Abstract
Purpose
Cardiovascular disease is the leading cause of mortality in dialysis patients with left ventricular hypertrophy (LVH) being an important predictor of mortality. We wanted to determine the prevalence of LVH in peritoneal dialysis (PD) patients and factors contributing to it.
Methods
This is a cross-sectional study assessing LVH using echocardiogram in PD patients. Left ventricular mass index (LVMI) was calculated to determine LVH. Chronic fluid overload (overhydration) was assessed using the body composition monitor, and blood pressure (BP) was measured using 24-h ambulatory BP monitoring.
Results
Thirty-one patients (21 females:10 males, 48.97 ± 14.50 years and dialysis vintage 40.0 ± 28.9 months) were studied. More than two-thirds (77.4 %) were hypertensive, and a third (35.5 %) were diabetic. Baseline data included mean serum albumin (37.34 ± 4.43 g/l), weekly Kt/V (2.02 ± 0.23), residual renal function of 68 (0–880) ml and ultrafiltration of 1,606.9 ± 548.6 ml. Majority of patients (80.6 %) had LVH on echocardiogram with LVMI of 136.5 ± 37.8 g/m2 and overhydration of 2.23 ± 1.77 l. Average systolic BP, diastolic BP and mean arterial pressure were 141.2 ± 23.3, 90.8 ± 19.7 and 107.6 ± 19.6 mmHg, respectively. Patients with LVH had a lower serum albumin (p = 0.003), were more overhydrated (p = 0.010) and were on higher number of anti-hypertensive agents (p ≤ 0.001). Predictors of LVMI were overhydration (p = 0.002), the presence of diabetes (p = 0.008) and the number of anti-hypertensive agents used (p = 0.026). However, overhydration (p = 0.007) was the main predictor of LVH on multivariate analysis.
Conclusion
Overhydration is strongly associated with LVH in PD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite improvements in dialysis technology, patients on dialysis have a high morbidity and mortality with cardiovascular disease being the leading cause of death [1, 2]. Cardiovascular disease accounted for 57 % of dialysis-related mortality in Malaysia [2].
Diabetes mellitus, hypertension, dyslipidaemia and smoking are well-established risk factors for cardiovascular disease [3]. In addition, chronic kidney disease is increasingly being recognised as a risk factor for cardiovascular disease [4]. There are unique factors that make end-stage renal disease patients at risk of cardiovascular disease and these non-traditional risk factors include anaemia, hypoalbuminemia and deranged calcium–phosphate metabolism [5, 6]. Hypertension and left ventricular hypertrophy (LVH) are important predictors of mortality in end-stage renal disease [7, 8]. Hypertension is very common in patients with end-stage renal disease either as a primary cause, or secondary to the retention of salt and water while on dialysis and studies have shown improvements in BP control can be achieved by controlling fluid balance in dialysis patients [9].
Euvolemia is a predictor of better outcomes in peritoneal dialysis (PD) patients, and volume overload is associated with cardiac dysfunction [10, 11]. Peritoneal dialysis patients are believed to be more fluid overloaded and hypertensive than their haemodialysis counterparts [12]. We have previously demonstrated patients to be hypertensive and fluid overloaded while being on PD. [13, 14], and Ozkahya et al. [10] have earlier reported that chronic fluid overload is associated with hypertension and LVH.
Although studies have confirmed the strong association between LVH detected electrocardiographically and cardiovascular risk, it has also been recognized that the electrocardiogram is relatively insensitive for detecting prognostically important increases in left ventricular mass [15]. Our objective was to assess the prevalence of LVH in our stable asymptomatic PD patients and to look for factors that contribute to it.
Materials and methods
This was a cross-sectional observational study. All continuous ambulatory peritoneal dialysis (CAPD) patients followed up at nephrology outpatient clinics at the Universiti Kebangsaan Malaysia Medical Centre were screened when they attended their routine clinic visits between 1 September 2012 and 31 January 2013. Those who met the inclusion criteria and consented were recruited. The study protocol was reviewed and approved by the Human Research Ethics Committee of the Universiti Kebangsaan Malaysia, and written informed consent was obtained from all patients (FF-229-2012).
