Abstract
Background/aims
Anemia is common in patients with chronic kidney disease (CKD). Recently, the erythropoiesis-stimulating agent/hemoglobin level (ESA/Hb) index emerged as a new factor associated with increased morbidity and mortality in this population. In this study, we evaluated the factors that influence the ESA/Hb index in a pre-dialysis CKD population.
Methods
Ninety-five patients were evaluated for clinical and laboratory parameters, nutritional status and ESA/Hb index. For comparison, we divided our population into 3 groups: G I—no ESA treatment, G II—patients with ESA/index below 50th percentile and G III—patients with ESA/Hb index above 50th percentile. We performed single and multiple regression models and logistic regression analysis.
Results
In a multiple regression model, age (t = −3.456, P = 0.001), SGA (t = 2.059, P = 0.047), ferritin (t = 2.386, P = 0.027), Ca × P (t = 2.066, P = 0.043), TNF-α (t = 2.673, P = 0.009) and IL-6 (t = 2.939, P = 0.004) independently influenced the ESA/Hb index. At logistic regression analysis, gender, cardiovascular disease and TNF-α were independently associated with ESA/Hb higher than 50th percentile compared to the other patients (R 2 = 0.457).
Conclusion
In a pre-dialysis population, female gender, cardiovascular disease, malnutrition and inflammation are associated with a higher ESA/Hb index.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anemia is an almost universal finding in patients with CKD once the glomerular filtration rate (GFR) has fallen below 30 ml/min/1.73 m2 [1]. It is primarily due to insufficient production of erythropoietin (Epo) from diseased kidneys, and recombinant Epo has been shown to be useful in correction of anemia in patients with renal failure [2–4]. Anemia and its suboptimal correction have been associated with an increased prevalence of cardiovascular disease, which induces a higher morbidity and mortality in pre-dialysis patients, as well as in patients under renal replacement therapy [5–9]. In many patients with CKD, however, anemia seems to be resistant to ESA treatment despite adequate iron supplementation [10]. Several factors, such as hyperparathyroidism, aluminium intoxication, blood loss, hemoglobinopathies and hemolysis, are associated with ESA resistance [11]. Moreover, inflammation and malnutrition have been also described as factors that can be associated with anemia and can influence the ESA dose in patients with CKD [12–16]. On the other hand, recent studies indicated that the resistance to ESA [17, 18], evaluated by the ESA resistance index [19] is associated with a higher risk of death in renal patients [20].
The aim of our study was to evaluate the factors that influence the ESA/Hb index in a pre-dialysis CKD population.
Patients and methods
We included 95 patients from our outpatient “low-clearance” clinic at Serviço de Nefrologia of the Hospital de Faro, Algarve. Our Nephrology Unit, a tertiary center, is the only one in this most southern region of Portugal, the Algarve, with almost half a million inhabitants. The “low-clearance” clinic represents about 10% of our nephrology outpatient clinic.
Patients were referred whenever they had an estimated GFR (eGFR) below 30 ml/min/1.73 m2, from our own Nephrology Unit, from other Units of our Hospital or from general practitioners. During the 6-month recruitment period, patients who agreed to enter the study were included. This study was approved by our Hospital Ethics Committee, and informed consent was obtained from all patients.
At baseline, a complete clinical history and a physical examination were performed.
Fasting blood samples were collected to measure serum hemoglobin (Hb), albumin, creatinine, blood urea nitrogen (BUN), iron, ferritin, calcium (Ca), phosphate (P), parathyroid hormone (PTH), triglycerides and cholesterol (total and HDL).
The GFR was calculated according to the MDRD (Modification of Diet in Renal Disease) equation [21].
Plasma, collected using heparin as the anticoagulant, was separated within 30 min of drawing and stored at −80°C until measurements of IL-6 (interleukin 6) and TNF-α (tumor necrosis factor α) were performed. The Subjective Global Nutritional Assessment (SGA) was used to evaluate the nutritional status. We also evaluated anthropometric parameters like the body mass index (BMI).
