Abstract
Purpose
Obstructive sleep apnea (OSA) is highly prevalent in patients with chronic kidney disease and may lead to a loss of kidney function. However, it remains unclear whether or not continuous positive airway pressure (CPAP) treatment improves the estimated glomerular filtration rate (eGFR) in patients with OSA. This meta-analysis was designed to investigate the effect of CPAP therapy on eGFR in patients with OSA.
Methods
We searched the electronic databases Web of Science, Cochrane Library, PubMed, and Embase through June 1, 2022. Information about patients, CPAP duration, gender distribution, pre- and post-CPAP treatment eGFR, and age of patients were collected for further analysis. We applied the standardized mean difference (SMD) with a 95%confidence interval (CI) to analyze the pooled effects. Both Stata 12.0 software and Review Manager 5.2 software were employed for all statistical analyses.
Results
A sample of 13 studies with 519 patients was included in the meta-analysis. There was no significant change of eGFR levels before and after CPAP usage for patients with OSA (SMD = − 0.05, 95%CI: − 0.30 to 0.19, Z = 0.43, p = 0.67). However, subgroup analysis revealed that the level of eGFR was obviously decreased after CPAP therapy in patients with OSA and CPAP use duration > 6 months (SMD = − 0.30, 95% CI = − 0.49 to - 0.12, z = 3.20, p = 0.001), and elderly patients (> 60 years) (SMD = − 0.32, 95% CI = − 0.52 to − 0.11, z = 3.02, p = 0.002).
Conclusions
Meta-analysis confirmed that OSA treatment with CPAP has no clinically significant effect on eGFR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is a common sleep-related respiratory disease with substantial important clinical and public health implications. OSA is characterized by periodic reductions or cessations of respiration during sleep accompanied by chronic intermittent hypoxia (CIH), leading to excessive daytime sleepiness, nocturnal hypoxemia, nocturnal choking, and sleep disruption. The condition is known to affect about 9% and 24% of middle-aged females and males, respectively [1]. The high prevalence of OSA in middle-aged adults and its cardiovascular comorbidities make it a major public health burden for society. An increasing amount of evidence suggests that OSA is independently associated with an increased risk of cardiovascular mortality, because of its increased risks of heart failure, hypertension, myocardial ischemia, coronary artery disease, and arrhythmia [2,3,4,5].
Renal function decline, evaluated mainly by the estimated glomerular filtration rate (eGFR), is a massive economic problem. Chronic kidney disease (CKD) is recognized as eGFR less than 60 mL/min/1.73 m2 and/or pathological evidence of kidney damage, with at least a duration of 3 months. It is reported that the prevalence of both OSA and CKD is increasing [6]. Furthermore, the prevalence of OSA is higher among patients with end-stage renal disease (ESRD) [7]. 8]. OSA is considered to be a novel independent risk factor for development of CKD [8,9, 10]. Thus, a strong correlation may exist between OSA and CKD.
Continuous positive airway pressure (CPAP) treatment is currently the main therapy for patients with OSA. It is reported that CPAP is also associated with attenuation of glomerular hyperfiltration and downregulation of renin-angiotensin-aldosterone system (RAAS) activity [11, 12]. However, whether or not the eGFR levels can be reversed by CPAP is still under debate. The primary aim of the present meta-analysis was to evaluate the effect of CPAP treatment on eGFR among patients with OSA.
Materials and methods
PRISMA statement
The present meta-analysis was performed according to the PRISMA 2020 statement [13].
Search strategy
We searched four online databases from inception to June 1, 2022: Web of Science, PubMed, Embase, and Cochrane Library. All searches were conducted by using MeSH terms and free-text, and the combination of the following key terms was conducted: “estimated glomerular filtration rate OR eGFR” and “CPAP OR continuous positive airway pressure” and “sleep apnea OR obstructive sleep apnea”. No language or other restriction was imposed. All relevant articles listed in the reference of the pooled studies were also scrutinized attentively. Two independent investigators (JY, L and XY, C) identified the eligible studies.
Inclusion and exclusion criteria
We selected studies which met the following criteria: (1) All participants collected in the meta-analysis were diagnosed with OSA. (2) CPAP was applied. (3) The Mean and SD (or SE) of eGFR both before and after application of CPAP was measured. (4) All of the studies had to have enough data to enable our meta-analysis. (5) Reviews, abstracts, non-human studies, conference articles, case reports, letters to the editor, and non-English publications were excluded.