Patients
Criteria for inclusion in the study were age 18 years or older, on CAPD for at least 4 months with a stable PD prescription. Patients were excluded if they had an amputation, pacemaker or metal implants, as these would interfere with assessment of overhydration. Patients with known congestive cardiac failure with ejection fraction <55 %, known valvular heart disease (severe aortic valve stenosis, aortic regurgitation, mitral valve stenosis and mitral valve regurgitation) and cardiomyopathy were excluded. We also excluded patients on automated PD, due to the small number of patients on this modality at our centre.
Baseline demographics including age, gender, race, body weight and height, body mass index, primary kidney disease, co-morbidities like diabetes, hypertension, ischaemic heart disease, number of anti-hypertensive agents, including the use of angiotensin converting enzyme/angiotensin II receptor blockers were recorded. Medical records were reviewed for duration of PD, residual renal function, peritoneal membrane transport status and dialysis adequacy. Adequacy of dialysis was measured using the total weekly urea and creatinine clearance using standard methods [16]. Patients were classified into high or low transporters according to the 4 h D/P creatinine. Transporter status was based on D/P creatinine with 0.34–0.49 as low transporters; 0.50–0.64 as low average transporters; 0.65–0.80 as high average transporters and those >0.81 as high transporters [16].
Electrocardiogram (ECG)
Electrocardiography was done on the same day as the echocardiogram. ECGs were subsequently reviewed by a cardiologist who was blinded to the patient details including echocardiogram findings. LVH was diagnosed by fulfilling either Romhilt–Estes criteria, Cornell voltage criteria or Sokolow–Lyon voltage criteria [17–19].
Echocardiography
Two-dimensional, M-mode echocardiography was performed using Acuson Sequoia 512 ultrasound scan machine (Siemens, Germany) by a single experienced cardiologist. The cardiologist was unaware of the clinical details, ambulatory blood pressure monitoring (ABPM) and body composition monitor (BCM) findings. Left ventricular mass index (LVMI) was calculated based on the Devereux and Reichek formula [20].
where LVEDD, PWTD and IVSTD are left ventricle internal dimensions at end diastole, posterior wall thickness at end diastole and septal wall thickness at end diastole, respectively. LVMI was standardized by dividing left ventricular mass to body surface area that was calculated using DuBois formula [21]. LVH was defined as LVMI >104 g/m2 in women and >116 g/m2 in men and was reported according to American Society for Echocardiography criteria [22].
Blood pressure
ABPM was performed using the BPro machine (model T6400, Healthstats, Singapore). Patients were advised to carry out their normal activities as usual, and blood pressure (BP) readings were taken at 15-min intervals throughout the 24-h period. Mean 24-h daytime and night time systolic, diastolic and mean arterial BP readings were recorded. More than 60 % readings were required to be considered a successful 24-h ABPM recording. Patients were classified into dippers if their systolic or diastolic BP dropped >10 % during the night. Non-dippers were those who had either a drop by <10 % in night time BP or a paradoxical rise in BP.
Body composition monitor
BCM is a bio-impedance spectroscopy device that is simple, non-invasive and has been validated to assess overhydration in PD patients [23, 24]. Chronic fluid overload was assessed using the BCM (Fresenius Medical Care, Germany) which measures the impedance spectroscopy at different frequencies. BCM was performed once in the supine position with a full abdomen, and electrodes were attached to the ipsilateral arm and foot of the patient. The BCM device gives measurements including extracellular water, intracellular water and total body water. If the difference between measured and expected extracellular water was positive, patients were classified to be overhydrated.
Statistical analysis
Statistical analysis was performed using SPSS software version 20 (SPSS Inc., Chicago, IL, USA). All numerical data were subjected to normality testing using Shapiro–Wilk, and normally distributed data are expressed as mean ± standard deviation (SD), whereas non-normally distributed data are expressed as median (range). We used Student’s independent and paired t test for parametric data and Mann–Whitney U test for nonparametric data. One-way analysis of variance (ANOVA) was used for multiple categories between groups, whereas Chi-square was used to compare two categorical variables. Pearson and Spearman correlation coefficient was used for the degree of association between LVMI, clinical and laboratory variables. Multiple linear regression analysis was used to test for the relationship between overhydration, LVMI, number of anti-hypertensive agents and serum albumin. p values of <0.05 were considered statistically significant.