We calculated the average weekly darbepoetin dose, as well as the average hemoglobin level during the follow-up period.
The weekly darbepoietin dose/kg body weight was first multiplied by 200 and then divided by the Hb level, to calculate the ESA/Hb index [19].
The diagnosis of ischemic heart disease was based on clinical data plus at least one of the following: electrocardiographic signs of ischemic disease or myocardial infarction, at rest; positive stress test for ischemic heart disease (treadmill test, stress echocardiogram or myocardial scintigraphy); coronary angiography with a luminal stenosis greater than 50% in one of the main coronary arteries.
Statistical analysis
First we performed descriptive statistics; values were expressed as mean ± standard deviation; for comparison between the three groups, we used the one-way analysis of variance (ANOVA); the chi-square test was used to investigate the distribution of categorical variables in the groups; we used the ESA/Hb index as the dependent variable and the several biological and laboratory parameters as independent ones in a single regression model. Only the parameters with a statistically significant relationship in the single regression model (P ≤ 0.05) were later introduced in a multiple regression model. We also calculated in a multiple binary logistic regression analysis the odds and their 95% confidence intervals for ESA/Hb index higher than the 50th percentile in comparison with the other patients.
The statistical analysis was performed using SPSS 11.0 for Windows (SPSS, Chicago, IL).The null hypothesis was rejected below the 5% level (P < 0.05).
Results
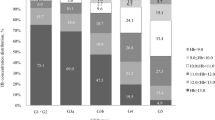
We included 95 patients (f = 41, m = 54), with an average age of 69.4 years, mean eGFR of 16.1 ml/min/1.73 m2 and a mean follow-up of 24.1 months. The original disease was unknown in 23 patients (24.2%); 30 patients (31.5%) had diabetic nephropathy, 19 patients (20%) had hypertensive renal disease, 15 patients (15.8%) had chronic interstitial disease, 5 (5.3%) had chronic glomerulonephritis and 3 (3.2%) had polycystic kidney disease.
The prevalence of ischemic heart disease in our population was 25.3% (n = 24).
Nutritional data obtained from subjective global nutritional assessment (SGA) was as follows: seven patients had normal nutritional status, 68 and 19 were mildly and moderately malnourished, respectively, and no patient had severe malnutrition. The mean value of SGA was 12.2 ± 3.6.
In Table 1, we can see the clinical and laboratory characteristics of the three groups. There were no significant differences between groups in terms of age, sex and diabetes distribution, as well as regarding albumin, PTH, Ca × P, phosphate, calcium and ferritin levels. We found significant differences concerning eGFR (P = 0.033), hemoglobin (P = 0.001), TNF-α (P = 0.018), IL-6 (P = 0.017), SGA (P = 0.02) and cardiovascular disease (P = 0.046). Using a single regression model (Table 2), we observed that age, SGA score, ferritin, TNF-α, IL-6, Ca × P and cardiovascular disease showed a significant correlation with the ESA/Hb index. However, in a multiple regression model (Table 3), only age (t = −3.456, P = 0.001), SGA (t = 2.059, P = 0.047), ferritin (t = 2.386, P = 0.02), Ca × P (t = 2.066, P = 0.043), TNF-α (t = 2.673, P = 0.009) and IL-6 (t = 2.939, P = 0.004) independently influenced the ESA/Hb index. Finally, using a multiple binary logistic statistics (Table 4), we found that female gender (P = 0.023), cardiovascular disease (P = 0.042) and TNF-α (P = 0.005) were independently associated with the higher than the 50th percentile compared to the other patients (R 2 = 0.457).
