Abstract
Testicular torsion is an important clinical urgency. Similar mechanisms occurred after detorsion of the affected testis as in the ischemia reperfusion (I/R) damage. This study was designed to investigate the effects of erythropoietin (EPO) treatment after unilateral testicular torsion. Fifty male Sprague-Dawley rats were divided into five groups. Group 1 underwent a sham operation of the right testis under general anesthesia. Group 2 was same as sham, and EPO (3,000 IU/kg) infused i.p., group 3 underwent a similar operation but the right testis was rotated 720° clockwise for 1 h, maintained by fixing the testis to the scrotum, and saline infused during the procedure. Group 4 underwent similar torsion but EPO was infused half an hour before the detorsion procedure, and in group 5, EPO was infused after detorsion procedure. Four hours after detorsion, ipsilateral and contralateral testes were taken out for evaluation. Treatment with EPO improved testicular structures in the ipsilateral testis but improvement was less in the contralateral testis histologically, but EPO treatment decreased germ cell apoptosis in both testes following testicular IR. TNF-α, IL-1β, IL-6 and nitrite levels decreased after EPO treatment especially in the ipsilateral testis. We conclude that testicular I/R causes an increase in germ cell apoptosis both in the ipsilateral and contralateral testes. Eryhropoietin has antiapoptotic and anti-inflammatory effects following testicular torsion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Testicular torsion is the twisting of the spermatic cord, which cuts off the blood supply to the testicle and surrounding structures within the scrotum. Surgery is usually required and should be performed as soon as possible after symptoms begin. If surgery is performed within hours, most testicles can be saved. But usually there is an increased risk of decreased sperm production and fertility, atrophy of testis [1, 2]. Many mechanisms like in the ischemia reperfusion (I/R) injuries, reactive oxygen species, activation of inflammatory cytokines etc. have been implicated in the testicular damage following torsion and detorsion process [3, 4]. Experimental testicular torsion models showed that it induces apoptosis and damage in the ipsilateral and contralateral testes [5].
Erythropoietin (EPO) is produced by the kidney in response to hypoxia and takes part in hematopoiesis [6]. EPO and its functional receptor (EPO-R) have been shown to be present in tissues outside the blood, including the brain, heart, gastrointestinal system, lung, testis, Leydig cells, suggesting potential roles of EPO beyond hematopoiesis and the treatment of anemia [7, 8]. It has been observed that EPO influences rat Leydig cell steroidogenesis by stimulating testosterone production and in humans, intravenous EPO administration increases testosterone production during renal failure [9]. Recent studies showed that EPO has anti-apoptotic, anti-inflammatory, antioxidant effects against I/R injuries of different tissues [10, 11]. Influence of EPO treatment during testicular torsion is not defined yet, in this study we made a testicular torsion model on rats and tried to identify effects of EPO treatment before detorsion and after detorsion procedure. Testicular TNF-α, IL-1β, IL-6 and nitrite levels were evaluated.
Materials and methods
Drugs
EPO (rHuEPO; Eprex, 4000 iu/flakon, Jansen-CILAG) was used in the experiment. Fifty adult male Sprague-Dawley rats (250–350 g) were used for torsion model. All animals were treated humanely and in compliance with the recommendations of the Animal Care Committee of our university and the principles of laboratory animal care of Helsinki by the permission of Ankara University Faculty of Medicine Ethics Committee. The rats were housed in a temperature-controlled room (22 ± 1°C) on a 12-h light and dark cycle, with free access to food and water.
The rats were anesthetized with pentobarbital (50 mg/kg) and placed on temperature controlled surgery tables. The body temperature was kept at 36 ± 0.5°C during the surgical procedures. Under aseptic conditions, surgical procedures were performed through a midline incision just above the testis. Sham and sham-EPO group were designed, respectively, as group 1 and 2. In group 3, which served as the ischemia reperfusion group, the animals were subjected to unilateral testicular torsion by rotating the right testis 720° in a clockwise direction. Then, this testis was maintained in the torsion position by fixing with a silk suture to the scrotal wall for 60 min. Then detorsion procedure was applied and reperfusion was maintained for 4 h. In group 4, EPO was given 30 min before detorsion; in group 5, it was given 30 min after detorsion procedure. EPO is given as 3,000 IU/kg i.p. according to group procedures. Both ipsilateral and contralateral testes were removed from the rats at the end of the experimental periods and tissue caspase-3, TNF-α, IL-1β, IL-6 and nitrite levels were measured. Histopathologic evaluations of testes were done.
