Abstract
Background
Glomerular hyperfiltration commonly associated with obesity is expected to improve postbariatric surgery. However, formula-based glomerular filtration rate (GFR) estimation in these patients is limited by body size confounders necessitating use of modified equations, the reliability of which remains uncertain.
Methods
In this study, various GFR-estimating formulae were compared in morbidly obese patients at baseline and postbariatric surgery. Through a retrospective chart review, we identified 220 patients who had undergone this procedure, with over 6-month follow-up, during which major weight reduction was achieved.
Results
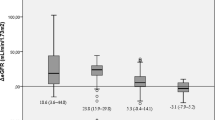
A significant decrease in BP and glomerular hyperfiltration was observed, though there was large variability in GFR estimation using the different formulae. Gross over and underestimation was observed which improved with correction for body size confounders especially lean body weight (LBW). Postoperatively, significant attenuation in estimated GFR was demonstrated when LBW or body surface area-adjusted versions were used. In a subgroup of patients with chronic kidney disease, a significant improvement in GFR was seen postoperatively with the LBW-modified formula but there were again inconsistencies when using other equations.
Conclusion
Though clinicians must be critical in the application of GFR estimates to patient care, LBW adjustment appears to be the most practical solution to its estimation in the obese patients. This is particularly true for patients with normal renal function but appears to be also applicable to those with compromised kidney function. Future studies are needed to compare these equations with a gold standard GFR measure as well as to explore whether the renal benefits from bariatric surgery are sustained or seen in more advanced CKD stages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well documented that obesity is associated with increased renal plasma flow and glomerular hyperfiltration [1–5]. This predisposes to micro- and macroalbuminuria in obese patients which is the hallmark of chronic kidney disease [6–10]. Some of the proposed mechanisms include increased angiotensin II levels produced by adipose tissue with its well-known effects on sodium retention and glomerular and systemic hemodynamics [4, 11, 12]. Additionally, hyperlipidemia and hyperinsulinemia, which are commonly coexistent in obese individuals as well as cytokines produced by adipocytes referred to as adipocytokines, all could contribute to hyperfiltration [4, 13, 14].
The quantitation of renal function to uncover hyperfiltration is clinically important [15], but its assessment via standard 24-h collection or nuclear studies is cumbersome or costly; hence, we rely on estimated glomerular filtration rate (GFR) calculated from various equations. Though each of these formulae has its own limitations, they can generally be used fairly reliably in the normal population [16, 17]. In the obese patients, however, assessing GFR is controversial as none of the standard formulae are reliable and require correction for body weight confounders [18]. For instance, the Cockcroft–Gault equation has ideal body weight as an integral component of the calculation, hence the difficulties with its estimation in the obese [19]. MDRD and CKD-Epi, on the other hand, have the tendency to underestimate GFR in obese individuals [20, 21] and thus require correction for body surface area. Recently, a new version of the Cockcroft–Gault formula has come on the horizon which corrects for lean body weight (CG-LBW) and is proposed to overcome some of these limitations to GFR estimation in the obese patients [22, 23].
More importantly, with the advent of bariatric surgery, significant benefits have been observed in controlling the cardiovascular risks and metabolic disorders of obese patients following weight reduction [24–26]. Nevertheless, its effect on renal parameters is not yet well determined. It is anticipated, however, that these would similarly follow suit with an attenuation of the hyperfiltration state and consequent reduction in albumin and protein excretion [27, 28]. This again underscores the importance of GFR measurement and the reassessment of its course over time.
This study aimed to assess the impact of bariatric surgery on renal function in morbidly obese patients. Specifically, the study intended to compare the different GFR-estimating formulae in obese individuals at baseline and 6 months after weight reduction surgery. This was analyzed in patients with normal renal function as well as a subgroup with mild renal impairment.
Subjects and methods
A retrospective chart review of 385 patients who had undergone bariatric surgery at Tawam Hospital between 2005 and 2010 was undertaken. Two hundred and twenty adult patients were included in the study, all of whom had at least 6-month follow-up. The rest excluded due to incomplete medical records, loss to follow-up, advanced CKD, chronic nephrotoxic medication use, underlying chronic illness or malignancy. Demographic and anthropometric data were extracted, as well as BP measures, serum creatinine and urinary parameters including albuminuria and proteinuria (where available), at baseline and 6 months postsurgery. Creatinine clearance was calculated as estimated GFR (eGFR) using the following formulae all with the appropriate correction for body size confounders: MDRD4 (IDMS), CKD-Epi equations, and their body surface area (BSA)-adjusted versions, Cockcroft–Gault (CG) and Cockcroft–Gault lean body weight-adjusted formula (CG-LBW). For the subgroup of patients with chronic kidney disease (CKD I–II), eGFR was calculated using GC-LBW as well as CKD-Epi and its BSA-adjusted version, preoperatively and postoperatively.