Discussion
Anemia is a common complication of CKD, and its prevalence increases as the renal function declines [22]. In the general population, the risk of cardiovascular disease is higher in the presence of lower hemoglobin levels [23]. In patients with CKD, the cardiovascular risk is influenced both by the decreasing renal function and by the presence of anemia [8]. In these patients, anemia has also been associated with a faster progression of renal disease [24, 25]. The importance of treating anemia in CKD pre-dialysis patients relies in the fact that its partial correction is associated with a decrease of progression of renal insufficiency [24, 26] and an increase of the general well-being and quality of life [27, 28]. Observational studies suggest that anemia treatment is also associated with a decrease of the risk of hospitalization and death in pre-dialysis patients [29, 30], although there are no controlled prospective studies to support these findings [31, 32]. Pfeffer et al. [33], in their study involving patients with type 2 diabetes, CKD and anemia who were not undergoing dialysis, found that in patients who received darbepoietin alfa to achieve a hemoglobin level of 13 g/dl there was no reduction in the risk of death, cardiovascular events or renal events. In this group of patients, there was an increased risk of stroke. This study emphasizes the importance of making a reasonable decision about the potential benefit of ESA in patients with CKD.
Recently, the ESA/Hb index has been used as a tool to evaluate the resistance to ESA therapy, and a high ESA/Hb index is associated with increased morbidity and mortality in chronic hemodialysis patients [20, 34].
In our group of CKD stages 4 and 5 pre-dialysis patients, 72 out of 95 were under ESA therapy. When we compared the 3 groups regarding the ESA/Hb index, we observed that the main differences between groups, were related to inflammatory and nutritional parameters. These findings have been well described in patients undergoing maintenance renal replacement therapy [14, 34–36]. Inflammation can interact with the hematopoietic system at different levels, inhibiting erythropoietin secretion and erythroid progenitor cells maturation, increasing red blood cells destruction and decreasing iron delivery from reticuloendothelial to hematopoietic cells [12, 37]. Malnutrition can also influence the response to ESA. There has been described an association of anemia and ESA resistance with poor nutritional status [14, 15, 34], supporting the malnutrition-inflammation-atherosclerosis (MIA) concept [38]. Remarkable to verify that it was described that a poor appetite was related with anemia, similar to previous reports [39]. When looking at the results of our multiple regression analysis, we can also see that the inflammatory and nutritional parameters independently influenced the ESA dosage. More interesting is the fact that in our population we found a significant relationship between the presence of cardiovascular disease and a higher ESA/Hb index, in the ANOVA analysis and also in the single regression model. Probably, the well-described relationship between cardiovascular disease, nutritional status and inflammation, the so-called “MIA” syndrome [38], decreased the influence of cardiovascular disease on the ESA/Hb index, in the multiple regression model. The relationship of ferritin with ESA resistance can be more difficult to interpret, because ferritin level can reflect both the status of iron deposits and inflammation [34]. Worthy of note was that we found that lower age and a higher Ca × P were associated with ESA resistance. These findings could be explained, at least in part, by the fact that younger people are less compliant and have more severe hyperparathyroidism [40, 41]. As it was explained previously, we divided our patients under darbepoietin therapy in two groups, above and below the 50th percentile according to the ESA/Hb index. We took the 50th percentile and not the 90th percentile because of the small number of patients above the 90th percentile. However, we could find, in the logistic regression analysis, that female sex, inflammation and cardiovascular disease were associated with ESA resistance. Regarding the influence of female gender on ESA dose, our results are in agreement with other [42, 43], but not with all authors [44]. In our study, the role of inflammation on the ESA/Hb index in the logistic analysis confirmed the results of the multiple regression model. Even if we could not find an influence of cardiovascular disease in the multiple regression model, in the logistic analysis a statistically significant relationship was found and this can explain the higher morbidity and mortality associated with ESA resistance [18–20].
In conclusion, in a pre-dialysis CKD population, we observed that malnutrition and inflammation can independently influence the ESA/Hb index. Moreover, we also found that female gender and cardiovascular disease were associated with ESA resistance.
