Abstract
Purpose
The efficacy and safety of vibegron, which is a novel β3-adrenoceptor agonist, need to be systematically evaluated in the treatment of overactive bladder (OAB) patients. This study aimed to assess, using meta-analytic methods, the efficacy and safety of vibegron for OAB compared with placebo or antimuscarinics, considering all available data from comparative studies.
Methods
A systematic search was performed on MEDLINE, Web of Science, and Cochrane Central Register of Controlled Trials database to identify studies from their date of inception before 15 March 2020. Meta-analysis was performed based on eligible studies.
Results
Six studies derived from four clinical trials with 4314 randomized patients were finally included in our analysis. We found that vibegron 50 mg and 100 mg were both significantly more efficacious than placebo for all efficacy outcomes. Furthermore, no significant differences were found between vibegron 50 mg or 100 mg and placebo for all the AEs assessed. In addition, the efficacy between vibegron 50 mg or 100 mg and antimuscarinics were comparable except for voided volume. Moreover, vibegron was associated with a decreased risk of dry mouth and an increased risk of nasopharyngitis versus antimuscarinics. Our study also demonstrated that the vibegron 50 mg and 100 mg were equally effective and safe across all the efficacy and AEs’ outcomes.
Conclusions
Vibegron is effective and safe for treating patients with OAB. Based on the current evidence, we recommended that the initial use of vibegron 50 mg was the optimal algorithm in the pharmacologic management of OAB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Overactive bladder (OAB) is a prevent disorder worldwide. It was estimated that up to 17% in adults suffered from OAB in Europe and the USA [1, 2]. The symptom complex, including urinary urgency, frequency, and (or) urgency urinary incontinence (UUI), has a significant negative impact on the quality of life. There are kinds of treatments for OAB, including the pharmacological treatments, minimally invasive procedures (e.g., intravesical injections with botulinum toxin A) and neuromodulation (e.g., tibial nerve stimulation). The doctor or the patients can choose one of them by discussing with each other among these different treatments of OAB. Whereas the management of OAB has a treatment algorithm by beginning with the pharmacologic treatment, in cases of refractory situations or for special reasons related to the patients, the minimally invasive treatment can be applied.
Antimuscarinics have proven to be effective in the treatment of OAB. However, side effects reduce patient satisfaction leading to relatively poor adherence to most antimuscarinics [3]. To improve the adherence rates [4], Mirabegron, a β3-adrenoceptor (AR) agonist, offers an alternative to antimuscarinics. A network meta-analysis [5] showed a significant therapeutic benefit of mirabegron 50 mg over placebo and was comparable to common antimuscarinics. Besides, it was better tolerated with fewer anticholinergic side effects such as dry mouth and constipation.
Acting as a novel potent and highly selective β3-AR agonist, the pharmacological activity of vibegron was proved both in vitro and in vivo [6, 7]. Since then, multiple studies have been performed to compare vibegron with placebo or antimuscarinics for patients with OAB [8,9,10,11]. Our systematic review and meta-analysis aimed to assess the efficacy and tolerability of vibegron in patients with OAB. To our knowledge, this is the first systematic review exploring the role of vibegron in the treatment of OAB.
Materials and methods
We performed this study following the Preferred Reported Items for Systematic Reviews and Meta-analysis guidelines [12]. This study was registered in PROSPERO with an ID of CRD42020175574.
Literature search
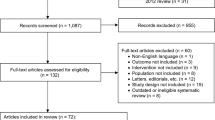
Two independent reviewers performed the electronic literature searches restricting to English language study on MEDLINE, Web of Science, and Cochrane Central Register of Controlled Trials database to identify studies from their date of inception prior to 15 March 2020. In case of any disagreement, they were resolved by consulting with a senior author (WK). Keywords used for the search were “[(Detrusor, Overactive) OR Overactive Bladder OR Overactive Urinary Bladder OR (Bladder, Overactive) OR Overactive Detrusor] AND vibegron”. These studies were then screened for inclusion and exclusion criteria.
