Abstract
Purpose
Left ventricular hypertrophy (LVH) represents one of the main risk factors for cardiovascular mortality in dialysis patients. Low serum magnesium Mg is related with increased mortality in general and dialysis population. Aim of our study was to evaluate the association of Mg with LVH and cardiac geometry in dialysis patients.
Methods
Hemodialysis (HD) and peritoneal dialysis (PD) patients from nine nephrology departments were included. Echocardiographic LVH was defined by LV mass index > 95 g/m2 in women and > 115 g/m2 in men. Four LV geometric patterns were defined: normal, concentric remodeling, eccentric LVH and concentric LVH. Demographic and laboratory data were collected.
Results
133 patients (68 HD, 65 PD) with a median age of 63 years (IQR 52–74) were studied. Mg correlated positively with creatinine, HDL and negatively with CRP levels and BMI. There were no significant differences in Mg between the modality groups. 80 patients presented LVH (43 HD and 37 PD patients). Patients with LVH were older (median age 68 vs 55 years, p < 0.001), with higher BMI (median 26.9 vs 24.7 kg/m2, p = 0.009), had a history of PVD or CAD (55% vs 30.2%, p = 0.003), had higher pulse pressure (median 60 vs 50, p = 0.017), MIS score (median 5 vs 4, p = 0.011), lower albumin (median 3.5 vs 3.8 g/dl, p = 0.011) and Mg levels (median 2.1 vs 2.4 mg/dl, p < 0.001). In univariate analysis age, CVD comorbidities, pulse pressure, CRP, BMI, albumin, Mg, MIS and use of b-blockers or calcium blockers were LVH predictors. In multivariate analysis, Mg was an independent predictor of LVH, adjusted for age, MIS and b-blockers. Considering LV geometry, lower Mg levels were mainly correlated with concentric LVH.
Conclusion
Low serum magnesium levels seem to be an independent factor for LVH in hemodialysis and peritoneal dialysis patients.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease is the leading primary cause of death in both hemodialysis (HD) and peritoneal dialysis (PD) patients, as it accounts for almost 55% of all deaths [1]. Left ventricular hypertrophy (LVH) represents one of the main risk factors responsible for the high incidence of cardiovascular disease (CVD) and is highly prevalent in the chronic kidney disease (CKD) population from the early stages of renal disease [2, 3]. Regarding geometrical pattern of left ventricular mass (LVM), four patterns can be distinguished; a) normal LVM b) “normal” geometry (NG) and concentric remodeling (CR) c) concentric left ventricular hypertrophy (cLVH) and d) eccentric left ventricular hypertrophy (eLVH) [4]. This information is clinically relevant, because according to studies in cohorts of the general population and patients with essential hypertension, abnormal patterns of LV geometry adversely affect prognosis, with higher risk of CV events and all-cause mortality particularly evident in patients with concentric LVH [5, 6]. Similar data in CKD populations are sparse [7, 8] and eccentric LVH is most prevalent in dialysis-dependent CKD stage 5 (CKD5D) while in non- CKD patients cLVH is the commonest. In the same population the risk of sudden death, which accounts for one quarter of all deaths and one half of CVD mortality, is fivefold greater in patients with eLVH than with cLVH [9].
The mechanisms that are involved in the pathophysiology of LVH in CKD patients are quite complex and interactive [10]. Systemic arterial resistance, hypertension and large-vessel compliance due to aortic calcification (afterload-related factors) and hypervolemia and anemia (preload-related factors) are the most important ones. However, processes seemingly unrelated to both afterload or preload, like inflammation, oxidative stress and the parathyroid hormone (PTH)–vitamin D–phosphate axis are also emerging as important in the production of LVH in patients with CKD. An underrated pathogenetic factor that could link vascular calcification, LVH and high mortality in this population might be serum magnesium [11,12,13]. In a population-based longitudinal study, LVH was significantly higher in subjects with lower magnesium [14]. A recent meta-analysis adjusting for various prognostic factors showed that there was a strong association between hypomagnesemia and the risk of all-cause mortality–in hemodialysis patients [15], while in PD patients lower magnesium levels were also associated with CVD mortality [16]. In a cohort of European hemodialysis patients, hypomagnesemia was associated with sudden deaths [17].