Data extraction
Two authors (YY, F and JY, L) independently extracted the data. We resolved inconsistent decisions through a discussion with a third investigator. We collected the following variables from each eligible study: nationality, publication date, the number of patients, first author’s name, gender distribution, age range of study subjects, CPAP duration, daily CPAP usage time, mean AHI, eGFR values before and after CPAP, study design, and body mass index (BMI).
Statistical analysis
We performed statistical analyses with the use of RevMan v.5.2 and STATA 12.0. The standardized mean difference (SMD) and 95% confidence intervals (CI) were used by us to analyze the extracted data. Heterogeneity across studies was evaluated based on the chi2 and the I2 statistics, with I2 > 50% meaning substantial heterogeneity in our meta-analysis. If I2 ≤ 50%, a fixed-effects model was applied by our team to estimate an effect size. Otherwise, a random-effects model was then employed to obtain the SMD if I2 > 50%. At the same time, we further conducted sensitivity and subgroup analysis to clarify the possible sources of heterogeneity. Both the “Begg test” and the “Egger test” were conducted in the study to examine potential publication bias. p < 0.05 was set as statistical significance.
Results
Search results
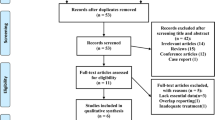
After the removal of duplicates, our initial search strategy yielded a total of 90 references. Of the 90 references, we found 40 possible relevant articles after a preliminary review of the abstracts and titles. Among the remaining 40 trials, 27 studies were ruled out. A total of 13 articles were included in the meta-analysis. The selection process of our literature search is outlined in Fig. 1.
Characteristics of included studies
In total, 13 trials with 18 cohorts and 519 patients were enrolled in the current meta-analysis. Our study included only one randomized clinical trial (RCT) [14]. The outcomes in the five studies were separately divided into two groups [11, 12, 14,15,16]. According to the severity of OSA, Li’s study was divided into mild OSA group and moderate/severe OSA group. According to the gender, Nicholl’s study (2020) was divided into women group and men group. According to the nocturnal hypoxemia, Nicholl’s study (2021) was divided into moderate group and severe group. Eight studies defined OSA based on AHI [15,16,17,18,19,20,21,22], four studies defined it according to the oxygen desaturation index (ODI) [11, 12, 14, 23], and Nicholl’s study defined OSA based on respiratory disturbance index (RDI) [24]. The study baseline characteristics (year, author, and country), therapy duration, daily CPAP time, sample size, inclusion criteria, and study design are included in Table 1. We found that most of the participants in this study were male. Most of the studies were SCT trials, which were carried out in western countries. BMI, AHI, age, and eGFR are shown in Table 2. Most of the included patients had a BMI greater than 28 kg/m2 and most of them were over 50 years old.
Pooled analysis
There were significant differences obtained in our study (chi2 = 64.14, p < 0.00001; I2 = 73%) in our heterogeneity test. Thus, a random effects model was applied for the pooled analysis. After pooling the data, there was no significant difference in eGFR before and after CPAP use among patients with OSA (SMD = − 0.05, 95%CI = − 0.30 to 0.19, Z = 0.43, p = 0.67) (Fig. 2). A similar result was found based on a fixed effects model (SMD = 0.60, 95%CI = − 1.34 to 2.54, Z = 0.61, p = 0.54).
Sensitive analysis and subgroup analysis
Sensitivity analysis indicated that removing every study at a time did not affect the present pooled analysis result (Fig. 3). Considering the effect of CPAP treatment might be influenced by multiple confounding factors, we then conducted subgroup analyses according to age (≤ 60 and > 60), baseline BMI (≤ 30 and > 30), CPAP duration (≤ 6 months and > 6 months), and gender distribution (women and men). eGFR was overtly decreased after CPAP therapy in elder patients (> 60 years) (SMD = − 0.32, 95% CI = − 0.52 to − 0.11, z = 3.02, p = 0.002), and in patients with CPAP usage duration > 6 months (SMD = − 0.30, 95% CI = − 0.49 to − 0.12, z = 3.20, p = 0.001). However, we found that the differences in BMI, and gender distribution did not have an effect on the efficacy of CPAP therapy (Table 3).
Publication bias
Figure 4 suggests that there may have been a publication bias. However, both the Begg and Egger tests demonstrated that no evidence of publication bias was detected in the study (p = 0.705 and 0.145, respectively).
