Abstract
Purpose
Tissue damage in testicular torsion/detorsion is caused not only by the ischemia, but also by the ischemia/reperfusion injury after detorsion. Erythropoietin and sildenafil are considered to protect against ischemia/reperfusion injury. Here, we studied and compared their actions in testicular torsion/detorsion in adult rats.
Methods
Twenty-two adult male Wistar Albino rats were divided into four groups. Rats in group A (n = 5) were sham operated. Rats in group B (n = 5), group C (n = 6) and group D (n = 6) underwent torsion of the right testis and detorsion after 90 min. No pharmaceutical intervention was performed in group B. Erythropoietin (1,000 IU/kg) and sildenafil (0.7 mg/kg) were injected intraperitoneally in groups C and D, respectively, after 60 min of torsion. All animals were killed 24 h after detorsion, and their right testis was extracted, placed into 10 % formalin solution and sent for histopathological examination. The histological changes in the testes were scored according to the four-point grading system proposed by Cosentino et al.
Results
All rats in group A had normal testicular architecture (grade 1). The untreated group B had a mean grade of 3.81 (range 3.65–4). The treated groups C (mean grade 3.24; range 3.05–3.45) and D (2.69, range 2.4–2.9) presented statistically significant better results (lower grades) compared with the untreated group B. Group D had significantly better results (lower grades) than group C.
Conclusions
The intraperitoneal injection of erythropoietin and sildenafil protects against ischemia/reperfusion injury after testicular torsion and detorsion. Sildenafil may have a stronger action than erythropoietin at the doses used in this study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Testicular torsion is an emergent condition occurring primarily in the pediatric and the young adult male population. It causes rotation of the testicular vascular pedicle due to twisting of the spermatic cord, leading initially to the obstruction of venous return and subsequently to compromise of arterial flow, which result in ischemia of the testis [1–9]. Emergent surgical treatment is necessary, because the testis suffers significant ischemic damage after 4–8 h [2, 3, 6]. Apart from ischemia, reperfusion after surgical testicular detorsion is also considered to be involved in the pathophysiological changes within the affected testis. Reactive oxygen species are produced after reperfusion, and the release of cytokines, such as interleukin-1β and tumor necrosis factor-α, results in recruitment of neutrophils and macrophages, causing testicular atrophy, germ cell apoptosis and disruption of spermatogenesis [1, 4, 5, 8, 9]. Erythropoietin and sildenafil are considered to prevent ischemia/reperfusion injury by suppressing these mechanisms [10, 11]. In this study, we investigate the potential protective effect of erythropoietin and sildenafil against the unilateral ischemia/reperfusion testicular injury in rats and we also compare the results of their administration.
Methods
Animals
The experimental protocol was reviewed by the Ethics Committee of the Medical School of the University of Athens and by the Veterinary Directorate of the Region of Attica and conformed to the ethical recommendations of the European Communities Council Directive of November 24, 1986 (86/609/EEC). The animals were divided in four groups. Twenty-two adult male Wistar Albino rats, aged between 12 and 14 weeks and weighing between 250 and 300 g, were used in this study. The animals were obtained from the Hellenic Pasteur Institute (Athens, Greece) and were acclimatized for 1 week prior to the beginning of the experiment. They were housed under standard laboratory conditions of temperature (22 ± 2 °C) and relative humidity (60 %) with 12-h light and dark cycles. They were anesthetized with an intramuscular injection of ketamine (100 mg/kg) and xylazine (5 mg/kg), and they breathed spontaneously throughout the procedures. All rats received chemoprophylaxis (enrofloxacin/Baytril 10 mg/kg) subcutaneously and analgesia (carprofen/Rimadyl 0.08 ml/kg) preoperatively. The skin of the scrotum was shaved, disinfected with betadine solution, and all procedures were performed under sterile conditions.
Group A (normal controls)
Group A included 5 sham-operated rats. The right testis was exposed through a vertical paramedian incision of the scrotum. No torsion was performed, the testis was placed back in its anatomical position, and the scrotal incision was closed by 4/0 silk sutures.