Despite magnesium link with LVH and mortality in CKD patients, magnesium status is infrequently assessed in clinical practice while sparse trials have addressed the issue and its potential target for treatment. In dialysis populations there is only one study in hemodialysis patients [18] where lower magnesium concentrations were independent predictors of LVM and vascular calcification score. To our knowledge, there are no relevant data in PD patients. So far, no study has demonstrated a possible relation of magnesium with different LVH patterns in CKD populations.
The aim of our study was to evaluate the role of serum magnesium on LVH both in HD and PD patients. Moreover we aimed to assess any correlation of serum magnesium with different cardiac geometry patterns in dialysis patients.
Methods
This cross sectional study included prevalent patients on dialysis (HD and PD) from nine nephrology departments in Greece from 01/01/2018 to 31/12/2018. Inclusion criteria were (a) age > 18 years old (b) dialysis vintage more than 3 months and (c) stable clinical condition (asymptomatic patients without recent hospitalization for any reason). Exclusion criteria were (a) incident patients (b) recent (1–6 months) major surgical procedure (c) active cancer (d) autoimmune disease (e) short life expectancy (less than a year). This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving patients were approved by the Ethics Committee of the University Hospital of Athens, Attikon. Written informed consent was obtained from all participants at enrolment.
Demographic and biochemical data
Demographic and clinical characteristics, including age, sex, primary renal disease, dialysis duration, comorbidity including arterial hypertension, diabetes, and CVD (coronary artery disease CAD, congestive heart failure, stroke and peripheral vascular disease (PVD)] at enrolment were reviewed. Biochemical data, including serum albumin, serum magnesium (Mg), high-sensitivity C-reactive protein (CRP), serum lipid profile, serum calcium (corrected for albumin), serum phosphate, serum potassium (K) and sodium(Na), intact PTH, serum creatinine and urea, were collected at enrolment before midweek session in hemodialysis patients and at the routine visit at the unit in peritoneal dialysis patients. Data about medication such as statins, b-blockers, calcium channel blockers (CCB) and renin-angiotensin system (RAS) inhibitors (ACE/ARBs) were also collected. The normal reference range for Mg levels in this study was 1.5–2.5 mEq/L. Serum Mg values (as well as all biochemical parameters) were defined as the mean of all predialysis measurements available during the preceding 3 months. Blood pressure (systolic, diastolic and pulse pressure) were defined as the mean of all predialysis (in HD) and monthly office (in PD) measurements during the preceding 3 months. Pulse pressure (PP) was calculated by the formula PP = SBP − DBP (SBP, systolic blood pressure; DBP, diastolic blood pressure).
Malnutrition-Inflammation Score-MIS assessments were performed in all patients by their physician at enrolment. MIS score is a tool for assessing nutritional status and inflammation in CKD patients. It has four sections (nutritional history, physical examination, BMI, and laboratory values) and 10 components. Each component has four levels of severity, from 0 (normal) to 3 (severely abnormal). The sum of all 10 MIS components can range from 0 (normal) to 30 (severely malnourished); a higher score reflects a more severe degree of malnutrition and inflammation [19].
Echocardiographic readings were performed by trained and certified personnel according to published recommendations [4]. Echocardiographic LVH was defined by LV mass index (LVMI) > 95 g/m2 in women and > 115 g/m2 in men. Based on LVMI and relative wall thickness (RWT), four LV geometric patterns were defined: normal (normal LVMI and RWT), concentric remodeling (normal LVMI and increased RWT > 0.42), eccentric LVH (increased LVMI and normal RWT) and concentric LVH (increased LVMI and RWT > 0.42).