Caspase-3 levels
Protein content of homogenates was determined with the Bradford method. Then 100 μg of proteins was diluted with assay buffer (50 mM HEPES, pH 7.4, 100 mM NaCl, 0.1% CHAPS, 10 mM DTT, 2 mM EDTA, 2 mM EGTA, Triton X-100, 0.1%) and incubated at 25°C with the colorimetric substrates (sigma): Ac-DEVD-pNA, Ac-YVAD-pNA, IETD-pNA, or LEHD-pNA. The final concentration was 200 μM for all substrates in 96-well microtiter plates. Cleavage of the p-nitroaniline (pNA) dye from the peptide substrate was determined by the measurement of absorbance of pNA at 405 nm in a microplate reader. The results were calibrated with known concentrations of p-NA and expressed in pm substrate cleaved/minute and per microgram protein at 25°C.
Measurement of nitrite
Nitrite was quantitated in testis tissue by using the diazotization method which depends on the diazotization of nitrite with an aromatic amine (sulphonamide) and formation of a colorful azo derivative with N-(1-Naftil) etilendiamine (Griess reaction). The tissue samples were homogenized and centrifuged in 0.1 M phoshate buffered saline pH 7.4 at 10,000 rcf for 10 min. Absorbance of the supernatants was measured spectrophotometrically at 550 nm wave length [12].
Measurement of Cytokine levels
Protein extracts are prepared from fresh tissue samples in Brij 150 lysis solution (tris 1 M, EDTA 0.5 M, NaCl 5 M, 10% brij 96, 10% NP40 in H2O) which contains a coctail of protease inhibitors aprotinin, leupeptine, pepstatine, PMSF (2 μg/ml each). Lysis solution was added as two folds of tissue weight and homogenised using homogenizer (Fisher Scientific)
Tissue supernatants were analyzed for cytokine levels according to kit procedures for TNF-α, IL-1β, IL-6 in triplicate by ELISA (Cytolab/PeproTech, Israel).
Histopathological examination
To obtain the testis to be sectioned for histopathology, rats were killed 4 h after reperfusion of the testis. The testes were removed and the specimens were fixed in Bouin’s fixative for 24 h and subsequently paraffin embedded. Five micrometer sections were cut and stained with H&E. They were photographed by Zeiss Axioscope Photomicroscope. Bilateral testis tissues were examined for histopathological features. Acute cell injury, apoptosis and edema development were evaluated for all groups.
Statistical analysis
Results are expressed as means ± SD. Statistical analysis was performed using one-way ANOVA and Tukey multiple range tests for comparison of different groups. The variance analyses using unpaired and paired Student’s t-test were used for comparison of the ipsilateral and contralateral testis cytokine results. Correlation between variables was assessed by calculating the parametric Pearson’s correlation coefficient (SPSS 10.0). P < 0.05 was accepted as statistically significant.
Results
Histopathological examinations
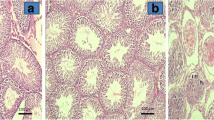
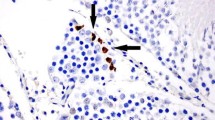
Histomorphological examination of groups, showed that the group 1 and 2 had the thickest seminiferous epithelium with Sertoli cells and orderly spermatogenic cell lineages. The region between the seminiferous tubules are occupied by richly vascularized loose connective tissue and interstitial cells (Fig. 1a, b, c). Group 2 showed minimal intertubular edema and inflammation especially at the ipsilateral testis (Fig. 1d). Testis from I/R (group 3) group and contralateral testis had extensively high-level intertubular edema, extravasation and inflammation (Fig. 1e, f). The tortioned testis and the contralateral testis showed two conspicuous features, first had empty vacuolar spaces between Sertoli cells and germ cells in multilayered seminiferous epithelium and the other was apoptotic cells. Type A spermatogonia showed intact morphology but Sertoli cells, some primary spermatocytes and round spermatids showed apoptotic signs that were identified by nuclear and cytoplasmic condensation. Basal membrane morphology of seminiferous tubules was destroyed by I/R injury and blood cells invaded intraepithelial spaces (Fig. 2a, b).
