Abstract
Background and aim
Previous studies showed that renal dysfunction was associated with both a reduction in serum high-density lipoprotein (HDL) cholesterol concentration and increased circulating monocyte count. We aimed to investigate the effect of circulating monocyte to serum HDL cholesterol ratio (M/H ratio) on fatal and composite cardiovascular events, in an observational cohort study of chronic kidney disease (CKD) patients.
Materials and methods
A total of 340 subjects with stage 1–5 CKD were followed for a mean follow-up period of 33 (range 2–44) months and assessed for fatal and nonfatal CV events. M/H ratio was calculated for all patients. All-cause mortality and CVE were also analyzed in relation to M/H ratio.
Results
Monocyte/HDL cholesterol ratio was negatively correlated with estimated glomerular filtration rate (eGFR) (r = −0.43, P < 0.001). Notably, both fatal and combined fatal and nonfatal cardiovascular events were more common in patients having a M/H ratio in the third tertile was associated with a hazard ratio of 2.24 and 4.91, respectively, for fatal and composite cardiovascular events compared to being in the first tertile.
Conclusion
Monocyte/HDL cholesterol ratio was increased with decreasing eGFR in predialytic CKD patients. Most importantly, we report for the first time that an increased M/H ratio was cross-sectionally associated with a worse cardiovascular profile and arose as independent predictors of major cardiovascular events during follow-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Macrophages play a major role in the pathogenesis of atherosclerotic plaque formation. Plaque macrophages account for the majority of leukocytes in plaques and are believed to be different from monocytes recruited from circulating blood [1]. During development of atherosclerotic lesions, blood monocytes are recruited into the intima and subintimal layers of the vessel wall, where these cells can take up oxidized low-density lipoprotein (oxLDL) and other lipids through their scavenger receptors. Recruited monocytes facing fatty deposits undergo activation and accumulate as foam cells [2, 3]. It has been shown that the circulating monocyte count is predictive of new plaque development [4].
High-density lipoprotein (HDL) molecules counteract the migration of macrophages and remove cholesterol from these cells. Murphy et al. [5] showed in an experimental study that HDL and its major protein component—apolipoprotein A–I (apo A–I)—exhibit an antiinflammatory effect on human monocytes by inhibiting activation of CD11b.
Ganda et al. [6] analyzing data from the Malmö Diet and Cancer study found that subjects with mild renal dysfunction have elevated monocytes and more severe atherosclerosis. At the same time, individuals with higher HDL cholesterol levels had a 42 % decreased odds of being in the top quartile of monocyte count. Overall, renal dysfunction was associated with both a reduction in serum HDL cholesterol concentration and increased circulating monocyte count.
A reduced HDL cholesterol level is one of the hallmarks of ESRD-related dyslipidemia [7]. Additionally, the uremic milieu seems to cause monocytosis and monocyte activation—combination that could account for the accelerated atherosclerosis present in advanced chronic kidney disease (CKD). No study to date has evaluated the impact of the monocyte count/HDL cholesterol ratio on cardiovascular endpoints, in moderate to severe CKD. Thus, we aimed to investigate the prognostic impact of circulating monocyte count to serum HDL cholesterol ratio on fatal and composite cardiovascular events, in an observational cohort study of CKD patients.
Materials and methods
Study design and subjects
This was an observational prospective cohort study in which data were derived from a larger dataset of a prospectively maintained cohort of CKD patients. The initial dataset was reassessed after generation of the study hypothesis. The CKD patient cohort reported in this study was created between January 2008 and January 2012. Patients were referred to the Renal Unit of the Gulhane School of Medicine Medical Center, Ankara, Turkey, for the first time because of suspected or manifest renal failure. All patients were diagnosed as having CKD according to their estimated glomerular filtration rate (eGFR) and the presence of kidney injury as defined by National Kidney Foundation K/DQOI Guidelines [8]. None of the patients in stage 5 CKD were on hemodialysis or peritoneal dialysis at the time of the inclusion. Stages of CKD were determined using eGFRs which were calculated via modification of diet in renal disease (MDRD) equation. Exclusion criteria including acute infections and unwillingness to participate in the study were applied (n = 53). One hundred and sixty-five eligible patients dropped out for the following reasons: lost to follow-up or transferred to other nephrology unit (n = 82), viral hepatitis (n = 15), vasculitis (n = 9), withdrew consent (n = 59). Hypertension was defined as systolic blood pressure (SBP) ≥140 mmHg or diastolic blood pressure (DBP) ≥90 mmHg on repeated measurements, or the use of antihypertensive drugs.