Statistical analysis
Descriptive statistical analysis was performed for all data; continuous variables were summarized with the use of medians (25th–75th percentiles) and categorical variables were displayed as frequency tables (N, %). Standard statistical tests were used to assess univariate associations between categorical (Fisher’s exact tests or Pearson chi-square test or categorical and continuous variables (Two-sample Wilcoxon rank-sum test, T-test). Shapiro–Wilk W test was performed to evaluate the normality for each continuous variable combined with graphical methods. Box plots and histograms were used for graphical presentation of differences and trends between covariates. Laboratory variables (apart from CRP levels) were presented and analyzed only in continuous forms. Logistic regression was implemented to describe the relationship between LVH (dependent variable) and our covariates. Selection of influential variables was done by a combination of clinical and statistical criteria; all variables with p-value in univariate analysis of less than 0.15 were selected. A cut-off point for serum Mg was chosen according to the Youden Index (J) which optimizes the differentiating ability when equal weight is given to sensitivity and specificity. All covariates apart from potassium and sodium had a percentage of missing values less than 7%; potassium and sodium were both excluded from candidates for the multivariate model. Stepwise backward-selection method was chosen, starting with all candidate explanatory variables and removing terms with p ≥ 0.05. Our final model was checked for overfitting, multicollinearity and influential observations. Interactions between variables were checked by including interaction terms in the multivariate model. Pearson residuals, Deviance residuals and leverage were graphed against either the predicted probabilities or the case numbers. Multiple imputations methods for missing data were not used. Results with p-value of < 0.05 were considered statistically significant, and results with 0.05 ≤ p ≤ 0.10 suggestive. All statistical analyses were performed using STATA/SE 16.1 software (Copyright 1985–2019; StataCorp LP, College Station, Texas, USA).
Results
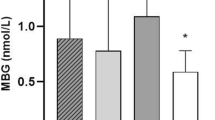
The study included 133 patients (79 male/54 female) with stage CKD- 5D (68 on HD and 65 on PD). The median age was 63 years old (IQR 52–74) and median total dialysis time was 37 months (IQR 20–67). 28.6% of patients were diabetics and 75.9% hypertensive, while 32.3% had a history of PVD, 27.8% CAD, 12.8% stroke and 18.8% were current smokers. All HD patients used dialysis solution with 0.5 mEq/L Mg concentration, while half of PD patients used dialysis solution of 0.5 mEq/L Mg and rest of them 0.25 mEq/L Mg concentration. Regarding calcium dialysis solution concentration, the majority of PD patients (64.6%) had a 1.75 mEq/L solution, while 47% of HD used a 1.5 mEq/L calcium concentration dialysis solution. Table 1 presents the main demographic and laboratory characteristics of the patients in the whole and according to the dialysis modality. Overall, Mg levels correlated positively with creatinine levels, HDL, use of statins and negatively with CRP levels and body mass index (BMI). There were no significant differences in Mg levels between the modality groups (Fig. 1).
80 patients presented LVH (43 HD and 37 PD patients)-median LVMI 126 gr/m2 (116.5–140.5). There were no differences in LVH prevalence in the dialysis modalities (57% in PD vs 63% in HD) while overall eccentric and concentric patterns were the commonest (30.8 and 29.3% respectively). PD patients had higher prevalence of concentric remodeling and concentric LVH comparing with HD patients (24.6 vs 10.3% and 34 vs 25%, respectively) while eLVH was more common in HD comparing with PD patients (38 vs 23%). Patients with LVH were older (median age 68 vs 55 years, p < 0.001), with higher BMI (median 26.9 vs 24.7 kg/m2, p = 0.009), had a history of PVD or CAD (55 vs 30.2%, p = 0.003), had higher pulse pressure (median 60 vs 50, p = 0.017), MIS score (median 5 vs 4, p = 0.011), lower albumin (median 3.5 vs 3.8 g/dl, p = 0.011) and Mg levels (median 2.1 vs 2.4 mg/dl, p < 0.001) (Table 2).