Seminiferous Tubules sections in all tortioned testes (ipsilateral) and other (contralateral) testes groups. Arrow head apoptosis, arrow vacuolization, A A Spermatogonium, asteriks oedoma and inflammation (Fig. 1 a, b, e, f H&E, X50; c, d, g, h, i, j (H&E, X250)
Vacuolization in seminiferous epithelium and apoptosis decreased in group 4 when compared with the I/R group. Intertubular edema and extravasation were minimal (Fig. 1g).
In group 5, intraepithelial vacuolization and intertubular edema were mild but apoptosis was significantly decreased (Fig. 1i). In groups of 4 and 5 intraepithelial vacuolization and intertubular edema were also high but less than contralateral testis of the I/R group (Fig. 1h, j).
Caspase-3 levels
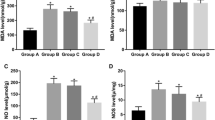
Apoptosis was evaluated by caspase-3 levels. As shown in Fig. 3, caspase-3 levels increased after I/R in both of the testes. EPO treatment during IR procedure significantly decreased apoptosis of testes compared to IR group (P < 0.001).
Tissue nitrite levels
The nitrite levels of both ipsilateral and contralateral testes in the IR group increased significantly (P < 0.001; Fig. 4). EPO treatment decreased nitrite production after detorsion procedure.
Cytokine levels
Significant increase was seen in TNF-α, IL-1β, IL-6 protein levels of ipsilateral torsion–detorsion group testis when compared to other groups (P < 0.001; Table 1).
TNF-α, IL-1β, IL-6 protein levels also increased in the contralateral testis of the I/R group. EPO treatment decreased cytokine production especially in the ipsilateral testis. There was no difference between different time applications of EPO.
Testis tissue TNF-α and IL-1β levels were correlated with nitrite levels and also with caspase-3 levels (P < 0.001).
Discussion
Testicular torsion is a very common medical emergency. Most of the cases result in infertility. Mechanism of the damages after testicular torsion is similar to ischemia reperfusion injury [2]. Ischemia reperfusion of rat testis results in production of reactive oxygen species, stimulation of proinflammatory cytokines, secretion of TNF-α and IL-1β, activation of nitric oxide synthase, apoptosis, testicular atrophy and decreased spermatogenesis [3, 4, 13]. In this study we studied whether EPO treatment is effective during testicular torsion and tried to find out correct application time of treatment. For this purpose we examined apoptosis levels and measured proinflammatory cytokine levels of IR damaged testis tissues without and with EPO treatment.
In this study, apoptosis, confirmed by levels of caspase-3. Caspase-3 levels increased after detorsion procedure in both testes of untreated group. There are multiple factors that are known to induce testicular apoptosis during testicular torsion. Neutrophils and macrophages recruited during reperfusion have been implicated as mediators of parenchymal injury, and because of their ability to release a variety of toxic materials such as oxygen-free radicals and inflammatory cytokines, can induce testicular cell apoptosis. After extravasation of neutrophils, the increased number of activated leukocytes can release a lot of proinflammatory cytokines, oxidative substances and bioactive molecules that lead to damage. Also damaged spermatozoa can trigger further oxidative damage and cause expansion of damage and apoptosis [3, 4]. In our study, extravasation and edema were prominent in the I/R group testes. EPO is an endogenous molecule, and has been known to reduce cellular infiltrations in inflammatory conditions such as various models of inflammation and has antiinflammatory, antioxidant and antiapoptotic effects during ischemic injuries of kidney, gastrointestinal systems etc. [10, 11, 13].
The increased levels of these cytokines are an indirect measurement of tissue inflammation. Secretion of TNF-α and IL-1β act as proinflammatory cytokines that induce IL-6 production and activate stress-related intracellular and extracellular pathways [14]. It was shown that these cytokines were produced from testicular cells as well as activated interstitial macrophages [15, 16]. Especially TNF-α is a potent modulator of normal and pathological apoptosis. TNF-α receptor and TRAIL receptors activate a cascade leading to apoptosis [14]. Similar to TNF-α, IL-1β production activated upon appropriate extracellular stimuli causes activation of inflammatory pathways. Both of them are important for regulation of other cytokines, chemokines, adhesion molecules, growth factors and inducible enzymes. IL-6 is an acute phase reactant, enhances monocytes and macrophages functions after stimulation. But it also has a modulatory effect on the TNF-α and IL-1β production [17]. The results of our experiment showed that TNF-α, IL-1β, IL-6 levels increased after I/R of the testis and levels of these cytokines decreased after EPO treatment without depending on the application time. We found that EPO has anti-inflammatory and antiapoptotic effects during testicular torsion.
