Abstract
Previous studies often report lower responses to erythropoietin (EPO) therapy in pediatric patients on chronic dialysis than those of adults. Because of the greater capacity for hematopoiesis in the younger population, these studies may be confounded by poorly identified variables. Thus, we made parallel studies of pediatric and adult cohorts to explore the relationship between age, gender and other risk factors with EPO resistance. Thirty pediatric subjects (aged 8–20 years) and 66 adult subjects (aged 22–85 years) on chronic hemodialysis and EPO were enrolled. After stratification by 50th percentile of EPO response, the best predictive model was identified by backward elimination of the risk factors with the least contribution to the regression. Relationship between age, gender and EPO resistance was examined by analysis of covariance (ANCOVA). The most predictive model of EPO response for the pediatric cohort had, as the major variables, urea clearance × dialysis duration/total body water (Kt/V), urea reduction ratio (URR), intact parathyroid hormone (iPTH), blood loss, normalized protein catabolic rates (nPCR) and indices of malnutrition and inflammation, whereas adults had iron and folate deficiencies as the dominant variables. Although EPO resistance was more common in female subjects than in male subjects, relationship with neither age nor gender was significant. Furthermore, the prescription of a larger (initiating) EPO dose by pediatric physicians compared with adult nephrologists confounded the interaction between age and EPO resistance. In summary EPO resistance in the pediatric dialysis cohort was predicted by nutritional deficits, inflammation, poor dialysis, and hyperparathyroidism, while iron and folate deficits were the major determinants in adults. Although confounded by the pattern of EPO prescription, neither age nor gender was predictive of EPO resistance in the two study groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The routine use of erythropoietin (EPO) in anemia control has reduced morbidity and mortality rates in patients with chronic kidney disease (CKD) [1–3]. Risk factors for EPO resistance include dosing modality, malnutrition, inflammation, hyperparathyroidism, and dialysis adequacy [4–9]. The non-modifiable predictors are age, gender, and race [4, 8, 10–12]. Recent study suggests an association of EPO resistance with higher mortality rates in dialysis subjects [13].
Compared with that in adults, EPO resistance is more commonly reported in retrospective data analysis of children on hemodialysis (HD) [8, 14, 15]. However, effects of confounding variables were seldom controlled in these studies [4]. In addition, these observations are not supported by the superior capacity for hematopoiesis in younger subjects [16].
Using multiple regression statistics, our study examined the influence of confounding variables in the risk-factor analysis of EPO resistance in dialysis cohorts. Furthermore, relationships between age, gender and EPO resistance were explored in an extended population of pediatric, adult and geriatric subjects.
Objectives
(1) To determine the risk factors for EPO resistance in a parallel study of pediatric and adult dialysis cohorts, and (2) to analyze the contributory roles of age and gender in the two study groups.
Hypotheses
(1) Young age per se is not an etiological factor in EPO resistance; the relationship is likely confounded by other variables. (2) Because of estrogenic influence and menstrual blood loss, larger EPO doses may be required for women than for men.
Methods
The study was approved by the Institutional Review Board (IRB) of the Montefiore Medical Center, Bronx, New York, USA. A total of 30 pediatric subjects who were on HD and EPO for a minimum of 2 months between July 2002 and January 2005 were enrolled. In addition, a cohort of 163 adults was stratified by gender and age group. As shown in Table 1, 12 subjects were then randomly selected from each of the age- and gender-specific strata. Six of the 72 adults were not suitable for analysis because of inadequate data.
Anemia management
Anemia was managed according to the guidelines of the National Kidney Foundation/Dialysis Outcome Quality Initiatives [17]. Dose of EPO was increased by 25% every 2–4 weeks to achieve a target hemoglobin (Hb) level of 12.0 g/dl. Ten intravenous doses of 100–125 mg iron were given with consecutive HD sessions if iron saturation was < 20% and/or serum ferritin was < 100 ng/ml. If iron saturation was > 20% and/or serum ferritin > 100 ng/ml, 25–125 mg iron was administered weekly. If iron saturation was > 50% and/or serum ferritin > 800 ng/ml, iron therapy was withheld. In furtherance of an ongoing study, oral doses of 5 mg folate were given to all pediatric subjects with every HD session.