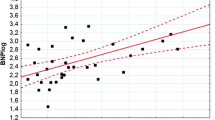
In univariate analysis age, CVD comorbidities, pulse pressure, CRP, BMI, albumin, Mg, MIS and use of b-blockers or CCB were LVH predictors. Factors with p < 0.15 such as creatinine, CRP, diabetes, ACE/ARB, CCB, smoking, calcium were also included in a stepwise multivariate logistic regression model. BMI and albumin levels were not included in the multivariate analysis because MIS score entails these parameters. Mg was an independent predictor of LVH, along with age, MIS and use of b-blockers (Table 3). Each increase of serum Mg by 1 mg/dl was associated with 89% lower odds of having LVH. Considering LV geometry, over Mg levels were mainly correlated with concentric LVH (Fig. 2) and not with eccentric LVH or concentric remodeling.
Discussion
Our study found that low serum magnesium levels are strongly correlated with LVH in HD and PD patients. This finding was stable and robust even adjusted for multiple factors. HD patients had a higher prevalence of eccentric LVM pattern comparing with PD and low serum magnesium was mainly correlated with concentric LVH, a finding which is reported for the first time in literature. Low Mg in CKD patients could be due to inadequate diet intake, medication and low dialysate Mg concentration. In most relevant studies, it is correlated with malnutrition markers like serum albumin, creatinine and phosphorus levels (14) or inflammation- mainly assessed by CRP (15–17). In our study, Mg levels in hemodialysis and peritoneal dialysis patients were negatively correlated with CRP and BMI, but we did not find any correlation with albumin. On the other hand, Mg was positively correlated with creatinine, HDL cholesterol and statin use. BMI is an inaccurate index of nutritional status as higher levels could imply only hypervolemia and not increased fat mass or better nutrition (18). Regarding higher HDL cholesterol levels apart from their association with survival (19), it seems to be related with higher albumin [20] and higher Mg levels [21]. So overall our results are in concordance with other studies (15, [18, 22], as all these interrelated factors are involved in the pathogenesis of malnutrition and inflammation.
In the general [23, 24] and HD populations [25, 26] several studies have shown an association between low serum Mg levels and higher all-cause and cardiovascular mortality and sudden cardiac death. In contrast, the data for patients undergoing PD are substantially more limited. In a prospective study, lowest Mg levels were independently associated with 2.28 fold increased cardiovascular mortality [27] while in a large USA database lower serum Mg levels were associated with higher risk of hospitalization particularly among those with hypoalbuminemia [28]. In HD, the pathophysiological link between hypomagnesemia and mortality may be attributed to endothelial dysfunction [29], higher pulse pressure and vascular calcification [18], more arrhythmias [30] and malnutrition-inflammation syndrome [31] as hypomagnesemia correlates with hypoalbuminemia, hypophosphatemia and higher CRP levels. In PD populations vascular calcifications seem to be again the important connection between Mg levels and mortality, as higher serum Mg level was associated with a lower abdominal aortic calcification score [32].
Another important link appears to be the development of LVH. In dialysis patients, the severity and persistence of LVH are strongly associated with mortality risk and cardiovascular events [2]. LVH is a multifactorial complication which etiology involves interrelated factors like anemia, hypertension, hypervolemia, increased systemic arterial resistance and reduced large-vessel compliance due to aortic calcification [33]. Reffelman et al. [14] was the first to study magnesium levels and LVH prospectively (5 years) in a large general population cohort. The authors found that in the lowest Mg -quintile (Mg ≤ 0.73 mmol/l), LVM (187.4 ± 3.1 g at baseline) increased by 14.9 ± 1.2 g, while in the highest Mg-quintile (Mg = 0.85 mmol/l) LVM (186.7 ± 3.4 g at baseline) decreased by − 0.5 ± 2.8 g (p < 0.0001 between quintiles). By multivariable analysis including several cardiovascular risk factors and antihypertensive treatment, serum Mg was associated with the increase in LVM at a statistically high significant level. In dialysis populations there is only one study in 200 HD patients [18] where lower Mg concentrations were independent predictors of LVMI (≥ 140 g/m2, p = 0.03), increased PP (≥ 65 mm Hg, p = 0.002) and higher vascular calcification score (≥ 3, p = 0.01).