Nitric oxide has a very short life span. In this study we measured nitrite that is stable end product of nitric oxide. Many cell types such as endothelial cells, phagocytic cells, hepatocytes as well as sertoli cells are capable of NO release [18]. NO can easily react with the other radicals especially with superoxide ion and produce very harmfull substance peroxynitrite. Peroxynitrite reacts with oxidative enzymes, thiol esters and glutathione. Nitrating agents may have strong inhibitory potential on the spermatozoa mitochondrial functions [19]. Nitrite production was found to be increased as a response to increased cytokine production during inflammatory and pathological conditions. NO especially via iNOS, can activate many inducible intracellular pathways like NFkb which leads to cellular apoptosis. Inhibitors of nitric oxide synthase and reactive oxygen radicals have been shown to protect tissues from ischemia reperfusion induced damage [20–22]. Nitric oxide plays an important role during testicular-torsion-induced damage and has a vasodilatory effect also [23]. In our study we found that the testes of the I/R group had a high level of intertubular edema, extravasation and inflammation which was correlated with high levels of nitrite and proinflammatory cytokines like TNF-α and IL-1β. Also in the same group nitrite levels were significantly higher than the sham-operated group. The high nitrite levels may have been a result of the inflammation of testis tissue. This finding was supported by the histopathological appearance of same tissues. Acute or chronic infection and inflammation of the genitourinary tract usually ends with infertility. EPO treatment decreased nitrite and proinflammatory cytokine production from both testes.
It has been shown that unilateral testicular torsion may have an adverse effect on the contralateral testis; which has been previously explained as immunologic and oxidative mechanisms [5]. Although still little is known about mechanisms of the effect of ischemia on apoptosis of the contralateral testes. Our data demonstrate that unilateral testicular ischemia/reperfusion produces prominent contralateral damage, which includes a decrease in the number of germ cells, edema, disorganization, increased apoptosis. We found that inflammatory cytokine levels of the contralateral testes were higher than ipsilateral testis. But it was interesting to note that the protective effect of EPO treatment was more prominent at the ipsilateral testis although EPO treatment was applied systemically.
As a conclusion, in the present study, we have strong evidence that EPO is a potential protective agent against acute testicular damage after testis torsion. Further studies are needed for explaining the mechanisms of protection.
References
Horica CA, Hadziselimovic F, Kreutz G, Bandhauer K (1982) Ultrastructural studies of the contorted and contralateral testicle in unilateral testicular torsion. Eur Urol 8(6):358–362
Thomas WE, Williamson RC (1983) Diagnosis and outcome of testicular torsion. Br J Surg 70(4):213–216
Filho DW, Torres MA, Bordin AL, Crezcynski-Pasa TB, Boveris A (2004) Spermatic cord torsion, reactive oxygen and nitrogen species and ischemia-reperfusion injury. Mol Aspects Med 25(1–2):199–210
Lysiak JJ (2004) The role of tumor necrosis factor-alpha and interleukin-1 in the mammalian testis and their involvement in testicular torsion and autoimmune orchitis. Reprod Biol Endocrinol 10(2):9
Akgur FM, Kilinc K, Tanyel FC, Buyukpamukcu N, Hicsonmez A (1994) Ipsilateral and contralateral testicular biochemical acute changes after unilateral testicular torsion and detorsion. Urology 44(3):413–418
Lacombe C, Mayeux P (1999) The molecular biology of erythropoietin. Nephrol Dial Transplant 14(suppl. 2):22–28
Chikuma M, Masuda S, Kobayashi T, Nagao M, Sasaki R (2000) Tissue- spesific regulation of erythropoietin production in the murine kidney,brain and uterus. Am J Physiol Endocrinol Metab 279:E1242–E1248