Results
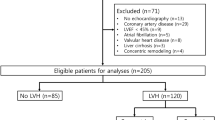
Although fifty-two patients were screened, only thirty-one were recruited into the study and their demographic characteristics are demonstrated in Table 1. Nine patients were excluded as they were on APD, two patients were amputees, and one patient had a pacemaker. Two patients were on CAPD <4 months duration, 1 patient was <18 years, one patient was admitted with peritonitis, and five patients had documented cardiovascular disease and therefore excluded (of which four had failed haemodialysis due to vascular access problems and switched to CAPD). Results of ECG, echocardiogram, BCM and ABPM are tabulated in Table 2. Over 70 % of patients were on anti-hypertensive agents with the majority being on either angiotensin converting enzyme inhibitors or angiotensin II receptor blockers. All our patients were on lactate-based PD solutions and none were on icodextrin.
We found that ECG was not a sensitive investigation to detect LVH, with only 32.3 % patients having ECG changes of LVH as compared to echocardiogram that detected LVH in 80.6 % of our patients. We found patients with LVH had a significantly lower serum albumin, were more overhydrated and were on a higher number of anti-hypertensive agents than those without LVH, as demonstrated in Table 3 . The non-dippers on ABPM had a higher LVMI compared to dippers (non-dippers 145.67 ± 37.55 vs. dippers 117.3 ± 31.94 g/m2, p = 0.049).
Patients with diabetes were more overhydrated than non-diabetics (diabetics 3.54 ± 1.63 vs. non-diabetics 1.51 ± 1.42 l, p = 0.001), with a higher LVMI (diabetics 159.82 ± 31.86 vs. non-diabetics 123.7 ± 35.12 g/m2, p = 0.008). The mean HbA1c of the diabetic patients was 6.582 ± 1.63 %.
Men were more overhydrated than women (3.23 ± 1.41 vs. 1.76 ± 1.75, p = 0.028) with a trend towards higher LVMI (154.8 ± 32.77 vs. 127.81 ± 37.57 g/m2, p = 0.062). There were no differences in serum albumin between the genders, but men had a higher total ultrafiltration compared to women (2,092.1 ± 447.18 vs. 1,375.9 ± 433.29 ml, p ≤ 0.001) with a non-significant trend towards higher D/P creatinines (0.66 ± 0.10 vs. 0.60 ± 0.10, p = 0.079). There was no difference in the presence of diabetes or hypertension between men and women that may have explained this. Higher transporters had a lower serum albumin (35.50 ± 5.35 vs. 38.48 ± 3.65 g/l, p = 0.026) and had a trend towards overhydration (p = 0.071) and higher LVMI (p = 0.073).
Overhydration correlated well with left atrium size (p ≤ 0.001) and reduced ejection fraction (p = 0.038). Predictors of overhydration on multivariate analysis included serum albumin (p = 0.005), presence of diabetes (p = 0.006) and male gender (p = 0.019). Hypertension did not correlate with overhydration or LVH. There was no relationship between haemoglobin and LVH. Predictors of LVMI were overhydration (p = 0.002), presence of diabetes (p = 0.008) and number of anti-hypertensive agents (p = 0.026). However, multivariate analysis revealed overhydration (p = 0.007) as the main predictor of LVMI. Figure 1 demonstrates the correlation between LVMI and overhydration.
Discussion
Cardiovascular disease remains the leading cause of death amongst dialysis patients and has been reported to be several times higher than the general population [1, 7]. LVH is a strong predictor of myocardial infarction, heart failure, sudden death and stroke in patients with end-stage renal disease [5, 6, 25–27]. Our study demonstrated a high prevalence of LVH in PD patients with fluid overload being the main predictor of LVH which is in keeping with findings reported by others [28, 29].
We also found that ECG was not a sensitive test for the determination of LVH, as majority did not have LVH on ECG criteria and this is in keeping with reported literature [15]. We demonstrated patients with LVH had a lower serum albumin, were more overhydrated and were on a higher number of antihypertensive agents. On multivariate analysis, overhydration was the main predictor for LVH, which is in keeping with evidence that chronic fluid overload contributes to LVH [30]. Chronic fluid overload leads to an increase in blood volume and left ventricular end diastolic volume which results in an enlarged left ventricle and myocardial hypertrophy. The mean LVMI was high in our cohort compared to other studies, but our median PD duration was longer [31]. We also noted a significantly enlarged left atrium in patients with LVH which is associated with a higher morbidity and mortality [32]. The left atrium becomes dilated due to left ventricular diastolic dysfunction that occurs as a result of LVH and chronic fluid overload.