Our study confirmed these results in HD population and for the first time in PD patients. In univariate analysis age, CVD co -morbidities, pulse pressure, Mg, CRP, BMI, albumin, MIS and use of b-blockers or calcium blockers were LVH predictors. In multivariate analysis -apart from lower Mg levels- higher age, higher MIS and use of b-blockers remained predictors of LVH. Age is a standard risk factor for LVH in all studies, while b-blockers (the most common prescribed medication in our population − 68%) represent a group of patients with CAD and hypertension. On the other hand, MIS score characterizes inflammation and malnutrition which have a main role in the pathophysiology of LVH too [34]. For the first time in literature, we found that PD patients had mainly concentric LVH pattern, while HD presented mainly eccentric LVH, which agrees with literature data and is associated with sudden death [9]. However lower Mg levels correlated mainly with concentric LVH, a finding which is reported for the first time in literature and it needs further studies to be confirmed.
How can low Mg be associated with LVH? Magnesium and vascular calcification may be the main indirect pathogenetic mechanism that could explain LVH. Moreover, Mg correlates with LVH directly via fibrinogenesis in animal studies mediated by the renin–angiotensin–aldosterone system, as in magnesium-deficient rats circulating angiotensin II and aldosterone stimulate fibroblast activity in the heart [35, 36]. Magnesium prevents osteogenic vascular smooth muscle cell transdifferentiation and calciprotein particle maturation and mineralization of the extracellular matrix in vitro and in vivo models, which may be the mechanism underlying its anti-calcification properties [37, 38]. In limited and small studies, supplementation of Mg in CKD [39, 40] and hemodialysis [41] patients seems to provide a protective role in slowing calcification of the arteries.
Our study has limitations. It is a retrospective, observational study and Mg levels were not assessed longitudinally. Nevertheless, the study shows that Mg is an independent predictor of LVH in both dialysis populations. Despite magnesium link with LVH and mortality in CKD patients, Mg status is infrequently assessed in clinical practice. Furthermore there is lack of randomized trials about the optimal Mg levels in dialysis patients which could be beneficial in terms of outcomes.
In conclusion, the development of LVH in dialysis patients is a major mortality risk factor. Several complicated mechanisms are involved in the pathogenesis of LVH and low serum magnesium levels seem to be an independent risk factor. Randomized controlled trials examining the optimal serum magnesium in dialysis patients are needed.
References
Saran R, Robinson B, Abbott KC, Bragg-Gresham J, Chen X, Gipson D, Gu H, Hirth RA, Hutton D, Jin Y, Kapke A, Kurtz V, Li Y, McCullough K, Modi Z, Morgenstern H, Mukhopadhyay P, Pearson J, Pisoni R, Repeck K, Schaubel DE, Shamraj R, Steffick D, Turf M, Woodside KJ, Xiang J, Yin M, Zhang X, Shahinian V (2020) US Renal data system 2019 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 75(1 Suppl 1):A6–A7. https://doi.org/10.1053/j.ajkd.2019.09.003
Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Giacone G, Cataliotti A, Seminara G, Stancanelli B, Malatino LS (2004) Prognostic value of echocardiographic indicators of left ventricular systolic function in asymptomatic dialysis patients. J Am Soc Nephrol 15(4):1029–1037. https://doi.org/10.1097/01.asn.0000117977.14912.91
Cai QZ, Lu XZ, Lu Y, Wang AY (2014) Longitudinal changes of cardiac structure and function in CKD (CASCADE study). J Am Soc Nephrol 25(7):1599–1608. https://doi.org/10.1681/ASN.2013080899