Formulae used
where Scr is serum creatinine (mg/dl)
where Scr is serum creatinine (mg/dl), κ is 0.7 for women and 0.9 for men, α is −0.329 for women and −0.411 for men, min indicates the minimum of Scr/κ or 1, max indicates the maximum of Scr/κ or 1
The adjusted versions were modified to correct for actual body surface area rather than the standard 1.73 m2
Cockcroft–Gault
CG corrected for lean body weight (CG-LBW)
where lean body weight (LBW) in kg was calculated as follows:
Study population
Adult patients with morbid obesity (BMI > 30) and follow-up at least 6 months postbariatric surgery were included. The exclusion criteria were incomplete medical records, loss to follow-up, advanced CKD stages III–V with estimated GFR < 60 ml/min, chronic nephrotoxic medication use, underlying chronic illness or malignancy.
Statistical analysis
Variables with normal distribution were expressed as mean ± SD while non-parametric variables as median. Student’s t test was used for the comparison between 2 means and Pearson correlation to correlate different variables with ANOVA test used for multiple comparisons.
Results
About 145 female and 75 male patients with mean age of 34.7 ± 10 years underwent weight reduction surgery, with gastric banding in 45, gastric bypass in 59 and sleeve resection in the remaining 116 patients. We identified 45 patients with dyslipidemia, 41 with mild CKD, 25 hypertensives and 8 diabetics, 3 of whom were also hypertensive. Over mean follow-up of 7.2 ± 3 months, a significant weight reduction was achieved which was associated with a decrease in systolic and diastolic BP. Baseline and post-op data are shown in Table 1.
There was a significant drop in serum creatinine associated with weight loss, as well as a decrease in BMI and BSA postoperatively which correlated significantly with the decline in both adjusted CKD-Epi and MDRD as well as the CG-LBW (P < 0.001). This achieved statistical significance at BMI reduction of at least 40% (4.35 kg ml−2) postsurgery. The lean body weight, on the other hand, also decreased postoperatively but did not achieve statistical significance. Similarly however, it showed a significant correlation with CG-LBW and adjusted versions of CKD and MDRD equations (P < 0.001).
Subgroup analysis
From 220 patients, 41 were found to have mild CKD with pre-op eGFR 60−90 ml/min. Forty patients were women and only one man with mean age of 45.15 ± 8 years. Seven patients had diabetes and another 7 were hypertensive. After mean follow-up of 7.2 ± 3 months, a significant decrease in weight, BMI and BP was achieved. Baseline and post-op data are shown in Table 2.
There was a significant increase in GFR estimation postoperatively using CG-LBW formula which was also apparent with the CKD-Epi but disappeared when corrected for BSA.
Discussion
Hyperfiltration is well described in obese individuals, typically defined as eGFR > 120 ml/min, and has been associated with higher risk of mortality and ESRD [29] hence the importance of its quantitation. In normal weight individuals, various equations are available to estimate GFR, and despite each having its own limitations, they can generally be used fairly reliably [16, 17]. In the obese patients, however, assessing GFR using the standard formulae is not reliable and requires correction for body weight confounders [18]. These include lean body weight, ideal body weight, body surface area, body mass index, fat-free mass, percent ideal body weight, adjusted body weight and predicted normal body weight [30]. Though any of these can be used for body weight correction, there is considerable variation in GFR estimation using the different formulae [18–20]. The fat-free mass, lean body weight and body surface area appear to be the main parameters requiring correction and might serve as surrogates for ideal body weight in the obese patients. Though CDK-Epi has been proposed as the most accurate method for estimating GFR for diverse populations [31], lean body weight (LBW) has recently emerged as a preferred adjustment confounder for total body weight [30] and might hence overcome some of the limitations to GFR estimation [22, 23]. This is used with a modified version of the Cockcroft–Gault formula namely CG-LBW.
In our study, the correlation in eGFR between the BSA corrected versions of CKD-EPi and MDRD was compared to that obtained with CG-LBW estimation.
The expected supernormal GFR was apparent preoperatively, in estimators which took into account body size descriptors. This was grossly overestimated by CG but was attenuated, though still supranormal, with adjustment for lean body weight. Conversely, MDRD and CKD-Epi equations failed to show the expected hyperfiltration which subsequently became manifest with BSA correction. A strong correlation was observed for measurements obtained using both the adjusted MDRD and CKD-Epi and those with CG-LBW. These might therefore be considered as alternative options given their less cumbersome calculation.