Majority of our patients on PD were fluid overloaded with a mean overhydration of 2.23 ± 1.77 l, which is in keeping with what was reported by Van Biesen et al. [33]. Our cohort was generally younger but on PD for a long duration. Given the relatively long time period on PD and presence of overhydration, one would expect that a large proportion of patients would be hypertensive (77 % patients were hypertensive at the time of the study) and would also contribute to LVH. Hypertension in chronic kidney disease is due to multitude of factors including activation of the renin–angiotensin–aldosterone system due to renal ischaemia, increased levels of endothelium derived vasoconstrictors in the uraemic milieu and the use of erythropoietin stimulating agents, amongst others. Secondly, it is well recognized with increasing time on PD, patients become more fluid overloaded as a result of ultrafiltration failure and loss of residual renal function [34]. We noted that a low serum albumin was a predictor of overhydration. This is in keeping with other studies where low serum albumin levels were both a predictor of overhydration and cardiovascular morbidity and mortality [33, 35].
Although multiple factors play a role in the development of hypertension, it has been well established that hypertension is mainly associated with fluid overload in dialysis patients [36]. In hypertensive patients, ABPM has been reported to correlate better with LVMI than office BP measurements [37, 38]. Contrary to some other studies, our study did not demonstrate a correlation between presence of hypertension and LVH [39]. However, presence of LVH on echocardiogram was associated with use of higher number of anti-hypertensive agents, suggesting an association between LVH and severity of hypertension, with up to a third (32.3 %) of patients being on three or more agents. As some patients were not compliant with their salt and fluid restrictions, their BPs were controlled with anti-hypertensive agents in an attempt to reduce their cardiovascular risks, thereby achieving acceptable mean BP of 140/90 mmHg in our study. Majority of our patients were non-dippers with a significantly higher LVMI compared to dippers, which is associated with adverse cardiovascular outcomes [40]. However, this could be confounded by the presence of diabetes as all diabetics were non-dippers [41]. Blood pressure and more importantly fluid overload need to be addressed to prevent the development of LVH, by restricting salt and fluid intake and using diuretics in patients with residual renal function.
Our diabetic PD patients were more fluid overloaded with a higher LVMI than their non-diabetic counterparts, a finding reported by others as well [42]. We found no differences in gender, mean serum albumin or residual renal function between diabetics and non-diabetics to account for the occurrence of fluid overload in our diabetic population.
Interestingly, in our study, men were more overhydrated than women and others have reported similar findings [43]. On sub-analysis, there were no differences in age, Kt/V, mean BPs, duration of PD, BMI or serum albumin levels between male and female patients. There were equal numbers of diabetics in both genders, but men had significantly better residual renal function and ultrafiltration as compared to the women. Men also had a trend towards higher D/P creatinines, and this may have partially contributed to the fluid overload. However, it is also possible that men were less compliant with their fluid and salt restrictions as compared to women. It is well recognised that salt intake contributes to fluid overload [34, 44] but assessment of dietary salt intake and daily sodium balance was beyond the scope of this study.
Some studies have shown high peritoneal membrane transport status to be associated with fluid overload, and we saw a trend towards high transport status being associated with higher LVMIs and overhydration. In addition, high peritoneal transport status was associated with low serum albumin, which is a strong predictor of fluid overload and LVH [35]. Icodextrin, a glucose polymeric solution, is known to improve ultrafiltration and fluid overload in PD patients [45] but none of our patients were on icodextrin. We found no association between haemoglobin and LVMI which may be explained by the fact that our patients achieved target haemoglobin levels with the use of erythrocyte stimulating agents.
In our study, multivariate analysis revealed low serum albumin, presence of diabetes and male gender to be predictors of overhydration, and this finding is in keeping with results from the largest BCM study to date [33].
In conclusion, chronic fluid overload continues to remain a problem in PD patients and is associated with LVH. Majority of PD patients have LVH on echocardiogram, which is a strong predictor of cardiovascular mortality. Effective control of fluid overload may reduce LVH and have a positive impact on reducing cardiovascular mortality in patients on PD.