Eligibility criteria
Two authors reviewed the relevant articles obtained from the literature search independently. Inclusion criteria used in this study were stated as follows: (1) participants: patients with OAB; (2) interventions: treated with vibegron; (3) comparison: any other drug therapy or placebo; (4) endpoints: the efficiency and safety evaluations; (5) study design: comparative studies. Case reports, reviews, meta-analysis, meeting abstracts, comments, and letters were excluded.
Data extraction and study assessment
Data included in the meta-analysis were extracted independently by two reviewers. The primary outcome was micturition episodes/24 h. Other efficacy outcomes included urgency urinary incontinence (UUI) episodes/24 h, urgency episodes/24 h, incontinence episodes/24 h, and voided volume/micturition. Safety and tolerability outcomes included the most commonly reported adverse events (AEs), including dry mouth, constipation, diarrhea, nasopharyngitis, and cystitis. Information including the name of the first author and trial number, each intervention and number of randomized patients, trial design, and location, patient population, and treatment duration are extracted and showed in Table 1.
Data analysis
Effect measures for the outcomes were calculated as the mean difference (MD) for continuous variables and odds ratio (OR) for dichotomous variables together with their 95% confidence interval (95% CI), respectively. Both least-squares mean change or mean change from baseline were extracted from eligible studies and were pooled into meta-analysis as previously reported [13]. For studies with standard error (SE) or 95%CI but no published standard deviation (SD), we employed a widely used method to estimate SD according to the Cochrane handbook [14].
Primary fixed model (inverse variance method) for pooled estimates was used based on a low level of statistical heterogeneity among included studies (I2 < 50% and P > 0.1). Otherwise, the random model (DerSimonian and Laird method) was applied to report estimates when substantial heterogeneity was observed [15].
Sensitivity analyses and publication bias tests were performed as previously reported [16]. Briefly, we first examined studies that used vibegron 50 mg and 100 mg dose. Then, a subgroup analysis enrolling one study [10] using vibegron 75 mg was performed to explore whether this had an impact on the meta-analysis results of vibegron 50 mg and 100 mg, respectively. Besides, the influence of the individual study on overall meta-analysis results by omitting study one by one was conducted. Potential publication bias was identified using the Begg funnel plot and Egger test. Assessment for sensitivity analyses and publication bias would not be done if the studies included in meta were less than 3.
Results
Finally, six studies [8,9,10,11, 17, 18] derived from four clinical trials were obtained through systematic literature search (Supplementary material S1) and the characteristics were showed in Table 1. Among these studies, three were RCTs [8,9,10] and one [9] of them conducted two independent parts of research (part 1 and part 2). The last trial [11] was a 1 year, phase III, open-label, non-controlled, multicenter study. In this study, patients were treated with vibegron 50 mg for 8 weeks. The dose would be increased to 100 mg for the rest 44 weeks if the efficacy was insufficient. Otherwise, 50 mg would be maintained for a total 52 weeks. Another two studies were post hoc analysis on nocturia [17] and post hoc analysis on severe urgency urinary incontinence [18] of the same trial JapicCTI-152936 [8], respectively. The quality of RCTs included in the meta-analysis was relatively high and summarized in Supplementary material S2.
Efficacy
Micturition
Compared with placebo, both vibegron 50 mg (WMD = − 0.82; P < 0.001) and 100 mg (WMD = − 0.83; P < 0.001) showed more significant efficacy using fixed model. Although there was a trend in favor of vibegron 100 mg, efficacy of vibegron 50 mg (WMD = − 0.09; P = 0.528) and 100 mg (WMD = − 0.33; P = 0.084) did not differ significantly from the antimuscarinics. Based on 3 studies (2 RCTs and 1 non-RCT), vibegron 100 mg was not significantly more efficacious than vibegron 50 mg (WMD = − 0.06; P = 0.576) (Fig. 1).
Urgency urinary incontinence, urgency, incontinence, and voided volume
No significant differences were observed for vibegron 50 mg or 100 mg compared with the antimuscarinics with regard to UUI episodes/24 h (WMD = 0.04; P = 0.721 and WMD = − 0.11; P = 0.278, respectively), urgency episodes/24 h (WMD = − 0.06; P = 0.691 and WMD = − 0.34; P = 0.018, respectively), and incontinence episodes/24 h (WMD = − 0.10; P = 0.636 and WMD = − 0.17; P = 0.137, respectively). However, both of which were more efficacious than placebo (Table 2). There was also no significant difference between vibegron 50 mg and 100 mg for all of these efficacy outcomes (Table 2). The voided volume/micturition with mirabegron 50 mg (WMD = 8.24; P < 0.001) or 100 mg (WMD = 8.06; P < 0.001) were both significantly higher compared with antimuscarinics.
