Abstract
Purpose
We carried out a systematic review and meta-analysis to assess the efficacy and safety of imidafenacin for treating overactive bladder in adult.
Methods
A literature review was performed to identify all published randomized placebo-controlled trials of imidafenacin for the treatment of OAB. The search included the following databases: MEDLINE, EMBASE. The reference lists of retrieved studies were also investigated.
Results
Five publications involving a total of 1,428 patients were used in the analysis, which compared imidafenacin with propiverine and solifenacin. We found that imidafenacin was effective in treating OAB in our meta-analysis, which was similar to propiverine in its efficacy. The mean number of UI per week (the standardized mean difference (SMD) = 1.23, 95 % CI −0.19 to 2.65, p = 0.09), the mean number of urgency episodes per day (SMD = 0.26, 95 % CI −0.11 to 0.63, p = 0.17), the mean number of micturitions per day (SMD = 0.01, 95 % CI −0.30 to 0.31, p = 0.96), and the mean urine volume (ml) per micturition (SMD = −13.04, 95 % CI −20.45 to −5.62, p = 0.0006) indicated that imidafenacin was similar to propiverine in its efficacy. Mean OABSS (SMD = 0.48, 95 % CI −0.08 to 1.03, p = 0.09) indicated that imidafenacin was also similar to solifenacin in its efficacy. Besides, imidafenacin was better tolerated than propiverine in the safety, indicated by dry mouth (OR 0.73, 95 % CI 0.54–0.98, p = 0.04) and any adverse events (OR 0.63, 95 % CI 0.46–0.88, p = 0.006). Moreover, imidafenacin was also better tolerated than solifenacin in the safety, indicated by constipation (OR 0.21, 95 % CI 0.08–0.53, p = 0.001) and any adverse events (OR 0.33, 95 % CI 0.15–0.71, p = 0.004).
Conclusions
This meta-analysis indicates that imidafenacin was similar to propiverine or solifenacin in its efficacy for OAB and was better tolerated than propiverine or solifenacin in the safety for OAB. We conclude that imidafenacin is preferable to propiverine or solifenacin from a perspective of safety.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Overactive bladder (OAB) is a highly prevalent, chronic, and debilitating condition, which, although not life-threatening, has a profound impact on the health-related quality of life of patients [1–4]. OAB syndrome is defined by the International Continence Society (ICS) as urgency, with or without urgency incontinence, usually with frequency and nocturia, in the absence of infection or other obvious pathology [5]. It is caused by spontaneous, involuntary contractions of the bladder wall during urinary filling that can be associated with reduced bladder wall compliance and elevated filling pressures [6]. Antimuscarinic agents are the current pharmacological mainstay for OAB [7, 8]. However, many patients discontinue their use due to inadequate efficacy and/or intolerable side effects [9–11].
Imidafenacin, as a novel anti-muscarinic agent to treat OAB, has high affinities for the M3 and M1 muscarinic receptor subtypes, a low affinity for M2 receptors, and a potent inhibitory activity against rhythmic bladder contractions in rats [12, 13]. Imidafenacin also inhibits the contractions of isolated human detrusor smooth muscles by blocking both postjunctional M3 receptors and prejunctional M1 receptors [14]. In addition, imidafenacin displays organ selectivity for the bladder over salivary gland tissues [13]. In OAB, imidafenacin has been shown to significantly decrease urgency, urgency urinary incontinence, and nocturia episodes at doses of 0.1 mg twice daily compared with placebo in several randomized placebo-controlled trials. Besides, the novel antimuscarinic agent imidafenacin at a dose of 0.1 mg twice daily was not inferior to propiverine and solifenacin for the reduction of incontinence episodes, and well tolerated for the treatment of OAB symptoms, which has also been shown in several randomized placebo-controlled trials.
Because of imidafenacin was more effective than placebo in many trials [15, 16], but the efficacy and safety compared with other antimuscarinic agents were unknown. The goal of the present study was to perform a meta-analysis to evaluate the safety and efficacy of imidafenacin compared with propiverine or solifenacin in treating OAB, which may resolve some of the current controversies over use of the drug.
Materials and methods
Inclusion criteria
Randomized controlled trials that met the following criteria were included: (1) a study design that included treatment with imidafenacin; (2) the study provided accurate efficacy and safety data that could be analyzed, including the total number of subjects and the values of each index, and (3) the full text of the study could be accessed. If these inclusion criteria were not met, then the study was excluded from the analysis.