Discussion
The current meta-analysis was the first study addressing the efficacy of CPAP treatment on eGFR in patients with OSA. We found that OSA treatment with CPAP exerted no effect on eGFR. Nevertheless, subgroup analyses revealed that CPAP therapy was linked to a marked decrease in eGFR in older patients (> 60 years), or patients with CPAP use duration > 6 months.
OSA is a highly prevalent disorder in patients with CKD. Heinzer et al. [25] found that sleep-disordered breathing had a high prevalence in elderly men. Previous studies demonstrated that its prevalence among CKD patients is higher than that in the general population [20, 26]. The presence of OSA in this population is related to a substantial reduction in quality of life, and a higher risk of cardiovascular morbidity and mortality. Numerous clinical studies found a correlation between OSA and renal dysfunction. Patients with OSA appeared to have elevated levels of albuminuria [27]. In addition, it was suggested that OSA is a significant predictor of the accelerated loss of kidney function, and may contribute to the progression of CKD.
Renal function decline, assessed by the eGFR, is linked to increased cardiovascular events and morbidity in the general population [28, 29]. Mild-to-moderate renal insufficiency is becoming a worldwide public and clinical health problem. Previous studies have reported that there is a bidirectional association between OSA and renal function decline. In clinical trials, nocturnal hypoxemia due to OSA has been shown to be related to the deterioration of kidney function [12, 30, 31]. Patients with ESRD often exhibit a high prevalence of OSA and can result in worsening of sleep apnea [8, 32]. The organ damage caused by OSA is associated with intermittent hypoxia, inducing oxidative stress, inflammation, increased sympathetic activity, RAAS, hemodynamic instability, and endothelial dysfunction [33,34,35,36,37], which may contribute to accelerated loss of renal function. Therefore, it is reasonable to speculate that OSA is related to a reduction in eGFR, which contributes to the worsening of kidney function.
Above all, clinicians should emphasize this mutual relationship to investigate patients with OSA at risk for CKD, as well as CKD patients for comorbid OSA. Recently,it has been gradually recognized that OSA is likely to be a contributing risk factor in the development of CKD. Therefore, early recognition and therapy of OSA may be an effective approach for blunting progression to ESRD.
CPAP plays a crucial role in the management of patients with OSA, reducing their symptoms and enhancing patients’ life quality. Recent research indicated that CPAP therapy might exert beneficial effects on eGFR for patients with OSA [19, 38]. As shown in the trial enrolled patients with CKD 4 or 5 stage, CPAP may significantly ameliorate the progression of CKD, especially in the patients with moderate/severe OSA [15]. There are several potential explanations for CPAP caused improvement of decreased eGFR. CPAP treatment may reverse the decreased eGFR by correcting sympathetic activity, eliminating apneic and/or hypopnea episodes, reducing renal RAAS activity, maintaining normal oxygenation, and reversing endothelial dysfunction [16, 22]. CPAP therapy may also affect diabetes and hypertension via the above possible mechanisms, and thus improve the decreased eGFR. However, our study found that CPAP treatment did not affect eGFR in patients with OSA. As the efficacy of CPAP was affected by multiple factors, we also conducted subgroup analyses in terms of treatment duration, age, BMI, and gender. The results indicated that 6 months of CPAP therapy was related to a decrease in eGFR. Besides CPAP duration, our findings showed that eGFR in elderly patients may respond worse to CPAP treatment. It was reported that eGFR declined with advancing age in all subjects. However, Marrone et.al found that fixed CPAP treatment could attenuate the decline in eGFR for patients with OSA [39]. Further studies focusing on the function of CPAP, or on alternative OSA treatment modalities are warranted, in order to find patients who may benefit from therapy.
The present meta-analysis has its important strengths. First, this is the first comprehensive meta-analysis to explore eGFR in response to CPAP therapy in a large number of patients with OSA patients. Second, compared to any individual study, pooling data from all eligible studies may yield more reliable results. Third, most trials pooled in our meta-analysis had a therapeutic CPAP treatment of 4 h/night or more, indicating good CPAP adherence. Fourth, the funnel plots in our meta-analysis did not indicate publication bias.
The study also has several limitations. First, the sample size of our pooled analyses was small, which may restrict the extrapolation of our conclusions. Second, most of the studies included in our report were self-control trials. Additional large-scale, well-designed RCTs are needed. Third, different studies used different measurement techniques for eGFR in the meta-analysis. Fourth, some potential confounders that could influence eGFR, like drugs, inflammation, diets, and comorbidities, were difficult to fully adjust for. Fifth, the treatment periods of included trials were varioused, which may cause the heterogeneity of the study. Sixth, average eGFR before CPAP differed highly among the sample populations of the analyzed studies, which should be taken in consideration when we explain theexamining the effect of CPAP treatment. Seventh, we only analyzed these articles written in English were included, which may have resulted in publication bias.