Safety
Regarding all the AEs as described below, no significant differences were observed between vibegron 50 mg and 100 mg (Table 2).
Dry mouth and nasopharyngitis
When compared with the placebo, vibegron 50 mg (OR 1.86; P = 0.085) and 100 mg (OR 1.04; P = 0.918) were not associated with an increased risk of dry mouth, both of which showed a significantly lower risk the antimuscarinics (OR 0.30; P < 0.001 and OR 0.18; P < 0.001, respectively).
On the contrary, the risk of nasopharyngitis with vibegron 50 mg (OR 1.86; P = 0.027) or 100 mg (OR 1.82; P = 0.029) was higher than that with antimuscarinics.
Constipation
Although meta-analysis based on random model due to a statistical heterogeneity (I2 = 81.2; P = 0.005) showed no significant differences between vibegron 50 mg (OR 0.88; P = 0.879) and antimuscarinics, vibegron 100 mg (OR 0.26; P < 0.001) was found to be associated with a lower risk of constipation compared with the antimuscarinics.
Diarrhea and cystitis
As shown in Table 2, The risk of diarrhea (OR 1.21; P = 0.605 and OR 1.18; P = 0.640, respectively) and cystitis (OR 1.99; P = 0.079 and OR 1.36; P = 0.426, respectively) was not statistically associated with vibegron 50 mg or 100 mg.
Sensitivity analyses and publication bias
For the results of the adding vibegron 75 mg to vibegron 50 mg and 100 mg subgroup analysis, the summary estimates did not substantially alter for micturition (Fig. 1) and other outcomes (data not shown). There was also no significant evidence of publication bias for micturition (Supplementary material S3) and any of the outcomes (data not shown) based on Begg funnel plot and Egger test.
Discussion
Based on current evidence, several significant findings in this first meta-analysis regarding the role of vibegron were demonstrated. First, vibegron 50 mg and 100 mg were both significantly more efficacious than placebo for all efficacy outcomes. No significant differences were found between vibegron 50 mg or 100 mg and placebo for all the AEs assessed. Second, the efficacy between vibegron 50 mg or 100 mg and antimuscarinics was comparable except for voided volume. Vibegron was associated with a decreased risk of dry mouth and an increased risk of nasopharyngitis versus antimuscarinics. Finally, the vibegron 50 mg and 100 mg were equally effective and safe across all the efficacy and AEs’ outcomes.
OAB is a common health disorder which typically requires long-term pharmacotherapy treatment. Nevertheless, patient persistence and adherence to most antimuscarinics are still relatively weak [3], while mirabegron showed a better persistence and adherence [4]. Mirabegron is the first approved β3-AR agonist for the treatment of OAB. A network analysis including 64 studies conducted by Kelleher et al. had demonstrated the efficacy and safety of mirabegron. For vibegron, no inhibitory effect on CYP enzymes is one of the advantages over mirabegron, which is known to inhibit CYP2D6. Therefore, drug interaction should be considered in patients treating with mirabegron.
In our study, it was more effective with vibegron 50 mg treatment than with placebo. Furthermore, there was no significant difference between vibegron 50 mg and vibegron 100 mg or antimuscarinics concerning micturition episodes/24 h, UUI episodes/24 h, urgency episodes/24 h, and incontinence episodes/24 h. Besides, vibegron provided statistically highly significant improvements in volume voided per micturition. As for safety outcomes, vibegron 50 mg was not associated with a higher risk of all AEs assessed compared with placebo. In addition, vibegron showed distinct advantages on dry mouth when compared with antimuscarinics. Therefore, we recommended that the initial use of vibegron 50 mg was the optimal algorithm in the pharmacologic management of OAB based on current evidence.