Magnanti M, Gandini O, Giuliani L, Gazzaniga P, Marti HH, Gradilone A, Frati L, Agliano AM, Gassmann M (2001) Erythropoietin expression in primary rat Sertoli and peritubular myoid cells. Blood 1 98(9):2872–2874
Yamamoto Y, Sofikitis N, Miyagawa I (1997) Effects of erythropoietin, bromocryptine and hydralazine on testicular function in rats with chronic renal failure. Andrologia 29(3):141–144
Sepodes B, Maio R, Pinto R, Sharples E, Oliveira P, McDonald M, Yaqoob M, Thiemermann C, Mota-Filipe H (2006) Recombinant human erythropoietin protects the liver from hepatic ischemia-reperfusion injury in the rat. Transpl Int 19(11):919–926
Liu X, Shen J, Jin Y, Duan M, Xu J (2006) Recombinant human erythropoietin (rhEPO) preconditioning on nuclear factor-kappa B (NF-kB) activation & proinflammatory cytokines induced by myocardial ischaemia-reperfusion. Indian J Med Res 124(3):343–354
Tunctan B, Abacioglu N (1998) J Pharm Sci 23:161–170
Johnson DW, Pat B, Vesey DA, Guan Z, Endre Z, Gobe GC (2006) Delayed administration of darbepoetin or erythropoietin protects against ischemic acute renal injury and failure. Kidney Int 69(10):1806–1813
Spierings DC, de Vries EG, Vellenga E, van den Heuvel FA, Koornstra JJ, Wesseling J, Hollema H, de Jong S (2004) Tissue distribution of the death ligand TRAIL and its receptors. J Histochem Cytochem 52(6):821–831
Grataroli R, Vindrieux D, Gougeon A, Benahmed M (2002) Expression of tumor necrosis factor-alpha-related apoptosis-inducing ligand and its receptors in rat testis during development. Biol Reprod 66(6):1707–1715
Rival C, Theas MS, Guazzone VA, Lustig L (2006) Interleukin-6 and IL-6 receptor cell expression in testis of rats with autoimmune orchitis. J Reprod Immunol 70(1–2):43–58
Zoja C, Wang JM, Bettoni S, Sironi M, Renzi D, Chiaffarino F, Abboud HE, Van Damme J, Mantovani A, Remuzzi G et al (1991) Interleukin-1 beta and tumor necrosis factor-alpha induce gene expression and production of leukocyte chemotactic factors, colony-stimulating factors, and interleukin-6 in human mesangial cells. Am J Pathol 138(4):991–1003
Ishikawa T, Morris PL (2006) Interleukin-1beta signals through a c-Jun N-terminal kinase-dependent inducible nitric oxide synthase and nitric oxide production pathway in Sertoli epithelial cells. Endocrinology 147(11):5424–5430
Vera Y, Erkkila K, Wang C, Nunez C, Kyttanen S, Lue Y, Dunkel L, Swerdloff RS, Sinha Hikim AP (2006) Involvement of p38 mitogen-activated protein kinase and inducible nitric oxide synthase in apoptotic signaling of murine and human male germ cells after hormone deprivation. Mol Endocrinol 20(7):1597–1609
Basar MM, Kisa U, Tuglu D, Yilmaz E, Basar H, Caglayan O, Batislam E (2006) Testicular nitric oxide and thiobarbituric acid reactive substances levels in obstructive azoospermia: a possible role in pathophysiology of infertility. Mediators Inflamm 3:27458
Reddy MM, Mahipal SV, Subhashini J, Reddy MC, Roy KR, Reddy GV, Reddy PR, Reddanna P (2006) Bacterial lipopolysaccharide-induced oxidative stress in the impairment of steroidogenesis and spermatogenesis in rats. Reprod Toxicol 22(3):493–500
Meroni SB, Subuo AM, Cigorraga SB (2000) Interleukin-1beta regulates nitric oxide production and gamma-glutamyl transpeptidase activity in sertoli cells. Androl 21(6):855–861
Ozokutan BH, Kucukaydin M, Muhtaroglu S, Tekin Y (2000) The role of nitric oxide in testicular ischemia-reperfusion injury. J Pediatr Surg 35(1):101–103
Acknowledgments
This study was supported by TUBITAK (The Scientific and Technological Research Council of Turkey) project no: 104S412 (SBAG-AYD-487).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yazihan, N., Ataoglu, H., Koku, N. et al. Protective role of erythropoietin during testicular torsion of the rats. World J Urol 25, 531–536 (2007). https://doi.org/10.1007/s00345-007-0200-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-007-0200-9