Statistics
Data were analyzed with the aid of Statistical Package for the Social Sciences (SPSS) software. All data sets were assessed for Gaussian distribution using the D’Agostino–Pearson’s test for kurtosis, skewness and omnibus normality. All data were essentially normally distributed.
Demographic characteristics
As shown in Table 1, demographic variables including age, gender, weight, height, body mass index (BMI), and ethnicity were compared in the two cohorts. Secondly, both groups (Table 2) were assessed for differences in dialysis adequacy, nutritional status, objective malnutrition inflammation score (OMIS), indices of bone disease, estimated blood loss (EBL), residual renal function (RRF), and type of vascular access [HD catheter or arterio-venous fistula (AVF)]. In addition, the pattern of EPO and iron prescription among the pediatric and adult physicians was examined (Table 3).
Univariate risk factor analysis
To assess their etiological role, we analyzed previously identified risk factors in the pediatric and adult literature for each cohort. The risk factor distribution was stratified into two sub-sets (by 50th percentile of EPO requirements), while differences in their mean values were examined. Only the risk factors with a P < 0.75 were selected for regression studies. These included age, gender, race, iron and folate indices, EBL, urea reduction ratio (URR), urea clearance × dialysis duration/total body water (Kt/V), RRF, normalized protein catabolic rates (nPCR), calcium (Ca), phosphorous (P) intact parathyroid hormone (iPTH), surgery grade, infection scores, pubertal status (pediatrics), diabetic mellitus (adults) and malnutrition inflammation indices. Excluded from the analyses (P > 0.75) were primary renal disease, human immunodeficiency virus (HIV) status, HD duration, use of angiotensin modifiers, thyroid dysfunction, nephrotic state, and vascular access modality.
Multiple regression studies
The EPO resistance index (defined as the number of units of EPO divided by the Hb level in grams per deciliter) was the dependent variable. All the risk factors with P < 0.75 (identified above) were initially included in the regression. The best set of predictors was determined by a serial elimination of variables with the least contribution to the significance (F value) of the model. The basic prerequisites for an accurate multivariate analysis (including goodness of fit) were met. Multi-collinearity (measure of variable similarity) was minimized by acceptance of only the predictors with a variance inflation factor (VIF) < 10. We avoided overfitting by seeking a minimum of ten possible outcomes for each variable. Separate analyses were performed for variables with similar attributes, e.g. Kt/V vs URR. In such cases, equivalent statistical results are reported as an entity.
-
(1)
Age vs EPO resistance index (ERI). The linear correlation between ERI and age was determined by Pearson’s statistic. The confounding effect of the different EPO dosing for children and adults was avoided by the use of analysis of covariance (ANCOVA). Unlike Student’s t-test, ANCOVA was predicated on variance rather than the mean, thereby eliminating error that might have resulted from EPO doses.
-
(2)
Gender vs ERI. The mean difference between the ERIs for each age category by gender, pre- and post-puberty, and menstrual status was examined for both study groups.
Composite scoring of variables
To facilitate (quantitative) regression analysis, we scored surgical procedures, infection severity, folate and iron deficits, malnutrition and inflammation indices (see Appendices). Indices for the scoring of the malnutrition–inflammation complex in adults were adapted for the pediatric population by exclusion of subjective items [18].
Sample size
The number of pediatric subjects (n = 30) required to determine the predictors of EPO resistance was estimated on the basis of a 30% prevalence (pilot study), statistical power of 0.80, and a P < 0.05. Because of the wide prevalence rates (5–67%) of EPO resistance found in the adult literature, twice the number of the pediatric sample (n > 60) was selected for study.