Except for systematically reviewing the efficacy and safety, patient satisfaction should also be focused on, because overactive bladder symptoms significantly affect the quality of life and sexual function of patients [19, 20]. However, we could not investigate it through meta-analysis method with the different questionnaires and limited data, because almost all studies paid attention to voiding parameters of OAB patients. Nevertheless, two studies [8, 11] reported by Yoshida et al. showed that Vibegron improved the quality of life compared with placebo. Therefore, these questions should be further explored.
Refer to vibegron for OAB, more questions should be further explored. First, although the AEs of vibegron was acceptable, the long-term effects on cardiovascular and cognitive function were needed to be answered, especially in the elderly. Second, since vibegron and antimuscarinics were demonstrated equally effective, whether combination therapy should be considered in patients with failure of monotherapy was worthy of answering. The only study about this issue conducted by Mitcheson et al. [9] reported that the improvement between vibegron 100 mg monotherapy and vibegron 100 mg/tolterodine 4 mg combination was comparable, while drug-related AEs were more common in patients with vibegron 100 mg plus tolterodine 4 mg. Third, since Yoshida et al. reported a 69.8% sufficient efficacy rate using vibegron 50 mg for the initial 8 weeks, the influence factors for a successful response to vibegron should be studied. Fourth, bladder dysfunctions are also common after radical hysterectomy and radiotherapy for the treatment of gynecological cancer [21, 22]. However, no study included paid attention to pharmacotherapy treatment among these cancer patients. In future, this topic should also be focused. Finally, since the benefit of mirabegron for OAB patients has been wildly proved, direct head-to-head comparison of efficacy and safety between vibegron and mirabegron is required in future.
Several limitations in this meta-analysis should be paid attention to. First, the small number of studies was one of the limitations of this study. Second, one non-RCT was included in the analysis for comparing vibegron 50 mg and 100 mg. Inclusion of this study might increase the risk of bias. Third, although we found no significant changes in subgroup analysis for vibegron 75 mg, results should be treated with caution. Finally, many clinical factors, including patient characteristics and treatment duration, would affect the efficacy outcomes.
Conclusion
In this meta-analysis, we have demonstrated that vibegron is effective and safe for treating patients with OAB. Compared with antimuscarinics, vibegron was associated with a higher volume voided per micturition and a lower rate of side effects (dry mouth and constipation). Based on the current evidence, we recommended that the initial use of vibegron 50 mg was the optimal algorithm in the pharmacologic management of OAB.
References
Stewart WF, Van Rooyen JB, Cundiff GW et al (2003) Prevalence and burden of overactive bladder in the United States. World J Urol 20(6):327–336
Milsom I, Abrams P, Cardozo L, Roberts RG, Thuroff J, Wein AJ (2001) How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int 87(9):760–766
Sexton CC, Notte SM, Maroulis C et al (2011) Persistence and adherence in the treatment of overactive bladder syndrome with anticholinergic therapy: a systematic review of the literature. Int J Clin Pract 65(5):567–585
Chapple CR, Nazir J, Hakimi Z et al (2017) Persistence and adherence with mirabegron versus antimuscarinic agents in patients with overactive bladder: a retrospective observational study in UK clinical practice. Eur Urol 72(3):389–399
Kelleher C, Hakimi Z, Zur R et al (2018) Efficacy and tolerability of mirabegron compared with antimuscarinic monotherapy or combination therapies for overactive bladder: a systematic review and network meta-analysis. Eur Urol 74(3):324–333
Edmondson SD, Zhu C, Kar NF et al (2016) Discovery of vibegron: a potent and selective beta3 adrenergic receptor agonist for the treatment of overactive bladder. J Med Chem 59(2):609–623
Di Salvo J, Nagabukuro H, Wickham LA et al (2017) Pharmacological characterization of a novel beta 3 adrenergic agonist, vibegron: evaluation of antimuscarinic receptor selectivity for combination therapy for overactive bladder. J Pharmacol Exp Ther 360(2):346–355