References
Eschbach JW, Adamson JW (1985) Anemia of end-stage renal disease. Kidney Int 28:1–5
Winearls CG, Oliver DO, Pippard MJ et al (1986) Effect of human erythropoietin derived from recombinant DNA on the anemia of patients maintained by chronic hemodialysis. Lancet 2:1175–1177
Eschbach JW, Egrie JC, Downing MR et al (1987) Correction of the anemia of end-stage renal disease with recombinant human erythropoietin: results of phase I and II clinical trial. N Engl J Med 316:73–78
Wingard RL, Parker RA, Ismail L et al (1995) Efficacy of oral iron therapy in patients receiving recombinant human erythropoietin. Am J Kidney Dis 25:433–439
Silverberg DS, Wexler D, Blum M et al (2003) The cardio-renal anaemia syndrome: does it exist? Nephrol Dial Transplant 18(Suppl 8):viii7–viii12
Collins AJ, Li S, Peter W et al (2001) Death, hospitalization and economic associations among incident haemodialysis patients with hematocrit values of 36 to 39%. J Am Soc Nephrol 12:2465–2473
Foley RN (2002) Anaemia: cardiovascular adaptations and maladaptative responses in chronic kidney disease. Nephrol Dial Transplant 17(Suppl 11):32–34
Astor BC, Coresh J, Heiss G et al (2006) Kidney function and anemia as risk factors for coronary heart disease and mortality: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 151:492–500
Leeder SR, Mitchell P, Liew G et al (2006) Low hemoglobin, chronic kidney disease, and risk for coronary heart disease-related death: the Blue Mountains Eye study. J Am Soc Nephrol 17:279–288
Tong EM, Nisseson AR (2001) Erythropoietin and anemia. Semin Nephrol 21:190–203
Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Section IV (2004) Failure to respond to treatment. Nephrol Dial Transplant 19(suppl 2):ii32–ii36
Macdougall IC, Cooper AC (2002) Erythropoietin resistance: the role of inflammation and pro-inflammatory cytokines. Nephrol Dial Transplant 17(Suppl 11):39–43
Stenvinkel P (2003) Anaemia and inflammation: what are the implications for the nephrologists? Nephrol Dial Transplant 18(Suppl 8):viii17–viii22
Kalantar-Zadeh K, Ikizler TA, Block G et al (2003) Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis 42:864–881
Neves PL, Morgado E, Faísca M et al (2006) Nutritional and inflammatory status influence darbepoietin dose in pre-dialysis elderly patients. Int Urol Nephrol 38:811–813
Kanbay M, Perazella MA, Kasapoglu B et al (2010) Erythropoiesis stimulatory agent-resistant anemia in dialysis patients: review of causes and management. Blood Purif 29(1):1–12
Cotter DJ, Stefanik K, Zhang Y et al (2004) Hematocrit was not validated as a surrogate end point for survival among epoietin-treated hemodialysis patients. J Clin Epidemiol 57:1086–1095
Zhang Y, Thamer M, Stefanik K et al (2004) Epoietin requirements predict mortality in hemodialysis patients. Am J Kidney Dis 44:866–876
Regidor DL, Kopple JD, Kovesdy CP et al (2006) Associations between changes in hemoglobin and administered erythropoiesis-stimulating agent and survival in hemodialysis patients. J Am Soc Nephrol 17:1181–1191
Lopez-Gomez JM, Porto J, Aljama P (2008) Factors that condition the response to erythropoeitin in patients on hemodialysis and their relation to mortality. Kidney Int 74:S75–S81
Levey AS, Bosch JP, Lewis JB et al (1999) A more accurate method to estimate glomerular filtration rate from serum craetinine: a new prediction equation. Ann Intern Med 130:461–470
Hsu C, McCulloch CE, Curhan GC (2002) Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol 13:504–510
Sarnak MJ, Tighiouart H, Guruprasad M et al (2002) Anemia as a risk factor for cardiovascular disease in the Atherosclerosis Risk in Communities (ARIC) study. J Am Coll Cardiol 40:27–33