Search strategy
MEDLINE (from 1966 to November 2014), EMBASE (from 1974 to November 2014), and the reference lists of retrieved studies were searched to identify RCTs that referred to the effects of imidafenacin treatment. The following search terms were used: imidafenacin, OAB, and randomized controlled trials.
Trial selection
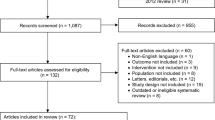
When the same study was published in various journals or in different years, the most frequently cited one was used for the meta-analysis. If the same group of researchers studied a group of subjects with multiple experiments, then each study was included. Together, we discussed each of the RCTs that were included and excluded studies that either failed to meet the inclusion criteria or could not be agreed upon by the authors. A flow diagram of the study selection process is presented in Fig. 1.
Quality assessment
The methodological quality of each study was assessed according to how patients were allocated to the arms of the study, the concealment of allocation procedures, blinding, and the data loss due to attrition. The studies were then classified qualitatively according to the guidelines published in the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [17]. Based on the quality-assessment criteria, each study was rated and assigned to one of the three following quality categories: A: if all quality criteria were adequately met, the study was deemed to have a low risk of bias; B: if one or more of the quality criteria were only partially met or were unclear, the study was deemed to have a moderate risk of bias; or C: if one or more of the criteria were not met, or not included, the study was deemed to have a high risk of bias. Sensitivity analyses were then performed on the basis of whether these quality factors were adequate, inadequate, or unclear. Differences were resolved by discussion among the authors.
Data extraction
The following information was collected: (1) the name of the first author and the publication year; (2) the study design and sample size; (3) the therapy that the patients received; (4) the source of the patients; (5) data including urgency incontinence episodes per week, urgency episodes per day, the mean number of micturitions per day, urine volume (ml) per micturition, OABSS, adverse events.
Statistical analysis
The meta-analysis of comparable data was carried out using Review Manager 5.1.0 [17]. Due to the large number of plots, we combined the 6 forest plots into 1 plot using Adobe Photoshop CS.
Results
Characteristics of individual studies
The database search and reference lists of retrieved studies found 142 potential articles to be used in our meta-analysis. Based on the inclusion and exclusion criteria, 117 articles were excluded after reading the titles and abstracts of the articles. Sixteen articles were not randomized controlled trials. Four articles lacked useful data. In all, five articles [15, 16, 18–20] with 10 RCTs that compared imidafenacin with propiverine and solifenacin were included in the analysis (Fig. 1). The baseline characteristics of the studies included in our meta-analysis are listed in Table 1.
Quality of individual studies
All ten RCTs were double blinded, and all described the randomization processes that they had used. All included a power calculation to determine the optimal sample size (Table 2). The level of quality of each identified study was A–B (Table 2). The funnel plot provided a qualitative estimation of publication bias of the studies, and no evidence of bias was found (Fig. 2).
Efficacy
Urgency incontinence episodes per week
There were four RCTs, representing 1,016 participants (519 in the imidafenacin group and 497 in the propiverine group) (Fig. 3). According to our analysis, no heterogeneity was found among the trials, and a fixed-effects model was thus chosen for the analysis. Based on our analysis, the pooled estimate of standardized mean difference (SMD) was 1.23, and the 95 % CI was –0.19 to 2.65 (p = 0.09). This result suggests that imidafenacin showed no significant reductions in the mean number of UI per week compared with propiverine.
Urgency episodes per day
There were four RCTs, representing 1,016 participants (519 in the imidafenacin group and 497 in the propiverine group) (Fig. 3). According to our analysis, no heterogeneity was found among the trials, and a fixed-effects model was thus chosen for the analysis. Based on our analysis, the pooled estimate of SMD was 0.26, and the 95 % CI was −0.11 to 0.63 (p = 0.17). This result suggests that imidafenacin showed no significant reductions in the mean number of urgency per day compared with propiverine.
The mean number of micturitions per day
There were four RCTs, representing 1,016 participants (519 in the imidafenacin group and 497 in the propiverine group) (Fig. 3). Based on our analysis, the pooled estimate of SMD was 0.01, and the 95 % CI was −0.30 to 0.31 (p = 0.96). This result suggests that imidafenacin showed no significant reductions in the mean number of micturitions per day compared with propiverine.
Urine volume (ml) per micturition
There were four RCTs, representing 1,016 participants (519 in the imidafenacin group and 497 in the propiverine group) (Fig. 4). According to our analysis, no heterogeneity was found among the trials, and a fixed-effects model was thus chosen for the analysis. Based on our analysis, the pooled estimate of SMD was −13.04, and the 95 % CI was −20.45 to −5.62 (p = 0.0006). This result suggests that imidafenacin showed statistically significant increases in the mean urine volume per micturition compared with propiverine.