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ, Chamber Quantification Writing G, American Society of Echocardiography’s G, Standards C, European Association of E (2005) Recommendations for chamber quantification: a report from the American Society of Echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European association of echocardiography, a branch of the European society of cardiology. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 18(12):1440–1463. https://doi.org/10.1016/j.echo.2005.10.005
Lieb W, Gona P, Larson MG, Aragam J, Zile MR, Cheng S, Benjamin EJ, Vasan RS (2014) The natural history of left ventricular geometry in the community: clinical correlates and prognostic significance of change in LV geometric pattern. JACC Cardiovasc Imaging 7(9):870–878. https://doi.org/10.1016/j.jcmg.2014.05.008
Gerdts E, Cramariuc D, de Simone G, Wachtell K, Dahlof B, Devereux RB (2008) Impact of left ventricular geometry on prognosis in hypertensive patients with left ventricular hypertrophy (the life study). Eur J Echocardiogr J Work Group Echocardiogr Eur Soc Cardiol 9(6):809–815. https://doi.org/10.1093/ejechocard/jen155
Paoletti E, De Nicola L, Gabbai FB, Chiodini P, Ravera M, Pieracci L, Marre S, Cassottana P, Luca S, Vettoretti S, Borrelli S, Conte G, Minutolo R (2016) Associations of left ventricular hypertrophy and geometry with adverse outcomes in patients with CKD and hypertension. Clin J Am Soc Nephrol CJASN 11(2):271–279. https://doi.org/10.2215/CJN.06980615
Nube MJ, Hoekstra T, Doganer V, Bots ML, Blankestijn PJ, van den Dorpel M, Kamp O, TerWee PM, de van RoijZuijdewijn CLM, Grooteman MPC (2018) Left ventricular geometric patterns in end-stage kidney disease: determinants and course over time. Hemodial Int Int Symp Home Hemodial 22(3):359–368. https://doi.org/10.1111/hdi.12644
de van RoijZuijdewijn CL, Hansildaar R, Bots ML, Blankestijn PJ, van den Dorpel MA, Grooteman MP, Kamp O, Ter Wee PM, Nube MJ (2015) Eccentric left ventricular hypertrophy and sudden death in patients with end-stage kidney disease. Am J Nephrol 42(2):126–133. https://doi.org/10.1159/000439447
Glassock RJ, Pecoits-Filho R, Barberato SH (2009) Left ventricular mass in chronic kidney disease and ESRD. Clin J Am Soc Nephrol CJASN 4(Suppl 1):S79-91. https://doi.org/10.2215/CJN.04860709
Meema HE, Oreopoulos DG, Rapoport A (1987) Serum magnesium level and arterial calcification in end-stage renal disease. Kidney Int 32(3):388–394. https://doi.org/10.1038/ki.1987.222
Massy ZA, Drueke TB (2012) Magnesium and outcomes in patients with chronic kidney disease: focus on vascular calcification, atherosclerosis and survival. Clin Kidney J 5(Suppl 1):i52–i61. https://doi.org/10.1093/ndtplus/sfr167
Sakaguchi Y, Hamano T, Isaka Y (2018) Magnesium in hemodialysis patients: a new understanding of the old problem. Contrib Nephrol 196:58–63. https://doi.org/10.1159/000485700
Reffelmann T, Dorr M, Ittermann T, Schwahn C, Volzke H, Ruppert J, Robinson D, Felix SB (2010) Low serum magnesium concentrations predict increase in left ventricular mass over 5 years independently of common cardiovascular risk factors. Atherosclerosis 213(2):563–569. https://doi.org/10.1016/j.atherosclerosis.2010.08.073
Xiong J, He T, Wang M, Nie L, Zhang Y, Wang Y, Huang Y, Feng B, Zhang J, Zhao J (2019) Serum magnesium, mortality, and cardiovascular disease in chronic kidney disease and end-stage renal disease patients: a systematic review and meta-analysis. J Nephrol 32(5):791–802. https://doi.org/10.1007/s40620-019-00601-6