Group B (control group)
Group B included 5 rats. The right testis was exposed through a vertical paramedian incision of the scrotum. Torsion of the testis was performed by rotating it 720° clockwise. The testis was then fixed to the internal spermatic fascia by 4/0 polyglycolic acid sutures, and it was placed back in its anatomical position. The scrotal incision was closed by 4/0 silk sutures. After 90 min of total torsion of the testis, the scrotum was reopened and the right testis was derogated to its normal position, placed back and the scrotum was closed.
Group C (erythropoietin group)
Group C included 6 rats. The right testis was exposed through a vertical paramedian incision of the scrotum. Torsion of the testis was performed by rotating it 720° clockwise. The testis was then fixed to the internal spermatic fascia by 4/0 polyglycolic acid sutures, and it was placed back in its anatomical position. The scrotal incision was closed by 4/0 silk sutures. Recombinant human erythropoietin (1,000 IU/kg) was injected intraperitoneally, after 60 min of torsion. After 90 min of total torsion of the testis, the scrotum was reopened and the right testis was derotated to its normal position, placed back and the scrotum was closed.
Group D (sildenafil group)
Group D included 6 rats. The right testis was exposed through a vertical paramedian incision of the scrotum. Torsion of the testis was performed by rotating it 720° clockwise. The testis was then fixed to the internal spermatic fascia by 4/0 polyglycolic acid sutures, and it was placed back in its anatomical position. The scrotal incision was closed by 4/0 silk sutures. Sildenafil (0.7 mg/kg) was injected intraperitoneally, after 60 min of torsion. After 90 min of total torsion of the testis, the scrotum was reopened and the right testis was derogated to its normal position, placed back and the scrotum was closed.
The animals were randomized into the four groups (A: normal controls, B: control group, C: erythropoietin group, D: sildenafil group) by drawing lots with their numbers on. Additional ketamine and xylazine administration was occasionally needed in a few rats in order to maintain the level of surgical anesthesia until the derotation of the testis. All animals were killed after 24 h from detorsion; the scrotum was opened, and the right testis was extracted and sent for histopathological examination.
Drugs
Recombinant human erythropoietin was obtained in the form of injections (Aranesp, Amgen, Holland), and a dose of 1,000 IU/kg was administered intraperitoneally. Sildenafil was obtained in the form of tablets (Viagra, Pfizer, England), each containing 100 mg of this substance. The tablets were grinded, dissolved in sterile 0.9 % NaCl solution, forming a 20 % w/v solution of sildenafil, and a dose of 0.7 mg/kg was administered intraperitoneally. We used doses of intraperitoneally administered erythropoietin and sildenafil reported by other researchers [12–16].
Histopathological examination
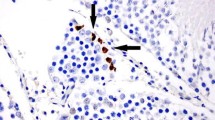
The right testis of each animal following extraction was immediately placed into 10 % formalin solution. Tissue specimens from each testis were embedded in paraffin, and 5-μm-thick sections were cut from these, stained with hematoxylin and eosin and examined with a light microscope. The histological changes in the testes caused by ischemia and reperfusion were scored according to the grading system proposed by Cosentino et al. (Table 1) [17]. Ischemia–reperfusion injury caused tissue damage whose severity ranged between areas in each testis. Therefore, each area was graded separately, and the final result for each testis was calculated by multiplying the grade for each area by the percentage of the total surface that it occupied. Apoptosis was defined as the presence of foci with pyknotic nuclei surrounded by apoptotic bodies (debri), whereas necrosis was defined as the presence of disrupted cell membranes. The pathologist who examined and graded the histological changes in the testes was blinded to the type of the treatment of each animal (no treatment, erythropoietin or sildenafil).
Statistical analysis
Shapiro–Wilk test was used for assessing normal distribution of data and Levene’s test for assessing homogeneity of variance. Analysis of variance (ANOVA) with the Bonferroni correction was performed for comparisons among groups. The results were considered statistically significant if p < 0.05.