Laboratory measurements
Blood samples were obtained from patients in the morning, after 12 h of fasting, for measurement of plasma glucose (FPG), serum albumin, total serum cholesterol (TC), triglyceride (TG), HDL, and low-density lipoprotein (LDL) cholesterol, and serum basal insulin for calculation of insulin resistance score homeostasis model assessment-insulin resistance (HOMA-IR).
The monocyte count was determined using the Pentra 120 Retic Hematology Analyzer (ABX, Montpellier, France), as part of the routine hemogram. The reference value for monocyte in our laboratory is 2–10 %.
Cardiovascular events
All included patients were followed up for time-to-event analysis until occurrence of fatal or nonfatal cardiovascular events of this prospectively maintained cohort were registered via routine outpatient clinic visits and regular telephone contacts starting from the enrollment date until analysis of the data. Fatal and nonfatal cardiovascular events including death, stroke, and myocardial infarction were recorded. Gulhane School of Medicine local ethical committee approved the study protocol, and all patients were included in the study after signing informed consent forms.
Statistical analysis
Analyses were performed using SPSS version 17.0 (SPSS, Inc., Chicago, Illinois). The study population was assigned into tertiles on the basis of monocyte/HDL ratio level. To test the distribution pattern of variables, the Kolmogorov–Smirnov test was used. Continuous data are presented as medians and interquartile ranges or as mean ± SD. Comparisons of multiple mean values were carried out using Kruskal–Wallis tests or analysis of variance (ANOVA) as appropriate. Categorical variables were summarized as percentages and compared using Chi square tests. Spearman’s correlation coefficient was computed to examine the association between two continuous variables. Effects of different variables on fatal and nonfatal events were analyzed in univariate analysis for each. Variables for which the unadjusted P values were <0.10 in univariate Cox regression analysis were identified as potential risk markers and included in the multivariable Cox regression model. A P value <0.05 was considered statistically significant, and the confidence interval was 95 %. The survival curve during follow-up for according to monocyte/HDL ratio tertiles was analyzed using the Kaplan–Meier method, and statistical assessment was performed using the log-rank test. A P value <0.05 was considered statistically significant.
Results
Demographic, clinical, and biochemical parameters of study population
A total of 340 patients with stage 1–5 CKD were included in the study. Mean age of the cohort was 51 ± 12 years. There were no statistically significant differences among the different CKD stages with regard to age, gender, BMI, history of CVD, etiology of CKD and smoking status. The demographic and clinical characteristics of the study groups are given in Table 1.
The monocyte/HDL cholesterol (M/H) ratio as a continuous variable was negatively correlated with eGFR (r = −0.43, P < 0.001)—Fig. 1. The whole cohort was divided into tertiles based on the M/H ratio. Clinico-demographic characteristics of the study population based on M/H ratio tertiles are shown in Tables 2 and 3. From tertile 1 with (the lowest M/H ratio) to tertile 3 (with the highest M/H ratio), eGFR values showed a significant decrease. The prevalence of smokers and diabetes mellitus was also significantly increased from the first tertile to the third tertile.
All-cause mortality and cardiovascular events according to M/H ratio
Cardiovascular outcomes were determined from the day of examination onwards, with a mean follow-up period of 33 (range 2–44) months. Forty-one patients died, 35 of which due to cardiovascular causes, four due to malignancies, and two due to infection. Cardiovascular mortality (n = 35) was defined as death due to coronary heart disease (n = 18), sudden death (n = 10), stroke (n = 4), or complicated peripheral vascular disease (n = 3).
In addition to the 35 cardiovascular deaths, 87 nonfatal cardiovascular events were registered during the follow-up as follows: stroke (n = 19); myocardial infarction (n = 59); peripheral vascular disease (n = 6); and aortic aneurysm (n = 3).
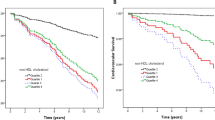
The predictors for time-to-cardiovascular event (n = 122, including a composite of fatal and nonfatal) were studied by multiple regression analysis. We constructed three models for the multiple regression analysis. Model 1 represents the unadjusted analysis. Model 2 is adjusting for age and gender, whereas in model 3, we made additional adjustments for other well-known established cardiovascular risk factors including diabetes mellitus, hypertension, eGFR, and smoking status. Having an M/H ratio in the third tertile was associated with a hazard ratio of 2.24 and 4.91, respectively, for fatal and composite cardiovascular events compared to being in the first tertile (Tables 4, 5; for further see Supplementary Tables 1 and 2). Kaplan–Meier analysis showed a significant survival advantage for the lowest M/H ratio in both fatal (Fig. 2) and nonfatal–fatal events (Fig. 3).