References
United States Renal Database System (USRDS) (2012) Annual data report
Seng WH, Meng OL (2011) 19th Report of the Malaysian dialysis and transplant registry
Hennekens CH (1998) Increasing burden of cardiovascular disease. Current knowledge and future directions for research on risk factors. Circulation 97:1095–1102
Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL et al (2003) Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, epidemiology and prevention. Hypertension 42:1050–1065
Wang AY, Wang M, Woo J, Lam CW, Lui SF, Li PK et al (2004) Inflammation, residual kidney function, and cardiac hypertrophy are interrelated and combine adversely to enhance mortality and cardiovascular death risk of peritoneal dialysis patients. J Am Soc Nephrol 15:2186–2194
Harnett JD, Kent GM, Barre PE, Taylor R, Parfrey PS (1994) Risk factors for the development of left ventricular hypertrophy in a prospectively followed cohort of dialysis patients. J Am Soc Nephrol 4:1486–1490
Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC et al (1995) Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int 47:186–192
Silberberg JS, Barre PE, Prichard SS, Sniderman AD (1989) Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int 36:286–290
Chazot C, Charra B (2007) Non-pharmacologic treatment of arterial hypertension in haemodialysis patients. Nephrol Therapeut 3(Suppl 3):S178–S184
Ozkahya M, Toz H, Qzerkan F, Duman S, Ok E, Basci A et al (2002) Impact of volume control on left ventricular hypertrophy in dialysis patients. J Nephrol 15(6):655–660
Konings CJ, Kooman JP, Schonck M, Dammers R, Cheriex E, Palmans Meulemans AP et al (2002) Fluid status, blood pressure, and cardiovascular abnormalities in patients on peritoneal dialysis. Perit Dial Int 22(4):477–487
Chen YC, Lin CJ, Wu CJ, Chen HH, Yeh JC (2009) Comparison of extracellular volume and blood pressure in haemodialysis and peritoneal dialysis patients. Nephron Clin Pract 113(2):c112–c116
Cader RA, Gafor HA, Kong NCT, Mohd R, Ibrahim S, Wan Haslina WH (2012) Blood pressure profile in continuous ambulatory peritoneal dialysis patients. EXCLI 11:116–124
Cader RA, Gafor HA, Mohd R, Kong NCT, Ibrahim S, Wan Haslina WH (2013) Assessment of fluid status in CAPD patients using the body composition monitor. J Clin Nurs 22(5–6):741–748
Okin PM, Roman MJ, Devereux RB, Pickering TG, Borer JS, Kligfield P (1998) Time voltage QRS area of the 12-lead electrocardiogram: detection of left ventricular hypertrophy. Hypertension 31:937–942
Twardowski ZJ (1989) Clinical value of standardized equilibration tests in CAPD patients. Blood Purif 7:95–108
Romhilt DW, Estes EH (1968) A point-score system for the ECG diagnosis of left ventricular hypertrophy. Am Heart J 75:752–758
Casale PN, Devereux RB, Kligfield P, Eisenberg RR, Miller DH, Chaudhary BS et al (1985) Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol 6:572–580
Sokolow M, Lyon TP (1949) The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J 37:161–186
Devereux RB, Reichek N (1977) Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 55:613–618
Dubois D, Dubois EF (1916) A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 17:863–871
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA et al (2005) Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group. J Am Soc Echocardiogr 18:1440–1463
Luo YJ, Lu XH, Woods F, Wang T (2011) Volume control in peritoneal dialysis patients guided by bioimpedance spectroscopy assessment. Blood Purific 31(4):296–302
Moissl UM, Wabel P, Chamney PW, Bosaeus I, Levin NW, Bosy-Westphal A et al (2006) Body fluid volume determination via body composition spectroscopy in health and disease. Physiol Meas 27(9):921–933
Kannel WB, Gordon T, Castelli WP, Margolis JR (1970) Electrocardiographic left ventricular hypertrophy and risk of coronary heart disease: the Framingham study. Ann Intern Med 72:813–822
Frohlich ED (1986) Left ventricular hypertrophy as a risk factor. Cardiol Clin 4:137–144
Casale PN, Devereux RB, Milner M, Zullo G, Harshfield GA, Pickering TG et al (1986) Value of echocardiographic measurement of left ventricular mass in predicting cardiovascular morbid events in hypertensive men. Ann Intern Med 105:173–178