Results
Baseline demographic characteristics of the study groups
-
(a)
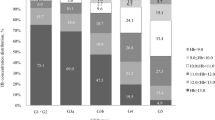
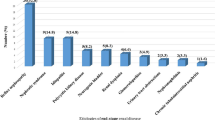
Pediatric cohort. As illustrated by Table 1, the male-to-female ratio was 1:1, mean age was 16 ± 3.2 years, average weight was 51.4 ± 16.5 kg, and the mean BMI was 21.4 ± 5 kg/cm2. Eight subjects had focal glomerulosclerosis (26.7%), six had chronic glomerulonephritis (20%), five had HIV nephropathy (16.7%), and four had renal dysplasia (13.3%), while two patients each had membrano-proliferative (6.7%), rapidly progressive (6.7%) or lupus glomerulopathy (6.7%). Four (13.3%) patients had suffered at least one episode of renal loss from graft rejection. Duration of dialysis was < 6 months in nine subjects (30%), 6–72 months in 17 (56.7%), and > 72 months in two (6.7%) patients.
-
(b)
Adult subjects. There were 29 men and 37 women with mean ages of 57 ± 15 years and 56 ± 15 years, respectively. Their mean weight was 73.5 ± 6.1 kg, mean height 165.5 ± 6.5 cm, and BMI was 27 kg/cm2. Polysynthane membrane dialyzers were used for all subjects.
Distribution of potential risk factors
The distribution of potential risk factors for EPO resistance was compared in the two groups. As shown by Table 2, a greater number of pediatric subjects had URR values < 65, but more of the adults had a minimum daily urinary output of 100 ml (defined as residual renal function), while nutritional indices (e.g. serum albumin) were not significantly different. Furthermore, in spite of a lower volume of blood loss, more adults had higher scores on the indices of iron deficiency. In addition, while 80% of the pediatric cohort used HD catheters as vascular access, five had AVFs. The ratio was reversed for the adults, with AVF in 56% and catheter use in 44%. There was a six-times more likelihood of greater blood loss in subjects with HD catheters (mean = 132.5 ± 96 ml) than those with AVFs (85 ± 38 ml) (P = 0.02).
Prescribed doses of EPO
The mean dose of EPO prescribed (for hemoglobin 11–12 g/dl) at the onset of HD for the pediatric group (19,524 U) was six-times larger than that for adults (3,508 U) (Table 3). In contrast, except for the larger doses of iron given to the adults with a saturation range of 36–45%, equivalent amounts were prescribed for both groups. Hence, the mean doses of iron for saturation < 26% and 26–35% were 119 mg [95% confidence interval (CI) = 79–159 mg] and 95 mg (95% CI = 67–122 mg), while corresponding adult doses were 120 mg (95% CI = 102–139 mg) and 90 mg (95% CI = 76–105 mg). Similarly, there was no significant difference in the amounts of iron prescribed for serum ferritin ranges 101–500 mg/dl (P = 0.41), 501–1,000 mg/dl (P = 0.72), and > 1,000 mg/dl (P = 0.3).
-
(1)
Predictors of EPO resistance in pediatric patients
-
(a)
Univariate analysis. Student’s t-test on the mean differences of each risk factor distributed across two subsets (of 50th percentile ERI) yielded the following: serum ferritin (P = 0.002), blood loss (P = 0.03), Kt/V (P = 0.006), serum albumin (P = 0.02), serum transferrin (P = 0.03), inflammation score (P = 0.002), and Tanner pubertal status (P = 0.02). The predictors with P > 0.05 but < 0.50 were age (P = 0.3), gender (P = 0.2), folate deficiency (P = 0.1), iPTH (P = 0.5), iron saturation (P = 0.5), URR (P = 0.1), C-reactive protein (P = 0.5), and infection score (P = 0.5) (Table 4).
-
(b)
Multiple regression. Multiple regression analysis of all the risk factors (with P < 0.75) had a coefficient of determination (R2) of 0.95 and an F value of 6.6 (P = 0.77). With the use of backward elimination, URR, Kt/V, blood loss, nPCR, inflammation scores, iPTH, serum ferritin and infection scores emerged as the most predictive (model) of EPO resistance (R2 = 0.8, F = 10.5, and P < 0.0001).