Discussion
The salient finding of this predialytic CKD cohort was that the M/H ratio increases in parallel with decreasing eGFR. Most importantly, we report for the first time that an increased M/H ratio is an independent predictor for both fatal and composite cardiovascular events in CKD patients.
Patients with CKD suffer from high cardiovascular morbidity and mortality, disproportionately increased to their classical CV risk profile. A number of nontraditional risk factors have been proposed to account for this discrepancy. Atherosclerosis is mainly fueled by increased inflammation, and CKD is considered as an inflammatory state. Several previous studies showed various abnormalities in monocyte count and function in patients with CKD [5, 6, 9–11]. Dysregulated monocytes are present in patients with renal insufficiency, further supporting the hypothesis of a significant detrimental contribution of monocytes in the excessive atherosclerotic burden typically seen in CKD. CD14+CD16+ monocytes are a subset of activated monocytes that are found in greater numbers in the peripheral blood of CKD patients [9]. This subset of monocytes—unlike the CD14+CD16− monocytes found in healthy subjects—show a high level of expression of chemokines, favoring their migration to the vascular wall and subsequent endothelial injury. In a small case–control study, Pereira et al. [10] found that compared to control subjects, HD patients presented a significant decrease in CXCR1 neutrophil expression, and in CD14 monocyte expression, accompanied by a significant increase in HLA-DR monocyte expression. The authors concluded that neutrophils and monocytes are activated in CKD patients and this may be related to erythropoietin resistance. Recently, Al-Chaqmaqchi et al. [11] described in stage 4–5 CKD patients a different monocyte gene profile, with a significant activation of the Wnt/β-catenin pathway—linked to dysregulation of monocyte adhesion, migration, and inflammatory status.
The protective role of serum HDL cholesterol levels in the general population has been described by numerous studies [12]. The anti-atherosclerotic properties of HDL have been mainly attributed to its role in the reverse cholesterol transport. A different profile is apparent in advanced CKD. Yamamoto et al. [13] investigated the cellular cholesterol efflux and inflammatory response of macrophages exposed to HDL, in patients on hemodialysis treatment, compared to healthy controls. HDL in hemodialysis patients had reduced anti-chemotactic ability and elicited increased macrophage cytokine response. The same authors also found that HDL from HD patients was significantly less effective in accepting cholesterol from macrophages [13]. The uremic milieu also causes some functional alterations in HDL particles. In the presence of excessive metabolic and oxidative stress (such as in uremia), HDL might lose its protective effect and may behave as a pro-inflammatory molecule [12]. In apparently healthy subjects, it has been shown that measurement of HDL inflammatory/anti-inflammatory properties could differentiate better the population with atherosclerotic cardiovascular disease than the classic measurement of HDL cholesterol levels. Inflammatory/anti-inflammatory properties of HDL also distinguish better patients that are favorably affected by simvastatin treatment [14]. Kalantar-Zadeh et al. [15] calculated the HDL proinflammatory index in 189 maintenance HD patients followed for 30 months and found that patients with a higher HDL proinflammatory index had a higher adjusted hazard ratio for death. Finally, Speer et al. [16] showed that symmetric dimethyl arginine (SDMA) could modify the HDL particle to mimic a damage-associated molecular pattern that activates toll-like receptor-2, linking abnormal HDL to innate immunity, endothelial dysfunction, and hypertension.
Recently Ganda et al. [6] investigated the association of mild renal dysfunction with monocytosis and HDL cholesterol levels in 4,757 patients stratified by Cystatin C quintiles. Lower levels of renal function were accompanied by higher monocyte counts that were independently associated with carotid intima-media thickness. Low levels of HDL cholesterol were independently associated with a 22 % increased risk of being in the top quartile of monocyte count.
Our results showed a significant trend of decreasing HDL and increasing monocyte count along with decreasing eGFR values. Consequently, the M/H ratio showed significant increase with decreasing kidney function. M/H ratio appeared as an independent predictor of fatal and composite CV events in our CKD patients.
In sum, the presence of renal dysfunction causes monocytosis and monocyte activation. On the other hand, uremia leads to both qualitative and quantitative changes in HDL particles. Moreover, it also renders HDL particles more proinflammatory and less anti-atherosclerotic. M/H ratio combines these two detrimental processes with impact on atherosclerosis. Although this ratio only takes into account quantitative changes in both parameters, it could nevertheless predict adverse clinical cardiovascular events in our CKD cohort. It is plausible to think that not only mere numeric changes in monocyte counts and HDL cholesterol level in blood but also monocyte activation and molecular changes in HDL cholesterol particles might have contributed to the predictive ability of this novel ratio in our study. Moreover, one may also argue that reduction in serum levels of so-called anomalous HDL particles in advanced uremia may counteract its untoward effects to some extent. It would be more detrimental in case of high concentrations of these dysfunctional HDL molecules were abounding in the bloods of the uremic patients. Thus, uremia, in addition to many other pathophysiologic pathways through novel CV risk factors, may render traditional risk factors more atherogenic.