Conclusions
The meta-analysis revealed that CPAP treatment did not impact affect eGFR in patients with OSA. However, longer duration of CPAP therapy for OSA patients iwas associated with a decline of eGFR. In elderly patients with OSA, CPAP was also linked to a significant decrease in eGFR. To clarify this issue, further large-sized RCTs with long-term follow-up may be warranted.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Cunningham J, Hunter M, Budgeon C, Murray K, Knuiman M, Hui J, Hillman D, Singh B, James A (2021) The prevalence and comorbidities of obstructive sleep apnea in middle-aged men and women: the Busselton Healthy Ageing Study. J Clin Sleep Med 17:2029–2039. https://doi.org/10.5664/jcsm.9378
Yeghiazarians Y, Jneid H, Tietjens JR, Redline S, Brown DL, El-Sherif N, Mehra R, Bozkurt B, Ndumele CE, Somers VK (2021) Obstructive sleep apnea and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 144:e56–e67. https://doi.org/10.1161/CIR.0000000000000988
Baker Smith CM, Isaiah A, Melendres MC, Mahgerefteh J, Lasso Pirot A, Mayo S, Gooding H, Zachariah J (2021) Sleep-disordered breathing and cardiovascular disease in children and adolescents. J Am Heart Assoc 10:e22427. https://doi.org/10.1161/JAHA.121.022427
Naito R, Kasai T, Dohi T, Takaya H, Narui K, Momomura S (2022) Factors associated with the improvement of left ventricular systolic function by continuous positive airway pressure therapy in patients with heart failure with reduced ejection fraction and obstructive sleep apnea. Front Neurol 13:781054. https://doi.org/10.3389/fneur.2022.781054
Zinchuk AV, Chu J, Liang J, Celik Y, Op De Beeck S, Redeker NS, Wellman A, Yaggi HK, Peker Y, Sands SA (2021) Physiological traits and adherence to sleep apnea therapy in individuals with coronary artery disease. Am J Resp Crit Care 204:703–712. https://doi.org/10.1164/rccm.202101-0055OC
Voulgaris A, Marrone O, Bonsignore MR, Steiropoulos P (2019) Chronic kidney disease in patients with obstructive sleep apnea A narrative review. Sleep Med Rev 47:74–89. https://doi.org/10.1016/j.smrv.2019.07.001
Wali S, Alkhouli A, Howladar M, Ahmad I, Alshohaib S, Al-Ghamdi S, Krayem A (2015) Risk of obstructive sleep apnea among Saudis with chronic renal failure on hemodialysis. Ann Thorac Med 10:263–268. https://doi.org/10.4103/1817-1737.164300
Lin C, Lurie RC, Lyons OD (2020) Sleep apnea and chronic kidney disease. Chest 157:673–685. https://doi.org/10.1016/j.chest.2019.09.004
Dou L, Lan H, Reynolds DJ, Gunderson TM, Kashyap R, Gajic O, Caples S, Li G, Kashani KB (2017) Association between obstructive sleep apnea and acute kidney injury in critically ill patients: a propensity-matched study. Nephron 135:137–146. https://doi.org/10.1159/000453367
Beaudin AE, Raneri JK, Ahmed SB, Hirsch Allen AJM, Nocon A, Gomes T, Gakwaya S, Series F, Kimoff J, Skomro RP, Ayas NT, Hanly PJ (2022) Risk of chronic kidney disease in patients with obstructive sleep apnea. Sleep 45:zsab267. https://doi.org/10.1093/sleep/zsab267
Nicholl DDM, Hanly PJ, Zalucky AA, Handley GB, Sola DY, Ahmed SB (2020) Sex differences in renal hemodynamics and renin-angiotensin system activity post-CPAP therapy in humans with obstructive sleep apnea. Am J Physiol-Renal 318:F25–F34. https://doi.org/10.1152/ajprenal.00290.2019
Nicholl DDM, Hanly PJ, Zalucky AA, Handley GB, Sola DY, Ahmed SB (2021) Nocturnal hypoxemia severity influences the effect of CPAP therapy on renal renin–angiotensin–aldosterone system activity in humans with obstructive sleep apnea. Sleep 44:zsaa228. https://doi.org/10.1093/sleep/zsaa228