-
(a)
-
(2)
Predictors of EPO resistance in adult patients
-
(a)
Univariate analysis. The variables with P less than 0.51 for the adult subjects are as follows: iron saturation < 25% (χ2 = −0.3, P = 0.01), folate deficiency (χ2 = −0.3, P = 0.05), iron deficiency (χ2 = −0.3, P = 0.1), Ca × P ion products (P = 0.1), EBL (P = 0.3), iPTH (P = 0.3), RRF [odds ratio = 1.7 (95% CI 0.5 - 5.9); P = 0.3], URR (P = 0.5), Kt/V (P = 0.4), OMIS (P = 0.3), serum transferrin (P = 0.5), and ferritin (P = 0.4).
-
(b)
Multiple regression analysis (with ERI as dependent variable) on the adult data, with age, gender, race, iron profile, folate indices, serum albumin, blood loss, dialysis adequacy, RRF, Ca × P ion product and iPTH as predictors, yielded a model with R2 = 0.95 (F = 1.4; P = 0.5). The best set of predictors (Table 5) after backward elimination were low serum transferrin, low iron saturation and folate deficiency status (F = 5.1, P = 0.001).
-
(a)
Age, gender and EPO responses
Age and ERI
Analysis of covariance on the mean ages of pediatric (16 ± 3.2 years) and adult (57 ± 15.6 years) subjects and the corresponding ERI yielded no statistical significance (Table 6). Similarly, there was no difference in the frequency distribution of the risk factors by 50th-percentile ERI across the four age groups of children and adults. Furthermore, neither the correlation between ERI and the age of pediatric patients (r = −0.13) nor that of adult patients (r = 0.03) was significant.
Gender and EPO resistance
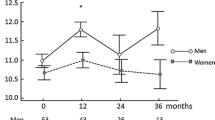
There were no significant differences in the ERI values of male and female subjects in each age stratum (Table 7). Similarly, the difference in the mean ERI of 2,056 (95% CI = 1,439–2,621) for the pre-pubertal children and 2,138 (95% CI = 1,079–3,135) for the post-pubertal children was not significant (P = 0.9). With similar hemoglobin mass, there was no difference in the ERI (P > 0.5) for either eight of 14 adolescents (2,431 ± 1,683 vs 3,099 ± 1,542) or seven of the 13 adults (301 ± 176 vs 287 ± 140) with and without regular menstrual cycles.
Discussion
Although there were similarities in the demographic attributes (e.g. gender and ethnicity) in the two study populations (Table 1), there were differences in their clinical characteristics. Whereas preliminary analysis had shown a lower dialysis dose and a greater (volume of) blood loss in pediatric subjects, statistical evidence showed that iron and folate deficiencies were more common in adults (Table 2). In addition, apparently due to a frequent catheter infection in pediatric subjects, a higher score was obtained for the malnutrition inflammation indices [5, 18–27]. Regression analysis of pediatric data had URR, Kt/V, blood loss, hyperparathyroidism, infection and inflammation scores as strong predictors of EPO resistance (Table 4). On the other hand, iron and folate deficits were the predominant variables in adults (Table 5). The occurrence of a potent set of predictors such as inflammation, malnutrition and poor dialysis (and the possible synergistic interaction between them) in pediatric subjects may explain its larger share of EPO requirement than the adults [20–23].
Earlier studies on dialysis subjects often failed to consider the impact of blood loss on EPO requirements. The larger amount of blood loss in our pediatric cohort was due to (a more frequent) laboratory sampling and use of HD catheters. Nevertheless, in spite of the disproportionate blood loss, iron deficiency was more prevalent in the adults [28]. Unlike EPO, intravenous dosing of iron was based on laboratory parameters [e.g. iron (Fe) saturation] rather than body weight in both groups. Consequently, because of the lower body mass of children, an equivalent dose of iron invariably results in a larger share. Furthermore, as part of an ongoing study, pediatric subjects routinely received folate supplements, and, therefore, had a lower prevalence of folic acid deficiency [9].