OABSS
There were six RCTs, representing 412 participants (204 in the imidafenacin group and 208 in the solifenacin group) (Fig. 5). According to our analysis, no heterogeneity was found among the trials, and a fixed-effects model was thus chosen for the analysis. Based on our analysis, the pooled estimate of SMD was 0.48, and the 95 % CI was −0.08 to 1.03 (p = 0.09). This result suggests that imidafenacin showed no significant reductions in the mean OABSS compared with solifenacin.
Safety
Dry mouth and any adverse events
Two RCTs, representing 787 participants (402 in the imidafenacin group and 385 in the propiverine group), included the dry mouth data (Fig. 6). According to our analysis, no heterogeneity was found among the trials, and a fixed-effects model was thus chosen for the analysis. Based on our analysis, the pooled estimate of odds ratio (OR) was 0.73, and the 95 % CI was 0.54–0.98 (p = 0.04). And two RCTs, representing 787 participants (402 in the imidafenacin group and 385 in the propiverine group), included the any adverse events data (Fig. 6). According to our analysis, no heterogeneity was found among the trials, and a fixed-effects model was thus chosen for the analysis. Based on our analysis, the pooled estimate of OR was 0.63, and the 95 % CI was 0.46–0.88 (p = 0.006). This result suggests that imidafenacin showed statistically significant reductions in the incidence of dry mouth and any adverse events compared with propiverine.
Constipation and any adverse events
Two RCTs, representing 145 participants (73 in the imidafenacin group and 72 in the solifenacin group), included the constipation data (Fig. 7). According to our analysis, no heterogeneity was found among the trials, and a fixed-effects model was thus chosen for the analysis. Based on our analysis, the pooled estimate of OR was 0.21, and the 95 % CI was 0.08–0.53 (p = 0.001). And two RCTs, representing 145 participants (73 in the imidafenacin group and 72 in the solifenacin group), included the any adverse events data (Fig. 7). According to our analysis, no heterogeneity was found among the trials, and a fixed-effects model was thus chosen for the analysis. Based on our analysis, the pooled estimate of OR was 0.33, and the 95 % CI was 0.15–0.71 (p = 0.004). This result suggests that imidafenacin showed statistically significant reductions in the incidence of constipation and any adverse events compared with solifenacin.
Discussion
OAB is highly prevalent and has a profoundly negative impact on ordinary quality of life. The major treatment for OAB is anticholinergics, including darifenacin, fesoterodine, oxybutynin, propiverine, solifenacin, and tolterodine. But their effectiveness has been limited by poor compliance due to side effects. Dry mouth is the most common and problematic side effect, and often leads to discontinuation of treatment [11]. Imidafenacin, as a novel antimuscarinic agent, is used to treat OAB and has high affinity for the M3 and M1 muscarinic receptor subtypes and low affinity for the M2 subtype [12, 13]. In addition, it shows organ selectivity for the bladder over the salivary glands [14]. Thus, it is highly likely that imidafenacin is safe, efficacious, and tolerable to control symptoms of OAB.
Our study reveals that imidafenacin was similar to propiverine for the reduction of UI episodes, urgency episodes, the mean number of micturitions, and was more effective than propiverine for the increase of urine volume (ml) per micturition. Our study also reveals that imidafenacin was similar to solifenacin for the reduction of OABSS. The results demonstrate that treatment with imidafenacin provides both statistically significant and, more importantly, clinically relevant improvements in the OAB symptoms. Furthermore, different trials assessing the effects of imidafenacin on nocturia and sleep quality showed that imidafenacin ameliorates sleep quality and duration in patients with OAB and nocturia [21–26]. We conclude that imidafenacin was effective in improving nocturia and sleep disorder in patients with OAB and in decreasing nocturnal urine volume and nocturnal polyuria index in patients with nocturnal polyuria [26], while solifenacin does not. And further more studies are needed to identify the efficacy of imidafenacin compared with other antimuscarinic agents on nocturia and sleep disorder.