Silaruks S, Sirivongs D, Chunlertrith D (2000) Left ventricular hypertrophy and clinical outcome in CAPD patients. Perit Dial Int 20:461–466
Enia G, Mallamaci F, Benedetto FA, Panuccio V, Parlongo S, Cutrupi S et al (2001) Long term CAPD patients are volume expanded and display more severe left ventricular hypertrophy than haemodialysis patients. Nephrol Dial Transpl 16(7):1459–1464
London G, Parfres PS (1997) Cardiac disease in chronic uraemia pathogenesis. Adv Ren Replace Ther 4:194–211
Asci G, Özkahya M, Duman S, Toz H, Erten S, Ok E (2006) Volume control associated with better cardiac function in long-term peritoneal dialysis patients. Perit Dial Int 26(1):85–88
Patel RK, Jardine AGM, Mark PB, Cunningham AF, Steedman T, Powell JR et al (2010) Association of left atrial volume with mortality among ESRD patients with left ventricular hypertrophy referred for kidney transplantation. Am J Kidney Dis 55(6):1088–1096
Van Biesen W, Williams JD, Covic AC, Fan S, Claes K, Lichodziejewska-Niemierko M et al (2011) Fluid status in peritoneal dialysis patients: the European Body Composition Monitoring (EuroBCM) study cohort. PLoS ONE 6(2):e17148
Chen YC, Lin CJ, Wu CJ, Chen HH, Yeh JC (2009) Comparison of extra-cellular volume and blood pressure in hemodialysis and peritoneal dialysis patients. Nephron. Clinical Practice 113:112–116
Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE (1996) Hypoalbuminemia, cardiac morbidity, and mortality in end-stage renal disease. J Am Soc Nephrol 7(5):728–736
Wizemann V, Wabel P, Chamney P, Zaluska W, Moissl U, Rode C et al (2009) The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transpl 24(5):1574–1579
Sorof JM, Cardwell G, Franco K, Portman RJ (2002) Ambulatory blood pressure and left ventricular mass index in hypertensive children. Hypertension 39:903–908
Cuspidi C, Lonati L, Sampieri L, Macca G, Michev I, Salerno M et al (2000) Impact of blood pressure control on prevalence of left ventricular hypertrophy in treated hypertensive patients. Cardiology 93:149–154
Cuspidi C, Meani S, Fusi V, Valerio C, Catini E, Magrini F et al (2005) Isolated ambulatory hypertension and changes in target organ damage in treated hypertensive patients. J Hum Hypertens 19:471–477
Bouhanick B, Bongard V, Amar J, Bousquel S, Chamontin B (2008) Prognostic value of nocturnal blood pressure and reverse dipping status on the occurrence of cardiovascular events in hypertensive diabetic patients. Diabetes Metab 34:560–567
Sturrock ND, George E, Pound N, Steven-son J, Peck GM, Sowter H (2000) Non dipping circadian blood pressure and renal impairment are associated with increased mortality in diabetes mellitus. Diabet Med 17:360–364
Davenport A, Willicombe MK (2010) Does diabetes mellitus predispose to increased fluid overload in peritoneal dialysis patients? Nephron Clin Pract 114:60–66
Tang W, Xue T, Lu XH, Luo YJ, Wang T (2011) Factors contributing to formation of oedema in volume overloaded continuous ambulatory peritoneal dialysis patients. Perit Dial Int 31:160–167
Ortega LM, Materson BJ (2011) Hypertension in peritoneal dialysis patients epidemiology, pathogenesis and treatment. J Am Soc Hypertens 5:128–136
Takatori Y, Akagi S, Sugiyama H, Inoue J, Kojo S, Morinaga H et al (2011) Icodextrin increases technique survival rate in peritoneal dialysis patients with diabetic nephropathy by improving body fluid management: a randomized controlled trial. Clin J Am Soc Nephrol 6:1337–1344
Acknowledgments
This study received a grant from Universiti Kebangsaan Malaysia towards the cost of echocardiogram and reimbursing patients for their clinic visits. We would like to thank our three CAPD nurses for their contributions in running this study. We would also like to thank Prof NCT Kong and Associate Prof Oteh Maskon for their guidance and our Dean for allowing us to publish the findings of this study.
Conflict of interest
All authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cader, R.A., Ibrahim, O.A., Paul, S. et al. Left ventricular hypertrophy and chronic fluid overload in peritoneal dialysis patients. Int Urol Nephrol 46, 1209–1215 (2014). https://doi.org/10.1007/s11255-013-0615-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-013-0615-8