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hrobjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Loffler KA, Heeley E, Freed R, Anderson CS, Brockway B, Corbett A, Chang CL, Douglas JA, Ferrier K, Graham N, Hamilton GS, Hlavac M, McArdle N, McLachlan J, Mukherjee S, Naughton MT, Thien F, Young A, Grunstein RR, Palmer LJ, Woodman RJ, Hanly PJ, McEvoy RD (2017) Effect of obstructive sleep apnea treatment on renal function in patients with cardiovascular disease. Am J Resp Crit Care 196:1456–1462. https://doi.org/10.1164/rccm.201703-0603OC
Li X, Liu C, Zhang H, Zhang J, Zhao M, Sun D, Xia M, Han M (2019) Effect of 12-month nasal continuous positive airway pressure therapy for obstructive sleep apnea on progression of chronic kidney disease. Medicine 98:e14545. https://doi.org/10.1097/MD.0000000000014545
Puckrin R, Iqbal S, Zidulka A, Vasilevsky M, Barre P (2015) Renoprotective effects of continuous positive airway pressure in chronic kidney disease patients with sleep apnea. Int Urol Nephrol 47:1839–1845. https://doi.org/10.1007/s11255-015-1113-y
Kinebuchi S, Kazama JJ, Satoh M, Sakai K, Nakayama H, Yoshizawa H, Narita I, Suzuki E, Gejyo F (2004) Short-term use of continuous positive airway pressure ameliorates glomerular hyperfiltration in patients with obstructive sleep apnoea syndrome. Clin Sci 107:317–322. https://doi.org/10.1042/CS20040074
Koga S, Ikeda S, Yasunaga T, Nakata T, Maemura K (2013) Effects of nasal continuous positive airway pressure on the glomerular filtration rate in patients with obstructive sleep apnea syndrome. Internal Med 52:345–349. https://doi.org/10.2169/internalmedicine.52.8468
Perticone M, Maio R, Scarpino PE, Mancuso L, Volpentesta M, Caroleo B, Suraci E, Sciacqua A, Sesti G, Perticone F (2021) Continuous positive airway pressure improves renal function in obese patients with obstructive sleep apnea syndrome. Front Med 8:642086. https://doi.org/10.3389/fmed.2021.642086
Pochetti P, Azzolina D, Ragnoli B, Tillio PA, Cantaluppi V, Malerba M (2020) Interrelationship among obstructive sleep apnea, renal function and survival: a cohort study. Int J Env Res Pub He 17:4922. https://doi.org/10.3390/ijerph17144922
Zhang X, Jiang X, Lin Q, Chen X, Zeng H (2014) Effect of continuous positive airway pressure on serum cystatin C among obstructive sleep apnea syndrome patients. Int Urol Nephrol 46:1997–2002. https://doi.org/10.1007/s11255-014-0779-x
Nowicki M, Zawiasa-Bryszewska A, Taczykowska M, Białasiewicz P, Nowak D (2020) The pattern of overnight changes in novel markers of acute kidney injury in patients with obstructive sleep apnea. Adv Clin Exp Med 29:1065–1072. https://doi.org/10.17219/acem/123356
Hanly PJ, Ahmed S, Fjell CD, Handley GB, Sola D, Nicholl D, Zalucky A (2020) Urine biomarkers of renal renin–angiotensin system activity: exploratory analysis in humans with and without obstructive sleep apnea. Physiol Rep 8:e14376. https://doi.org/10.14814/phy2.14376
Nicholl DDM, Hanly PJ, Poulin MJ, Handley GB, Hemmelgarn BR, Sola DY, Ahmed SB (2014) Evaluation of continuous positive airway pressure therapy on renin–angiotensin system activity in obstructive sleep apnea. Am J Resp Crit Care 190:572–580. https://doi.org/10.1164/rccm.201403-0526OC
Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, Mooser V, Preisig M, Malhotra A, Waeber G, Vollenweider P, Tafti M, Haba-Rubio J (2015) Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 3:310–318. https://doi.org/10.1016/S2213-2600(15)00043-0
Lin C, Perger E, Lyons OD (2018) Obstructive sleep apnea and chronic kidney disease. Curr Opin Pulm Med 24:549–554. https://doi.org/10.1097/MCP.0000000000000525
Chuang L, Lin S, Lee L, Chang C, Huang H, Hu H, Kao K, Hsieh M, Yang C, Li H, Chen N (2019) Elevated serum markers of acute kidney injury in patients with obstructive sleep apnea. J Clin Sleep Med 15:207–213. https://doi.org/10.5664/jcsm.7618