The most common adverse reactions of anticholinergics are dry mouth and constipation. According to the study, dry mouth was reported in 29.95 and 35.14 % of imidafenacin and propiverine group, significantly lower in the imidafenacin group (p = 0.04). Our study reveals that the incidence of dry mouth induced by imidafenacin was significantly lower than propiverine. And the incidence of moderate-to-severe dry mouth is significantly lower in patients taking imidafenacin (5.0 %) than in those receiving propiverine (9.2 %, p = 0.043) [15, 16]. Moreover, the episodes of dry mouth in patients treated with imidafenacin were milder than those observed in patients treated with solifenacin (p = 0.009), and the duration of dry mouth with imidafenacin was shorter than that with solifenacin (imidafenacin 2.16 ± 5.0 h, solifenacin 3.44 ± 5.9 h, 1 month after; p = 0.042) [18, 19]. We conclude that imidafenacin is related to the shortest duration of dry mouth symptoms compared with other AMs [18, 19, 27]. Besides, constipation was reported in 11 and 42.1 % of imidafenacin and solifenacin group, significantly lower in the imidafenacin group (p = 0.001). Moreover, the incidence of total adverse events induced by imidafenacin was significantly lower than propiverine (p = 0.006) or solifenacin (p = 0.004). The possible mechanisms of less dry mouth and constipation in our study are attributable to a higher selectivity of imidafenacin for the bladder over other organs. In addition, imidafenacin has higher affinities for M3 and M1 receptors and higher selectivity for the urinary bladder than for the salivary gland.
As the dose of imidafenacin was 0.1 mg twice daily, so we can conclude that imidafenacin 0.1 mg twice daily is an effective and well-tolerated treatment for OAB symptoms. Besides, efficacy and safety data were concluded from 4, 8, 12 and 28 weeks, and the data on longer-term safety and efficacy of imidafenacin cannot be extrapolated from the included RCTs. Homma et al. [28] conducted a RCT and reported that the rate of continuation of imidafenacin, evaluated in a long-term administration study at 52 weeks, was significantly high (78.7 %). Therefore, imidafenacin is expected to be useful for the long-term treatment of chronic OAB symptoms.
This meta-analysis includes studies which are all findings from randomized double-blind, placebo-controlled trials. According to the quality-assessment scale that we developed, the quality of the individual studies in the meta-analysis was conforming. The results of this analysis acquire great importance from scientific standpoint but also in the everyday clinical practice. However, the number of included studies was not many. The longer-term safety, efficacy, and persistence of imidafenacin cannot be extrapolated from this article. In addition, unpublished studies’ data were not included in the analysis. These factors may have resulted in a bias. More high-quality trials with larger samples are proposed to learn more about the efficacy and safety of the therapy on OAB.
Conclusion
This meta-analysis indicates that imidafenacin was similar to propiverine or solifenacin in its efficacy for OAB and was better tolerated than propiverine or solifenacin in the safety for OAB. We conclude that imidafenacin is preferable to propiverine or solifenacin from a perspective of safety.
References
Abrams P, Kelleher CJ, Kerr LA et al (2000) Overactive bladder significantly affects quality of life. Am J Manag Care 6(Suppl):S580–S590
Kobelt G, Kirchberger I, Malone-Lee J (1999) Quality-of-life aspects of the overactive bladder and the effect of treatment with tolterodine. BJU Int 83:583–590
Stewart WF, Van Rooyen JB, Cundiff GW et al (2003) Prevalence and burden of overactive bladder in the United States. World J Urol 20:327–336
Milsom I, Abrams P, Cardozo L et al (2001) How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int 87:760–766
Abrams P, Cardozo L, Fall M et al (2002) The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Neurourol Urodyn 21:167–178
Getsios D, El-Hadi W, Caro I et al (2005) Pharmacological management of overactive bladder. Pharmacoeconomics 23:995–1006
Marinkovic SP, Rovner ES, Moldwin RM et al (2012) The management of overactive bladder syndrome. BMJ 344:38–44
Andersson KE, Chapple CR, Cardozo L et al (2009) Pharmacological treatment of overactive bladder: report from the International Consultation on incontinence. Curr Opin Urol 19:380–394
Sexton CC, Notte SM, Maroulis C et al (2011) Persistence and adherence in the treatment of overactive bladder syndrome with anticholinergic therapy: a systematic review of the literature. Int J Clin Pract 65:567–585