The performance of each of these GFR estimators was compared 6 months following bariatric surgery since it is well documented that in addition to achieving effective weight loss and improvement in BP [27, 28, 31], it might also decrease glomerular hyperfiltration [28] and improve renal function [32, 33]. Not surprisingly, postoperatively, significant attenuation in eGFR was observed using the adjusted BSA versions which was also seen with the CG-LBW formula. In all three estimators, there was a decline in GFR to levels below hyperfiltration cutoffs. Interestingly, the correlation between BMI and GFR started to become apparent at BMI reduction of 40% from its baseline pre-op value suggesting a weight loss threshold.
In the subgroup analysis of patients with early chronic kidney disease, using CG-LBW formula, a significant increase in eGFR was seen after bariatric surgery as shown by recent studies [32, 33]. This was in contrast to the decrease which occurred in those with hyperfiltration preoperatively. Improvement in BP and overall metabolic profile may be partly responsible; however, firm conclusions cannot be drawn due to likely confounding effects of changes in muscle mass and protein intake on serum creatinine as well as the small sample size and short duration of follow-up. Though similar changes were seen with CKD-Epi-estimated GFR, this is clearly unreliable without BSA correction. When adjusted, an overestimation of GFR was observed preoperatively with no significant change post-op which is counterintuitive. This suggests that the CG-LBW-adjusted formula is perhaps a better estimator in the absence of a gold standard measure.
It is important to keep in mind however that the observed decrease in serum creatinine postoperatively in both groups is likely related to loss of muscle mass in addition to low protein intake. This most certainly plays an important role in the observed GFR changes and may not necessarily reflect actual alterations in the underlying renal function. A gold standard GFR quantitator, such as 24-h creatinine clearance, radioisotope assessment or the newer renal biomarkers such as cystatin C, is needed to confirm our findings. Some other limitations of our study include its retrospective nature, relatively small sample size and short duration of follow-up. In addition, there was inadequate data for retrieval regarding baseline as well as postoperative clinical and laboratory results including urinary protein excretion levels.
Conclusions
The results of our study show that bariatric surgery for obese patients is effective in weight loss and BP reduction and results in favorable effects on eGFR. However, significant variation in GFR estimation in the obese patients exists between methods, which the clinician must be cognizant of and critical in its application to patient care. CG-LBW equation appears to be the most practical solution to its estimation in the obese patients. This is because lean body weight can be more reliably assessed as compared with BSA. However, since the adjusted MDRD and CKD-Epi correlated well with it, these can be considered as alternative options given their less cumbersome calculation. Future studies are needed to compare each of these equations with a gold standard GFR measure.
In the subgroup of patients with mild chronic kidney disease, using CG-LBW formula, a significant increase in eGFR was seen after bariatric surgery, perhaps attributed to improvement in BP and metabolic profile. Whether the renal benefits from bariatric surgery are real, sustained or seen in more advanced CKD stages is uncertain. Long-term studies are needed to explore this further. Nevertheless, in the absence of a gold standard for eGFR measurement, CG-LBW appears to be a reasonable estimator in obese patients with CKD.
References
Wuerzner G, Pruijm M, Maillard M et al (2010) Marked association between obesity and glomerular hyperfiltration: a cross-sectional study in an African population. No association between BMI categories and GFR was found with adjustment for body surface area. Am J Kidney Dis 56(2):303–312
Levey AS, Kramer H (2010) Obesity, glomerular hyperfiltration, and the surface area correction. Am J Kidney Dis 56(2):255–258
Henegar JR, Bigler SA, Henegar LK, Tyagi SC, Hall JE (2001) Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol 12(6):1211–1217
Chagnac A, Weinstein T, Korzets A, Ramadan E, Hirsch J, Gafter U (2000) Glomerular hemodynamics in severe obesity. Am J Physiol 278:F817–F822
Henegar JR, Bigler SA, Henegar LK, Tyagi SC, Hall JE (2001) Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephro 12:1211–1217
Toto RD, Greene T, Hebert LA, et al, AASK Collaborative Research Group (2010) Relationship between body mass index and proteinuria in hypertensive nephrosclerosis: results from the African American Study of Kidney Disease and Hypertension (AASK) cohor. Am J Kidney Dis 56(5):896–906