However, the aforementioned differences in clinical characteristics (alone) do not justify the wide disparity in EPO requirement in both groups. In general, pharmacological dosing in pediatric patients is often based on body weight. Hence the initial EPO dose was calculated as 150 IU per kilogram of body mass. Once a large dose has been initiated, high maintenance dosing is perpetuated by the addition of a fraction of the original amount so that the hemoglobin targets recommended by the Kidney Disease Outcomes Quality Initiative (KDOQI) can be attained. On the other hand, for adults, the initial EPO dose is estimated on the basis of hemoglobin deficits (rather than body weight). Consequently, approximately 10,000–15,000 larger units of EPO (per subject) were prescribed by children’s physicians than by adults’ counterparts. Interestingly, even though there are limited data, there is no scientific basis for the use of body weight (rather than hemoglobin deficit) to derive EPO dose in pediatric subjects. In fact, as in adults, there is a median increase of 0.042 g/dl hemoglobin mass for every thousand units of EPO administered [29].
Unlike the findings in observational studies, there is no evidence to support the erroneous belief that younger subjects are intrinsically ‘more resistant to EPO’ than adults [6, 30]. In our study, we performed separate analysis on pediatric and adult data, and no relationship was found between EPO resistance and chronological age. If, indeed, there is a relationship, younger pediatric subjects (8–15 years) should have required larger doses than their older counterparts (16–21 years), but this was not the case. In like manner, there were no significant differences in the amount of EPO required by the three age categories of adults. However, in spite of a similar use of KDOQI protocol, there was a wide disparity in the prescribed doses of EPO by the children’s and adults’ physicians. In addition, while the combined pediatric and adult population showed a significant correlation between age and ERI, after data stratification by 50th-percentile (of EPO response), the influence of EPO prescription was effectively removed. Additional evidence in support of the confounding effect of EPO dosing (on age and ERI) was the loss statistical significance upon re-analysis of the stratified data [4, 5, 27, 31–35].
The relationship between gender and EPO resistance in chronic uremia is often confounded by multiple factors. Thus, even though androgens potentiate bone marrow response to EPO, dialysis subjects are often functionally hypogonadic [36, 37]. On the other hand, estrogenic inhibition of erythropoiesis may be obviated by uremic ovarian toxicity. Furthermore, delayed puberty in chronic dialysis may modulate gender effects on EPO response [38]. Nevertheless, in spite of control for puberty and menstrual status, an observed trend of a lower EPO response among female subjects was not statistically significant, apparently because of a small sample size [36–41].
Finally, there is a gradual evolution of our understanding of the roles (and appropriate use) of EPO in the dialysis population. Contrary to popular belief, our study suggests that chronological age is not a limiting factor in (hematologic) response to EPO. Although we do not know the downside of excessive EPO dosing in the face of poor clinical response, an attempt to normalize hemoglobin mass (> 12 g/dl) in adults with cardiovascular and kidney diseases results in a greater mortality rate [13, 42]. Hence, except for the small-sized pediatric patients (weight < 20 kg), EPO dosing should be based on hemoglobin deficit rather than body mass. In addition, EPO requirements in pediatric subjects may be curtailed by limiting use of HD catheters, enhancing dialysis adequacy, improving nutrition, and minimizing laboratory blood sampling.
Conclusion
Poor response to EPO in the pediatric population was influenced by clinical characteristics (such as chronic inflammation, inadequate dialysis, hyperparathyroidism, and blood loss) rather than chronological age. In contrast, due to inadequate supplementation, iron and folate deficiencies were the major determinants in adults. Nevertheless, the relationship between age and EPO resistance was confounded by a disproportionate prescription of larger EPO doses for the pediatric cohort than for the adults. Because of the clinical significance of this finding, a multi-institutional prospective study that includes a sizable proportion of infants and young children is warranted.
Study limitations
In spite of minimizing confounding interaction, risk-factor analysis by regression study does not establish a cause and effect relationship. On its face value, comparative analysis across the populations of pediatric and adult patients may have been prone to erroneous conclusions. However, the study was designed to analyze the influence of common characteristics on EPO resistance (age and gender) in the two populations. In addition, a larger sample size of pre-pubertal children (and women of reproductive age) might have increased the credibility of the findings.