Cai K, Luo Q, Dai Z, Zhu B, Fei J, Xue C, Wu D (2016) Hypomagnesemia is associated with increased mortality among peritoneal dialysis patients. PLoS ONE 11(3):e0152488. https://doi.org/10.1371/journal.pone.0152488
de van RoijZuijdewijn CL, Grooteman MP, Bots ML, Blankestijn PJ, Steppan S, Buchel J, Groenwold RH, Brandenburg V, van den Dorpel MA, Ter Wee PM, Nube MJ, Vervloet MG (2015) Serum magnesium and sudden death in European hemodialysis patients. PLoS ONE 10(11):e0143104. https://doi.org/10.1371/journal.pone.0143104
Joao Matias P, Azevedo A, Laranjinha I, Navarro D, Mendes M, Ferreira C, Amaral T, Jorge C, Aires I, Gil C, Ferreira A (2014) Lower serum magnesium is associated with cardiovascular risk factors and mortality in haemodialysis patients. Blood Purif 38(3–4):244–252. https://doi.org/10.1159/000366124
Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH (2001) A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis 38(6):1251–1263. https://doi.org/10.1053/ajkd.2001.29222
Bowden RG, Wilson RL (2010) Malnutrition, inflammation, and lipids in a cohort of dialysis patients. Postgrad Med 122(3):196–202. https://doi.org/10.3810/pgm.2010.05.2158
Yu L, Zhang J, Wang L, Li S, Zhang Q, Xiao P, Wang K, Zhuang M, Jiang Y (2018) Association between serum magnesium and blood lipids: influence of type 2 diabetes and central obesity. Br J Nutr 120(3):250–258. https://doi.org/10.1017/S0007114518000685
Nielsen FH (2014) Effects of magnesium depletion on inflammation in chronic disease. Curr Opin Clin Nutr Metab Care 17(6):525–530. https://doi.org/10.1097/MCO.0000000000000093
Reffelmann T, Ittermann T, Dorr M, Volzke H, Reinthaler M, Petersmann A, Felix SB (2011) Low serum magnesium concentrations predict cardiovascular and all-cause mortality. Atherosclerosis 219(1):280–284. https://doi.org/10.1016/j.atherosclerosis.2011.05.038
Zhang W, Iso H, Ohira T, Date C, Tamakoshi A, Group JS (2012) Associations of dietary magnesium intake with mortality from cardiovascular disease: the JACC study. Atherosclerosis 221(2):587–595. https://doi.org/10.1016/j.atherosclerosis.2012.01.034
Sato H, Takeuchi Y, Matsuda K, Saito A, Kagaya S, Fukami H, Ojima Y, Nagasawa T (2017) Evaluation of the predictive value of the serum calcium-magnesium ratio for all-cause and cardiovascular mortality in incident dialysis patients. Cardiorenal Med 8(1):50–60. https://doi.org/10.1159/000480739
Shimohata H, Yamashita M, Ohgi K, Tsujimoto R, Maruyama H, Takayasu M, Hirayama K, Kobayashi M (2019) The relationship between serum magnesium levels and mortality in non-diabetic hemodialysis patients: a 10-year follow-up study. Hemodial Int Int Symp Home Hemodial 23(3):369–374. https://doi.org/10.1111/hdi.12759
Ye H, Cao P, Zhang X, Lin J, Guo Q, Mao H, Yu X, Yang X (2018) Serum magnesium and cardiovascular mortality in peritoneal dialysis patients: a 5-year prospective cohort study. Br J Nutr 120(4):415–423. https://doi.org/10.1017/S0007114518001599
Yang X, Soohoo M, Streja E, Rivara MB, Obi Y, Adams SV, Kalantar-Zadeh K, Mehrotra R (2016) Serum magnesium levels and hospitalization and mortality in incident peritoneal dialysis patients: a cohort study. Am J Kidney Dis 68(4):619–627. https://doi.org/10.1053/j.ajkd.2016.03.428
Kanbay M, Yilmaz MI, Apetrii M, Saglam M, Yaman H, Unal HU, Gok M, Caglar K, Oguz Y, Yenicesu M, Cetinkaya H, Eyileten T, Acikel C, Vural A, Covic A (2012) Relationship between serum magnesium levels and cardiovascular events in chronic kidney disease patients. Am J Nephrol 36(3):228–237. https://doi.org/10.1159/000341868
Alhosaini M, Leehey DJ (2015) Magnesium and dialysis: the neglected cation. Am J Kidney Dis 66(3):523–531. https://doi.org/10.1053/j.ajkd.2015.01.029