Chen J, Munter P, Hamm LL et al (2004) The metabolic syndrome and chronic kidney disease in US adults. Ann Intern Med 140:167–174
Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD (2001) Obesity-related glomerulopathy: an emerging epidemic. Kidney Int 59:1498–1509
Praga M, Hernandez E, Morales E et al (2001) Clinical features and long-term outcome of obesity-associated focal segmental glomerulosclerosis. Nephrol Dial Transplant 16:1790–1798
Adelman RD, Restaino IG, Alon US, Blowley DL (2001) Proteinuria and focal segmental glomerulosclerosis in severely obese adolescents. J Pediatr 138:481–485
Tuck ML, Sowers J, Dornfeld L, Kledzik G, Maxwell M (1981) The effect of weight reduction on blood pressure, plasma renin activity, and plasma aldosterone levels in obese patients. N Engl J Med 304:930–933
Engeli S, Bohnke J, Gorzelniak K et al (2005) Weight loss and the renin–angiotensin–aldosterone system. Hypertension 45:356–362
Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R (2005) Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation 111:1448–1454
Wisse BE (2004) The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol 5:2792–2800
National Kidney Foundation (2002) K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39:S1–266
Verhave JC, Fesler P, Ribstein J, du Cailar G, Mimran A (2005) Estimation of renal function in subjects with normal serum creatinine levels: influence of age and body mass index. Am J Kidney Dis 46(2):233–241
Schold JD, Navaneethan SD, Jolly SE et al (2009) Implications of the CKD-EPI GFR estimation equation in clinical practice. J Nephrol 22(3):373–380
Hudson JQ, Mason DL, Huch KM (2011) Estimates of kidney function in obese African Americans with chronic kidney disease. Nephron Clin Pract 118(2):c101–c108
Rigalleau V, Lasseur C, Perlemoine C et al (2006) Cockcroft-Gault formula is biased by body weight in diabetic patients with renal impairment. Metabolism 55(1):108–112
Nair S, Mishra V, Hayden K et al (2011) The four-variable modification of diet in renal disease formula underestimates glomerular filtration rate in obese type 2 diabetic individuals with chronic kidney disease. Diabetologia 54(6):1304–1307
Stevens LA, Coresh J, Feldman HI et al (2007) Evaluation of the modification of diet in renal disease study equation in large diverse population. J Am Soc Nephrol 18:2749–2757
Lim WH, Lim EM, McDonald S (2006) Lean body mass-adjusted Cockcroft and Gault formula improves the estimation of glomerular filtration rate in subjects with normal-range serum creatinine). Nephrology (Carlton) 11(3):250–256
Ozmen S, Kaplan MA, Kaya H et al (2009) Role of lean body mass for estimation of glomerular filtration rate in patients with chronic kidney disease with various body mass. Scand J Urol Nephrol 43(2):171–176
O’Brien PE, Dixon JB, Laurie C et al (2006) Treatment of mild to moderate obesity with laparoscopic adjustable gastric banding or an intensive medical program: a randomized trial. Ann Intern Med 144:625–633
Giusti V, Suter M, Héraïef E, Gaillard RC, Burckhardt P (2004) Effects of laparoscopic gastric banding on body composition, metabolic profile and nutritional status of obese women: 12-months follow-up. Obes Surg 14(2):239–245
Karason K, Wikstrand J, Sjöström L, Wendelhag I (1999) Weight loss and progression of early atherosclerosis in the carotid artery: a four-year controlled study of obese subjects. Int J Obes Relat Metab Disord 23(9):948–956
Buchwald H, Avidor Y, Braunwald E et al (2004) Bariatric surgery: a systematic review and meta-analysis. JAMA 292:1724–1737 Published erratum appears in JAMA 293:1728, 2005
Serpa Neto A, Bianco Rossi FM, Dal Moro Amarante R, Alves Buriti N, Cunha Barbosa Saheb G, Rossi M (2009) Effect of weight loss after Roux-en-Y gastric bypass, on renal function and blood pressure in morbidly obese patients. J Nephrol 22(5):637–646
Matsushita K, Selvin E, Bash LD, Astor BC, Coresh J (2010) Risk Implications of the New CKD Epidemiology Collaboration (CKD-EPI) equation compared with the MDRD Study equation for estimated GFR: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis 55(4):648–659
Green B, Duffull SB (2004) What is the best size descriptor to use for pharmacokinetic studies in the obese? Br J Clin Pharmacol 58(2):119–133
Levey AS, Stevens LA (2010) Estimating GFR using the CKD epidemiology collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis 55(4):622–627
Currie A, Chetwood A, Ahmed AR (2011) Bariatric surgery and renal function. Obes Surg 21(4):528–539
Navaneethan SD, Yehnert H, Moustarah F, Schreiber MJ, Schauer PR, Beddhu S (2009) Weight loss interventions in chronic kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol 4(10):1565–1574
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abouchacra, S., Chaaban, A., Gebran, N. et al. GFR estimation in the morbidly obese pre- and postbariatric surgery: one size does not fit all. Int Urol Nephrol 45, 157–162 (2013). https://doi.org/10.1007/s11255-012-0131-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-012-0131-2