Yoshida M, Takeda M, Gotoh M, Nagai S, Kurose T (2018) Vibegron, a novel potent and selective beta3-adrenoreceptor agonist, for the treatment of patients with overactive bladder: a randomized, double-blind, placebo-controlled phase 3 study. Eur Urol 73(5):783–790
Mitcheson HD, Samanta S, Muldowney K et al (2019) Vibegron (RVT-901/MK-4618/KRP-114V) administered once daily as monotherapy or concomitantly with tolterodine in patients with an overactive bladder: a multicenter, phase IIb, randomized, double-blind, controlled trial. Eur Urol 75(2):274–282
Staskin D, Frankel J, Varano S, Shortino D, Jankowich R, Mudd PN Jr (2020) International phase III, randomized, double-blind, placebo- and active-controlled study to evaluate the safety and efficacy of vibegron in patients with symptoms of overactive bladder: EMPOWUR. J Urol. 101097JU0000000000000807
Yoshida M, Kakizaki H, Takahashi S, Nagai S, Kurose T (2018) Long-term safety and efficacy of the novel beta3-adrenoreceptor agonist vibegron in Japanese patients with overactive bladder: a phase III prospective study. Int J Urol 25(7):668–675
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(4):264–269 (W264)
Qiu C, Zhao X, She L et al (2019) Baricitinib induces LDL-C and HDL-C increases in rheumatoid arthritis: a meta-analysis of randomized controlled trials. Lipids Health Dis 18(1):54
Higgins JPT, Green S (eds) Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The cochrane collaboration, 2011. Available from www.cochrane-handbook.org
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Leow JJ, Martin-Doyle W, Fay AP, Choueiri TK, Chang SL, Bellmunt J (2014) A systematic review and meta-analysis of adjuvant and neoadjuvant chemotherapy for upper tract urothelial carcinoma. Eur Urol 66(3):529–541
Yoshida M, Takeda M, Gotoh M et al (2019) Efficacy of novel beta3-adrenoreceptor agonist vibegron on nocturia in patients with overactive bladder: a post-hoc analysis of a randomized, double-blind, placebo-controlled phase 3 study. Int J Urol 26(3):369–375
Yoshida M, Takeda M, Gotoh M et al (2020) Efficacy of vibegron, a novel beta3-adrenoreceptor agonist, on severe urgency urinary incontinence related to overactive bladder: post hoc analysis of a randomized, placebo-controlled, double-blind, comparative phase 3 study. BJU Int 125(5):709–717
La Rosa VL, Duarte de Campos da Silva T, Rosa de Oliveira A, Marques Cerentini T, Viana da Rosa P, Telles da Rosa LH (2020) Behavioral therapy versus drug therapy in individuals with idiopathic overactive bladder: a systematic review and meta-analysis. J Health Psychol 25(5):573–585
Coyne KS, Sexton CC, Irwin DE, Kopp ZS, Kelleher CJ, Milsom I (2008) The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: results from the EPIC study. BJU Int 101(11):1388–1395
Vitale SG, Capriglione S, Zito G et al (2019) Management of endometrial, ovarian and cervical cancer in the elderly: current approach to a challenging condition. Arch Gynecol Obstet 299(2):299–315
Kruppa J, Kavvadias T, Amann S, Baessler K, Schuessler B (2016) Short and long-term urodynamic and quality of life assessment after nerve sparing radical hysterectomy: a prospective pilot study. Eur J Obstet Gynecol Reprod Biol 201:131–134
Funding
This article is supported by grants from 1.3.5 project for disciplines of excellence, West China Hospotal, Sichuan University (ZYGD18011, ZY2016104 and ZYJC18015).
Author information
Authors and Affiliations
Contributions
JZ: project development, data collection and management, manuscript writing, and revising; YC: data collection, data analysis; LH: data collection, and data analysis; ZW: data collection and data analysis; WK: project design and development, data interpretation, manuscript editing, and revising. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors of this article as well as all the included studies declare that they have no conflict of interest.
Ethical standards
The study protocol is compliant with ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jian, Z., Yuan, C., Li, H. et al. Vibegron 50 mg is the optimal algorithm in the pharmacologic management of overactive bladder: outcomes from a systematic review and meta-analysis. Int Urol Nephrol 52, 2215–2221 (2020). https://doi.org/10.1007/s11255-020-02536-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-020-02536-5