A few limitations of the study deserve mentioning. Serum concentration of HDL cholesterol and percentage of monocytes in complete blood count are subject to change with time in a given subject, hence, relying only on one measurement may not completely reflect the real trend of the studied parameters. Several measurements at multiple points in time would be better. Owing to the observational nature of the study, some unknown or unincluded factors might have had an impact on the cardiovascular endpoints.
The findings of our study are novel as such for the first time, we showed that high circulating monocyte count and reduced HDL concentration jointly may predict adverse clinical cardiovascular events in patients with CKD. Our study added a new observation on top of previous clinical studies that monocyte count to HDL cholesterol ratio is relevant also in prediction of hard CV events in a CKD patient cohort and much more laboratory and clinical research now needs to be undertaken in order better to understand these findings.
References
Woollard KJ, Geissmann F (2010) Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol 7(2):77–86
Imhof BA, Aurrand-Lions M (2004) Adhesion mechanisms regulating the migration of monocytes. Nat Rev Immunol 4(6):432–444
Greaves DR, Gordon S (2009) The macrophage scavenger receptor at 30 years of age: current knowledge and future challenges. J Lipid Res 50(Suppl):S282–S286
Johnsen SH, Fosse E, Joakimsen O, Mathiesen EB, Stensland-Bugge E, Njolstad I et al (2005) Monocyte count is a predictor of novel plaque formation: a 7-year follow-up study of 2610 persons without carotid plaque at baseline the Tromso Study. Stroke 36(4):715–719
Murphy AJ, Woollard KJ, Hoang A, Mukhamedova N, Stirzaker RA, McCormick SP et al (2008) High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler Thromb Vasc Biol 28(11):2071–2077
Ganda A, Magnusson M, Yvan-Charvet L, Hedblad B, Engstrom G, Ai D et al (2013) Mild renal dysfunction and metabolites tied to low HDL cholesterol are associated with monocytosis and atherosclerosis. Circulation 127(9):988–996
Moradi H, Vaziri ND, Kashyap ML, Said HM, Kalantar-Zadeh K (2013) Role of HDL dysfunction in end-stage renal disease: a double-edged sword. J Ren Nutr 23(3):203–206
National Kidney Foundation (2004) K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42(Suppl 3):S1–S202
Ramirez R, Carracedo J, Merino A, Soriano S, Ojeda R, Alvarez-Lara MA et al (2011) CD14+CD16+ monocytes from chronic kidney disease patients exhibit increased adhesion ability to endothelial cells. Contrib Nephrol 171:57–61
Pereira R, Costa E, Goncalves M, Miranda V, do Sameiro Faria M, Quintanilha A et al (2010) Neutrophil and monocyte activation in chronic kidney disease patients under hemodialysis and its relationship with resistance to recombinant human erythropoietin and to the hemodialysis procedure. Hemodial Int 14(3):295–301
Al-Chaqmaqchi HA, Moshfegh A, Dadfar E, Paulsson J, Hassan M, Jacobson SH et al (2013) Activation of Wnt/beta-catenin pathway in monocytes derived from chronic kidney disease patients. PLoS ONE 8(7):e68937
Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J et al (2007) Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 370(9602):1829–1839
Yamamoto S, Yancey PG, Ikizler TA, Jerome WG, Kaseda R, Cox B et al (2012) Dysfunctional high-density lipoprotein in patients on chronic hemodialysis. J Am Coll Cardiol 60(23):2372–2379
Ansell BJ, Navab M, Hama S, Kamranpour N, Fonarow G, Hough G et al (2003) Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation 108(22):2751–2756
Kalantar-Zadeh K, Kopple JD, Kamranpour N, Fogelman AM, Navab M (2007) HDL-inflammatory index correlates with poor outcome in hemodialysis patients. Kidney Int 72(9):1149–1156
Speer T, Rohrer L, Blyszczuk P, Shroff R, Kuschnerus K, Krankel N et al (2013) Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity 38(4):754–768
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kanbay, M., Solak, Y., Unal, H.U. et al. Monocyte count/HDL cholesterol ratio and cardiovascular events in patients with chronic kidney disease. Int Urol Nephrol 46, 1619–1625 (2014). https://doi.org/10.1007/s11255-014-0730-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-014-0730-1