Results
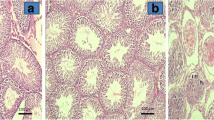
The results for each animal according to the percentage of the total testicular surface corresponding to each grade are shown in Table 2, and histological images from each group are demonstrated in Figs. 1, 2, 3 and 4. All rats in group A (sham operated) had a total score of 1, corresponding to grade I findings, which are normal testicular architecture with an orderly arrangement of germinal cells. Rats in group B (torsion and detorsion without pharmaceutical intervention: mean grade 3.81, range 3.65–4) had severe tissue lesions characterized by closely packed seminiferous tubules and coagulative necrosis of the germinal cells in the greatest part of the testes, along with some areas of ill-defined seminiferous tubule borders and apoptotic germinal cells. Rats in group C (torsion and detorsion with erythropoietin administration: mean grade 3.24; range 3.05–3.45) had moderate lesions in the largest part of the testes, consisting of ill-defined seminiferous tubule borders and disordered sloughed germinal cells with shrunken pyknotic nuclei. There was also a considerable portion with more severe changes consisting of closely packed seminiferous tubules with necrotic germinal cells and smaller areas with mild changes including closely packed, but better defined, seminiferous tubule borders with non-cohesive germinal cells. Rats in group D (torsion and detorsion with sildenafil administration: mean grade 2.69; range 2.4–2.9) showed milder lesions, with the greatest part of the testes occupied by ill-defined seminiferous tubule borders with disordered sloughed germinal cells with shrunken pyknotic nuclei, but also with large areas of mild changes, such as closely packed, but better defined, seminiferous tubule borders and disorderly arranged germinal cells. Comparing groups B, C and D with each other, we found statistically significant differences among them. The treated groups C and D presented significantly better results (lower grades) compared with the untreated group B (p = 0.0002 and p = 0.00000009, respectively). Furthermore, the group D treated with sildenafil had significantly better results (lower grades) than the group C treated with erythropoietin (p = 0.0002) (Fig. 5).
Discussion
Testicular torsion is a medical emergency affecting more often the pediatric and the young adult male population. The spermatic cord twists, and the consequent rotation of the testicular vascular pedicle causes venous congestion, which is followed by the interruption of arterial flow and ischemia of the testis. Emergent surgical detorsion is required, because testicular survival is reduced as time passes [1–9]. The testicular salvage rate falls from 90 % if detorsion occurs within 6 h from the onset of symptoms to 50 % after 12 h and 10 % after 24 h [1, 3, 7].
Reperfusion following detorsion also participates in testicular damage. When the oxygen supply is restored after detorsion, the production of reactive oxygen species is enhanced, causing damage to lipids, proteins and DNA of cells in the testis [1, 4, 5, 8]. Moreover, cytokines, such as tumor necrosis factor-α and interleukin-1β, are produced, leading to recruitment of neutrophils and macrophages and infiltration of the testis by inflammatory cells [4, 5, 8, 9]. These changes affect the function of Sertoli cells [9], cause apoptosis of germinal cells [4, 8, 9] and result in impaired spermatogenesis and testicular atrophy [1, 4, 5, 9].
It has been suggested that erythropoietin and sildenafil protect against ischemia/reperfusion injury [10, 11]. Several studies have demonstrated the protective role of erythropoietin in many tissues and organs, such as brain, heart, lung, liver, kidney, intestine and retina, due to its anti-oxidative, anti-inflammatory and anti-apoptotic properties. Several pathways have been implicated in this process, including Bcl-2, endothelial nitric oxide synthase, nuclear factor kappa B and vascular endothelial growth factor [10]. Sildenafil has also been found to have a protective role, especially in myocardium. Specifically, it has been proposed that the vasodilatory action of sildenafil and other phosphodiesterase type-5 inhibitors releases mediators of ischemic preconditioning, such as bradykinin and/or adenosine [11].
The potential protective role of erythropoietin and sildenafil against ischemia/reperfusion injury of the testis suffering torsion has been tested in rats. Concerning erythropoietin, it has been found that its intraperitoneal administration prior to detorsion reduces histological testicular damage by preserving the morphology of seminiferous tubules and decreasing the percentage of seminiferous tubules with necrotic lesions [12, 18, 19]. The intraperitoneal injection of erythropoietin before detorsion reduces damage and apoptosis of germinal cells [18–20]. Moreover, the levels of malondialdehyde and nitric oxide are decreased, and the levels of glutathione, which possesses antioxidant properties, are increased [19]. The protective role of erythropoietin is also confirmed when it is administered in the immediate interval after detorsion [18, 21]. Concerning sildenafil, it has been found that its intraperitoneal administration prior to detorsion prevents severe damages to seminiferous tubules and reduces the apoptosis of germinal cells [13–16, 22]. Malondialdehyde and nitric oxide levels, markers of oxidative stress, are decreased, glutathione levels are increased, and enzymes that protect against oxidative damage, such as glutathione peroxidase, catalase and superoxide dismutase, have increased activity in the affected testis [13, 14, 16]. Vardenafil, another phosphodiesterase 5 inhibitor, has similar protective results, either administered before or soon after detorsion [23, 24].