Kuriyama S, Tomonari H, Yoshida H et al (1997) Reversal of anemia by erythropoietin therapy retards the progression of chronic renal failure, especially in non diabetic patients. Nephron 77:176–185
Neves PL, Morgado E, Baptista A et al (2007) Anaemia and interleukin-6 are associated with a faster progression to end-stage renal disease. Dial Transplant 36:445–456
Jungers P, Choukroun G, Oualin Z et al (2001) Beneficial influence of recombinant human erythropoietin therapy on the rate of progression of chronic renal failure in predialysis patients. Nephrol Dial Transplant 16:307–312
Lim VS, DeGowin RL, Zavala D et al (1989) Recombinant human erythropoietin treatment in predialysis patients. A double-blind placebo-controlled trial. Ann Intern Med 110:108–114
Revicki DA, Brown RE, Feeny DH et al (1995) Health-related quality of life associated with recombinant human erythropoietin therapy in predialysis chronic renal disease patients. Am J Kidney Dis 25:548–554
Fink J, Blahut S, Reddy M et al (2001) Use of erythropoietin before the initiation of dialysis and its impact on mortality. Am J Kidney Dis 37:348–355
Maddux FW (2007) Effect of erythropoiesis-stimulating agents on healthcare utilization, costs, and outcomes in chronic kidney disease. Ann Pharmacother 41:1761–1769
Drueke TB, Locatelli F, Clyne N et al (2006) Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355(20):2071–2084
Singh AK, Szczech L, Tang KL et al (2006) Correction of anemia with epoietin alfa in chronic kidney disease. N Engl J Med 355:2085–2098
Pfeffer MA, Burdmann EA, Chen CY et al (2009) A trial of darbepoietin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361:2019–2032
Locatelli F, Andrulli S, Memoli B et al (2006) Nutritional-inflammation status and resistance to erythropoietin therapy in hemodialysis patients. Nephrol Dial Transplant 21:991–998
Barany P, Divino Filho JC, Bergstrom J (1997) High C-reactive protein is a strong predictor of resistance to erythropoeitin in hemodialysis patients. Am J Kidney Dis 29:565–568
Wei M, Bargman JM, Oreopoulus DG (2007) Factors related to erythropoietin hypo-responsiveness in patients on chronic peritoneal dialysis. Int Urol Nephrol 39(3):935–940
Stenvinkel P, Barany P (2002) Anaemia, rHuEPO resistance, and cardiovascular disease in end-stage renal failure: links to inflammation and oxidative stress. Nephrol Dial Transplant 17(suppl 5):32–37
Stenvinkel P, Heimbürger O, Lindholm B et al (2000) Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome). Nephrol Dial Transplant 15:953–960
Kalantar-Zadeh K, Block G, McAllister CJ et al (2004) Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am J Clin Nutr 80:297–307
McKevitt PM, Jones JF, Lane DA et al (1990) The elderly on dialysis. Some considerations in compliance. Am J Kidney Dis 16:346–350
Bonanno G, Urso S, Lo Faro F et al (1996) Parathyroid hormone in a population of elderly patients on hemodialysis (preliminary data). Arch Gerontol Geriatr 22:441–445
Di lorio B, Stellato D, De Santo N et al (2004) Association of gender and age with erythropoietin resistance in hemodialysis patients: role of menstrual status. Blood Purif 22:423–427
Ifudu O, Uribarri J, Rajwani I et al (2001) Gender modulates responsiveness to recombinant erythropoietin. Am J Kidney Dis 38:518–522
Bamgbola OF, Kaskel FJ, Coco M (2009) Analyses of age, gender and other risk factors of EPO resistance in pediatric and adult dialysis cohorts. Pediatr Nephrol 24:571–579
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Lurdes Agostinho Cabrita, A., Pinho, A., Malho, A. et al. Risk factors for high erythropoiesis stimulating agent resistance index in pre-dialysis chronic kidney disease patients, stages 4 and 5. Int Urol Nephrol 43, 835–840 (2011). https://doi.org/10.1007/s11255-010-9805-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-010-9805-9