Huang Y, Hsu Y, Chuang Y, Lin HYH, Chen Y, Chan T (2021) Association between renal function and cardiovascular mortality: a retrospective cohort study of elderly from health check-up. BMJ Open 11:e49307. https://doi.org/10.1136/bmjopen-2021-049307
Tunbridge MJ, Jardine AG (2021) Atherosclerotic vascular disease associated with chronic kidney disease. Cardiol Clin 39:403–414. https://doi.org/10.1016/j.ccl.2021.04.011
Hanly PJ, Ahmed SB (2014) Sleep apnea and the kidney. Chest 146:1114–1122. https://doi.org/10.1378/chest.14-0596
Zalucky AA, Nicholl DDM, Hanly PJ, Poulin MJ, Turin TC, Walji S, Handley GB, Raneri JK, Sola DY, Ahmed SB (2015) Nocturnal hypoxemia severity and renin–angiotensin system activity in obstructive sleep apnea. Am J Resp Crit Care 192:873–880. https://doi.org/10.1164/rccm.201502-0383OC
Voulgaris A, Bonsignore MR, Schiza S, Marrone O, Steiropoulos P (2021) Is kidney a new organ target in patients with obstructive sleep apnea? Research priorities in a rapidly evolving field. Sleep Med 86:56–67. https://doi.org/10.1016/j.sleep.2021.08.009
Mesarwi OA, Loomba R, Malhotra A (2019) Obstructive sleep apnea, hypoxia, and nonalcoholic fatty liver disease. Am J Resp Crit Care 199:830–841. https://doi.org/10.1164/rccm.201806-1109TR
Sanderson JE, Fang F, Lu M, Ma CY, Wei YX (2021) Obstructive sleep apnoea, intermittent hypoxia and heart failure with a preserved ejection fraction. Heart 107:190–194. https://doi.org/10.1136/heartjnl-2020-317326
Labarca G, Gower J, Lamperti L, Dreyse J, Jorquera J (2020) Chronic intermittent hypoxia in obstructive sleep apnea: a narrative review from pathophysiological pathways to a precision clinical approach. Sleep Breath 24:751–760. https://doi.org/10.1007/s11325-019-01967-4
Stanek A, Brożyna-Tkaczyk K, Myśliński W (2021) Oxidative stress markers among obstructive sleep apnea patients. Oxid Med Cell Longev 2021:1–8. https://doi.org/10.1155/2021/9681595
Catalan Serra P, Soler X (2022) Obstructive sleep apnea and cardiovascular events in elderly patients. Expert Rev Resp Med 16:197–210. https://doi.org/10.1080/17476348.2022.2030225
Rimke AN, Ahmed SB, Turin TC, Pendharkar SR, Raneri JK, Lynch EJ, Hanly PJ (2021) Effect of CPAP therapy on kidney function in patients with chronic kidney disease. Chest 159:2008–2019. https://doi.org/10.1016/j.chest.2020.11.052
Marrone O, Cibella F, Pépin JL, Grote L, Verbraecken J, Saaresranta T, Kvamme JA, Basoglu OK, Lombardi C, McNicholas WT, Hedner J, Bonsignore MR (2018) Fixed but not autoadjusting positive airway pressure attenuates the time-dependent decline in glomerular filtration rate in patients with OSA. Chest 154:326–334. https://doi.org/10.1016/j.chest.2018.04.020
Funding
The Joint Funds for the innovation of science and Technology, Fujian province (Grant number: 2021Y9027), Science and Technology Bureau of Quanzhou (Grant number: 2022C038R), Young and middle-aged backbone talents training project of Fujian Provincial Health Commission (Grant number: 2021GGA040), Science and Technology Project of Fujian Education Department (Grant number: JAT200136), Startup Fund for Scientific Research, Fujian Medical University (Grant number: 2018QH1105), and the project of Fujian Provincial Health Commission (Grant number: 2019-ZQN-66) provided financial support in the form of financial funding. The sponsor had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
All authors directly participated in the study and have reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fu, Y., Lin, J., Chen, L. et al. Meta-analysis of the effects of CPAP therapy on estimated glomerular filtration rate in patients with obstructive sleep apnea. Sleep Breath 27, 2155–2163 (2023). https://doi.org/10.1007/s11325-023-02811-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-023-02811-6