Our experimental study confirmed the protective role of erythropoietin and sildenafil against ischemia/reperfusion injury of the testis in adult rats. Both drugs, when administered intraperitoneally 30 min before detorsion, ameliorated significantly the deleterious effects of torsion and detorsion in the affected testis. This was confirmed by the lower grades of histological damage in the treated groups. Specifically, the seminiferous tubule borders were better defined and the presence of apoptotic and/or necrotic germinal cells was rarer in comparison with the control group. This suggests that erythropoietin and sildenafil protect the testis from the ischemia and reduce the effects of the reactive oxygen species generated by its reperfusion. We also went a step further by comparing the actions of these two drugs. Sildenafil exhibited better results (lower grades), corresponding to milder histological lesions, in comparison with erythropoietin. This suggests that sildenafil may protect the testis against ischemia/reperfusion injury better than erythropoietin although there is a possibility that the effects of erythropoietin (1,000 IU/kg) may not have been maximized [18].
In conclusion, both erythropoietin and sildenafil protect against ischemia/reperfusion injury after testicular torsion and detorsion. It seems that when these two drugs are administered intraperitoneally before detorsion, the testis is less affected by the ischemia and the deleterious effects of the reactive oxygen species generated by its reperfusion. However, there are several methodological variations in studies conducted on the effect of erythropoietin and sildenafil after ischemia/reperfusion injury of the testis regarding issues such as the dose of drug administration and the assessment of the drug administration timing. The exact dose and the administration timing for providing maximal effects of erythropoietin and sildenafil as individual treatments have not been clarified yet. It appears that sildenafil may have a stronger action than erythropoietin at the doses tested in this study. Future studies could concentrate on these aspects.
References
Kapoor S (2008) Testicular torsion: a race against time. Int J Clin Pract 62:821–827. doi:10.1111/j.1742-1241.2008.01727.x
Gatti JM, Patrick Murphy J (2007) Current management of the acute scrotum. Semin Pediatr Surg 16:58–63
Ringdahl E, Teague L (2006) Testicular torsion. Am Fam Physician 74:1739–1743
Turner TT, Bang HJ, Lysiak JL (2004) The molecular pathology of experimental testicular torsion suggests adjunct therapy to surgical repair. J Urol 172:2574–2578
Altavilla D, Romeo C, Squadrito F, Marini H, Morgia G, Antonuccio P, Minutoli L (2012) Molecular pathways involved in the early and late damage induced by testis ischemia: evidence for a rational pharmacological modulation. Curr Med Chem 19:1219–1224
Mellick LB (2012) Torsion of the testicle: it is time to stop tossing the dice. Pediatr Emerg Care 28:80–86. doi:10.1097/PEC.0b013e31823f5ed9
Reyes JG, Farias JG, Henríquez-Olavarrieta S, Madrid E, Parraga M, Zepeda AB, Moreno RD (2012) The hypoxic testicle: physiology and pathophysiology. Oxid Med Cell Longev 2012:929285. doi:10.1155/2012/929285.Epub2012Sep27
Filho DW, Torres MA, Bordin AL, Crezcynski-Pasa TB, Boveris A (2004) Spermatic torsion, reactive oxygen and nitrogen species and ischemia-reperfusion injury. Mol Aspects Med 25:199–210
Lysiak JJ (2004) The role of tumor necrosis factor-alpha and interleukin-1 in the mammalian testis and their involvement in testicular torsion and autoimmune orchitis. Reprod Biol Endocrinol 2:9