Chapple CR (2000) Muscarinic receptor antagonists in the treatment of overactive bladder. Urology 55:33–46 (discussion 50)
Madhuvrata P, Cody JD, Ellis G et al (2012) Which anticholinergic drugs for overactive bladder syndrome in adults? Cochrane Database Syst Rev 1:005429
Kobayashi F, Yageta Y, Segawa M et al (2007) Effects of imidafenacin (KRP-197/ONO-8025), a new anti-cholinergic agent, on muscarinic acetylcholine receptors. High affinities for M3 and M1 receptor subtypes and selectivity for urinary bladder over salivary gland. Arzneimittelforschung 57:92–100
Kobayashi F, Yageta Y, Yamazaki T et al (2007) Pharmacological effects of imidafenacin (KRP-197/ONO-8025), a new bladder selective anti-cholinergic agent, in rats. Comparison of effects on urinary bladder capacity and contraction, salivary secretion and performance in the Morris water maze task. Arzneimittelforschung 57:147–154
Murakami S, Yoshida M, Iwashita H et al (2003) Pharmacological effects of KRP-197 on the human isolated urinary bladder. Urol Int 71:290–298
Homma Y, Yamaguchi T, Yamaguchi O (2008) A randomized, double-blind, placebo-controlled phase II dose-finding study of the novel anti-muscarinic agent imidafenacin in Japanese patients with overactive bladder. Int J Urol 15:809–815
Homma Y, Yamaguchi O (2009) A randomized, double-blind, placebo- and propiverine controlled trial of the novel antimuscarinic agent imidafenacin in Japanese patients with overactive bladder. Int J Urol 16:499–506
Higgins JPT, Green S, (eds) (2011) Cochrane handbook for systematic reviews of interventions, v.5.1 [updated March 2011]. Cochrane Collaboration Web site. http://www.cochrane-handbook.org/
Zaitsu M, Mikami K, Ishida C et al (2011) Comparative evaluation of the safety and efficacy of long-term use of imidafenacin and solifenacin in patients with overactive bladder: a prospective, open, randomized, Parallel-group trial (the LIST Study). Adv Urol 2011:854697
Yokoyama T, Koide T, Hara R et al (2013) Long-term safety and efficacy of two different antimuscarinics, imidafenacin and solifenacin, for treatment of overactive bladder: a prospective randomized controlled study. Urol Int 90(2):161–167
Park C, Park J, Choo MS et al (2014) A randomised, prospective double-blind, propiverine-controlled trial of imidafenacin in patients with overactive bladder. Int J Clin Pract 68(2):188–196
Takeda M, Takahashi S, Nishizawa O et al (2009) Imidafenacin, a novel anticholinergic, significantly improves both nocturia and sleep disorders in OAB patients: ePOCH (Evaluation of anticholinergics in Patients with Overactive bladder and nocturia for Care Health) study. Jpn J Urol Surg 22:53–60
Nagaoka A, Sakurai T, Naito S et al (2011) Sleep disorders and HRQOL were significantly improved by imidafenacin, an anticholinergic agent, in OAB patients with nocturia. Jpn J Urol Surg 24:1649–1656
Shimizu N, Minami T, Uemura H et al (2011) A study of the efficacy and safety of imidafenacin in younger and older elderly patients with overactive bladder. Jpn J Urol Surg 24:639–648
Kuratsukuri K, Tsujimura A, Akino H et al (2012) Randomized controlled trial of nocturia in patients with benign prostatic hyperplasia with OAB using an alpha-blocker combined with a novel anticholinergic, imidafenacin In GOOD-NIGHT Study. Eur Urol (Suppl 11):E745–U584
Shimizu N, Tsujimura A, Akino H et al (2012) Imidafenacin reduces night time urine production as well as increase bladder capacity in nocturia with BPH and concomitant OAB. Results from prospective randomized controlled trial, GOOD-NIGHT study. Abs. 432 ICS
Wada N, Watanabe M, Kita M et al (2012) Effect of imidafenacin on nocturia and sleep disorder in patients with overactive bladder. Urol Int 89(2):215–221
Kase H, Arak S, Kitamura T et al (2010) A comparative study of anticholinergic drugs used for overactive bladder in routine clinical practice, with a focus on dry mouth. Jpn J Urol Surg 23:1299–1306
Homma Y, Yamaguchi O, Imidafenacin Study Group (2008) Long-term safety, tolerability, and efficacy of the novel anti-muscarinic agent imidafenacin in Japanese patients with overactive bladder. Int J Urol 15:986–991
Conflict of interest
The authors had no conflict of interest to declare in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, W., Zong, H., Zhou, X. et al. Efficacy and safety of imidafenacin for overactive bladder in adult: a systematic review and meta-analysis. Int Urol Nephrol 47, 457–464 (2015). https://doi.org/10.1007/s11255-015-0916-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-015-0916-1