Xu Q, Wang Z et al (2010) A potential regulatory single nucleotide polymorphism in the promoter of the Klotho gene may be associated with essential hypertension in the Chinese Han population. Clin Chim Acta 411:386–390
Wang Y, Sun Z (2009) Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension 54:810–817
Dammanahalli KJ, Sun Z (2008) Endothelins and NADPH oxidases in the cardiovascular system. Clin Exp Pharmacol Physiol 35(1):2–6
Sagar S, Kallo IJ, Kaul N, Ganguly NK, Sharma BK (1992) Oxygen free radicals in essential hypertension. Mol Cell Biochem 111:103–108
Saito Y, Nakamura T, Ohyama Y et al (2000) In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Commun 24(276):767–772
Bengtsson SH, Gulluyan LM, Dusting GJ, Drummond GR (2003) Novel isoforms of NADPH oxidase in vascular physiology and pathophysiology. Clin Exp Pharmacol Physiol 30:849–854
Mazighi M, Pelle A, Gonzalez W et al (2004) IL-10 inhibits vascular smooth muscle cell activation in vitro and in vivo. Am J Physiol Heart Circ Physiol 287:H866–H871
Sugiura H, Yoshida T, Mitobe M et al (2010) Klotho reduces apoptosis in experimental ischaemic acute kidney injury via HSP-70. Nephrol Dial Transplant 25:60–68
Dai S, Zou Y, Togao O et al (2011) Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem 286:8655–8665
Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A (2003) Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest 112:1486–1494
Yang K, Wang C, Nie L et al (2015) Klotho protects against indoxyl sulphate-induced myocardial hypertrophy. J Am Soc Nephrol 26:2434–2446
Guan X, Nie L, He T et al (2014) Klotho suppresses renal tubulo-interstitial fibrosis by controlling basic fibroblast growth factor-2 signalling. J Pathol 234:560–572
Zhao Y, Banerjee S, Dey N et al (2011) Klotho depletion contributes to increased inflammation in kidney of the db/db mouse model of diabetes via RelA (serine) 536 phosphorylation. Diabetes 60:1907–1916
Liu F, Wu S, Ren H, Gu J (2011) Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat Cell Biol 13:254–262
Xie J, Yoon J, An SW, Kuro-o M, Huang CL (2015) Soluble klotho protects against uremic cardiomyopathy independently of fibroblast growth factor 23 and phosphate. J Am Soc Nephrol 26:1150–1160
Sun CY, Chang SC, Wu MS (2012) Suppression of Klotho expression by protein-bound uremic toxins is associated with increased DNA methyltransferase expression and DNA hypermethylation. Kidney Int 81:640–650
Moreno JA, Izquierdo MC, Sanchez-Nino MD et al (2011) The inflammatory cytokines TWEAK and TNFalpha reduce renal klotho expression through NFkappaB. J Am Soc Nephrol 22:1315–1325
Hu MC, Shi M, Zhang J et al (2016) Renal production, uptake, and handling of circulating alphaklotho. J Am Soc Nephrol 27:79–90
Kadoya H, Satoh M, Haruna Y, Sasaki T, Kashihara N (2015) Klotho attenuates renal hypertrophy and glomerular injury in Ins2Akita diabetic mice. Clin Exp Nephrol. doi:10.1007/s10157-015-1202-3
Wang Y, Sun Z (2014) Antiaging gene Klotho regulates endothelin-1 levels and endothelin receptor subtype B expression in kidneys of spontaneously hypertensive rats. J Hypertens 32:1629–1636
Kim AJ, Ro H, Kim H et al (2016) Klotho and S100A8/A9 as discriminative markers between pre-renal and intrinsic acute kidney injury. PLoS ONE 11(1):e0147255
Semba RD, Cappola AR, Sun K et al (2011) Plasma klotho and cardiovascular disease in adults. J Am Geriatr Soc 59:1596–1601
Bernheim J, Benchetrit S (2011) The potential roles of FGF23 and Klotho in the prognosis of renal and cardiovascular diseases. Nephrol Dial Transplant 26:2433–2438
Gao LL, Ding X, Xie DM, Yang M, Dong BR (2015) G-395A polymorphism in the promoter region of the KLOTHO gene and hypertension among elderly (90 years and older) Chinese individuals. Genet Mol Res 14:15444–15452
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kalaitzidis, R.G., Duni, A. & Siamopoulos, K.C. Klotho, the Holy Grail of the kidney: from salt sensitivity to chronic kidney disease. Int Urol Nephrol 48, 1657–1666 (2016). https://doi.org/10.1007/s11255-016-1325-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-016-1325-9