Paschos N, Lykissas MG, Beris AE (2008) The role of erythropoietin as an inhibitor of tissue ischemia. Int J Biol Sci 4:161–168
Kukreja RC, Salloum F, Das A, Ockaili R, Yin C, Bremer YA, Fisher PW, Wittkamp M, Hawkins J, Chou E, Kukreja AK, Wang X, Marwaha VR, Xi L (2005) Pharmacological preconditioning with sildenafil: basic mechanisms and clinical implications. Vascul Pharmacol 42:219–232
Köseoğlu B, Yilmaz E, Ceylan K, Uzun E, Bayram I, Hizli F (2009) The protective effect of erythropoietin infusion on testicular torsion/detorsion: an experimental study. Int Urol Nephrol 41:85–91. doi:10.1007/s11255-008-9418-8
Beheshtian A, Salmasi AH, Payabvash S, Kiumehr S, Ghazinezami B, Rahimpour S, Tavangar SM, Dehpour AR (2008) Protective effects of sildenafil administration on testicular torsion/detorsion damage in rats. World J Urol 26:197–202. doi:10.1007/s00345-008-0243-6
Yıldız H, Durmus AS, Şimşek H, Yaman M (2012) Dose-dependent protective effect of sildenafil citrate on testicular injury after torsion/detorsion in rats. Andrologia 44:300–306. doi:10.1111/j.1439-0272.2011.01181.x
Yildiz H, Durmuş AS, Şimşek H, Yaman İ (2011) Effects of sildenafil citrate on torsion/detorsion-induced changes in red blood cell and plasma lipid peroxidation, antioxidants, and blood hematology of male rats. Eur J Obstet Gynecol Reprod Biol 159:359–363. doi:10.1016/j.ejogrb.2011.07.023
Yíldíz H, Durmus AS, Simşek H, Yaman M (2011) Protective effect of sildenafil citrate on contralateral testis injury after unilateral testicular torsion/detorsion. Clinics (Sao Paulo) 66:137–142
Cosentino MJ, Nishida M, Rabinowitz R, Cockett AT (1985) Histological changes occurring in the contralateral testes of prepubertal rats subjected to various durations of unilateral spermatic cord torsion. J Urol 133:906–911
Yazihan N, Ataoglu H, Koku N, Erdemli E, Sargin AK (2007) Protective role of erythropoietin during testicular torsion of the rats. World J Urol 25:531–536
Akcora B, Altug ME, Kontas T, Atik E (2007) The protective effect of darbepoetin alfa on experimental testicular torsion and detorsion injury. Int J Urol 14:846–850
Ergur BU, Kiray M, Pekcetin C, Bagriyanik HA, Erbil G (2008) Protective effect of erythropoietin pretreatment in testicular ischemia-reperfusion injury in rats. J Pediatr Surg 43:722–728. doi:10.1016/j.jpedsurg.2007.11.028
Rashed FK, Ghasemi B, Deldade Mogaddam H, Mesgari M (2013) The effect of erythropoietin on ischemia/reperfusion injury after testicular torsion/detorsion: a randomized experimental study. ISRN Urol 2013:351309. doi:10.1155/2013/351309
Halawa AM (2010) Effect of sildenafil administration on ischemia/reperfusion of the testis in adult Albino rat light and electron microscopic study. Egypt J Histol 33:380–395
Istanbulluoglu MO, Zor M, Celik A, Cicek T, Basal S, Ozgok A, Ustun H, Ozgok Y (2011) Effects of vardenafil on testicular torsion/detorsion damage: an experimental study in pigs. Urol Int 86:228–232. doi:10.1159/000321492
Erol B, Tokgoz H, Hanci V, Bektas S, Akduman B, Yencilek F, Mungan G, Mungan A (2009) Vardenafil reduces testicular damage following ischemia/reperfusion injury in rats. Kaohsiung J Med Sci 25:374–380. doi:10.1016/S1607-551X(09)70530-3
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Nick Zavras and Ioannis D. Kostakis have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zavras, N., Kostakis, I.D., Sakellariou, S. et al. Comparison of erythropoietin and sildenafil protective role against ischemia/reperfusion injury of the testis in adult rats. Int Urol Nephrol 46, 731–736 (2014). https://doi.org/10.1007/s11255-013-0569-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-013-0569-x