Pakfetrat M, Malekmakan L, Roozbeh J, Haghpanah S (2008) Magnesium and its relationship to C-reactive protein among hemodialysis patients. Magnes Res 21(3):167–170
Molnar AO, Biyani M, Hammond I, Harmon JP, Lavoie S, McCormick B, Sood MM, Wagner J, Pena E, Zimmerman DL (2017) Lower serum magnesium is associated with vascular calcification in peritoneal dialysis patients: a cross sectional study. BMC Nephrol 18(1):129. https://doi.org/10.1186/s12882-017-0549-y
McMahon LP, Roger SD, Levin A, Slimheart Investigators G (2004) Development, prevention, and potential reversal of left ventricular hypertrophy in chronic kidney disease. J Am Soc Nephrol 15(6):1640–1647. https://doi.org/10.1097/01.asn.0000130566.69170.5e
Ducros J, Larifla L, Merault H, Galantine V, Bassien-Capsa V, Foucan L (2020) N-terminal pro-B-type natriuretic peptide and malnutrition in patients on hemodialysis. Int J Nephrol 2020:9528014. https://doi.org/10.1155/2020/9528014
Sapna S, Ranjith SK, Shivakumar K (2006) Cardiac fibrogenesis in magnesium deficiency: a role for circulating angiotensin II and aldosterone. Am J Physiol Heart Circ Physiol 291(1):H436-440. https://doi.org/10.1152/ajpheart.01185.2005
Finckenberg P, Merasto S, Louhelainen M, Lindgren L, Vapaatalo H, Muller DN, Luft FC, Mervaala EM (2005) Magnesium supplementation prevents angiotensin II-induced myocardial damage and CTGF overexpression. J Hypertens 23(2):375–380. https://doi.org/10.1097/00004872-200502000-00020
Ter Braake AD, Vervloet MG, de Baaij JHF, Hoenderop JGJ (2022) Magnesium to prevent kidney disease-associated vascular calcification: crystal clear? Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc Eur Ren Assoc. https://doi.org/10.1093/ndt/gfaa222
Louvet L, Buchel J, Steppan S, Passlick-Deetjen J, Massy ZA (2013) Magnesium prevents phosphate-induced calcification in human aortic vascular smooth muscle cells. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc Eur Ren Assoc 28(4):869–878. https://doi.org/10.1093/ndt/gfs520
Bressendorff I, Hansen D, Schou M, Silver B, Pasch A, Bouchelouche P, Pedersen L, Rasmussen LM, Brandi L (2017) Oral magnesium supplementation in chronic kidney disease stages 3 and 4: efficacy, safety, and effect on serum calcification propensity-a prospective randomized double-blinded placebo-controlled clinical trial. Kidney Int reports 2(3):380–389. https://doi.org/10.1016/j.ekir.2016.12.008
Sakaguchi Y, Hamano T, Obi Y, Monden C, Oka T, Yamaguchi S, Matsui I, Hashimoto N, Matsumoto A, Shimada K, Takabatake Y, Takahashi A, Kaimori JY, Moriyama T, Yamamoto R, Horio M, Yamamoto K, Sugimoto K, Rakugi H, Isaka Y (2019) A randomized trial of magnesium oxide and oral carbon adsorbent for coronary artery calcification in predialysis CKD. J Am Soc Nephrol 30(6):1073–1085. https://doi.org/10.1681/ASN.2018111150
Spiegel DM, Farmer B (2009) Long-term effects of magnesium carbonate on coronary artery calcification and bone mineral density in hemodialysis patients: a pilot study. Hemodial Int Int Symp Home Hemodial 13(4):453–459. https://doi.org/10.1111/j.1542-4758.2009.00364.x
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors state that they do not have any financial or non-financial interests that are directly or indirectly related to the work submitted for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Balafa, O., Dounousi, E., Giannikouris, I. et al. Lower serum magnesium is a predictor of left ventricular hypertrophy in patients on dialysis. Int Urol Nephrol 55, 1015–1023 (2023). https://doi.org/10.1007/s11255-022-03391-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-022-03391-2