References
Eschbach JW (1989) The anemia of chronic renal failure: pathophysiology and the effects of recombinant erythropoietin. Kidney Int 35:134–148
Marsh J, Brown W, Wolcott D, Carr C, Harper R, Schweitzer S, Nissenson A (1991) rHuEPO treatment improves brain and cognitive function in anemic dialysis patients. Kidney Int 39:155–163
Revicki D, Brown R, Feeny D, Henry D, Teehan B, Rudnick M, Benz R (1991) Health related quality of life with human erythropoietin treatment for predialysis chronic renal disease patients. Am J Kidney Dis 25:548–554
Di Iorio B, Cirillo M, Bellizzi V, Stellato D, De Santo NG (2007) Prevalence and correlates of anemia and uncontrolled anemia in chronic hemodialysis patients: the Campania Dialysis Registry. Int J Artif Organs 30:325–333
Al-Hilali N, Al-Humoud H, Ninan VT, Nampoory MR, Puliyclil MA, Johny KV (2007) Does parathyroid hormone affect erythropoietin therapy in dialysis patients? Med Princ Pract 16:63–67
Seeherunvong W, Rubio L, Abitbol CL, Montane B, Strauss J, Diaz R, Zilleruelo G (2001) Identification of poor responders to erythropoietin among children undergoing hemodialysis. J Pediatr 138:710–714
Ifudu O, Uribarri J, Rajwani I, Vlacich V, Reydel K, Delosreyes G, Friedman EA (2000) Adequacy of dialysis and differences in hematocrit among dialysis facilities. Am J Kidney Dis 36:1166–1174
Varagunam M, McCloskey DJ, Sinnott PJ, Raftery MJ, Yaqoob MM (2003) Angiotensin-converting enzyme gene polymorphism and erythropoietin requirement. Perit Dial Int 23:111–115
Bamgbola OF, Kaskel F (2005) Role of folate deficiency on erythropoietin resistance in pediatric and adolescent patients on chronic dialysis. Pediatr Nephrol 20:1622–1629
Health Care Financing Administration (1998) 1998 Annual Report, End–Stage Renal Disease Core Indicators Project. Baltimore MD, Department of Health and Human Services, HCFA, Office of Clinical Standards and Quality
Frankfield DL, Rocco MV, Frederick PR, Pugh J, McClellan WM, Owen WF Jr (1991) Racial/ethnic analysis of elected intermediate outcomes for hemodialysis patients: results from the 1997 ESRD Core Indicators Project. Am J Kidney Dis 34:721–730
Ifudu O, Uribarri J, Rajwani I, Vlacich V, Reydel K, Delosreyes G, Friedman EA (2001) Gender modulates responsiveness to recombinant erythropoietin. Am J Kidney Dis 38:518–522
Zhang Y, Thamer M, Stefanik K, Kaufman J, Cotter DJ (2004) Epoietin requirements predict mortality in hemodialysis patients. Am J Kidney Dis 44:866–876
Van Damme-Lombaerts R, Broyer M, Businger J, Baldauf C, Stocker H (1994) A study of recombinant human erythropoietin in the treatment of anemia of chronic renal failure in children on hemodialysis. Pediatr Nephrol 8:338–342
Jabs K, Alexander S, McCabe D, Lerner G, Harmon WE (1994) Primary results from the US multicenter pediatric recombinant erythropoietin study. J Am Soc Nephrol 5:456–463
Palis J, Segel GB (1998) Developmental biology of erythropoiesis. Blood Rev 12:106–114
Collins AJ, Brenner RM, Ofman JJ, Chi EM, Stuccio-White N, Krishnan M, Solid C, Ofsthun NJ, Lazarus JM (2005) Epoetin alfa use in patients with ESRD: an analysis of recent US prescribing patterns and hemoglobin outcomes. Am J Kidney Dis 46:481–488
Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH (2001) A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis 38:1251–1263
El-Khatib M, Duncan HJ, Kant KS (2006) Role of C-reactive protein, reticulocyte hemoglobin content and inflammatory markers in iron and erythropoietin administration in dialysis patients. Nephrology 11:400–404
Lowrie EG, Chertow GM, Lew NL, Lazarus JM, Owen WF (1999) The urea [clearance x dialysis time] product (Kt) as an outcome-based measure of hemodialysis dose. Kidney Int 56:729–737
Cooper AC, Mikhail A, Lethbridge MW, Kemeny DM, Macdougall C (2003) Increased expression of erythropoiesis inhibiting cytokines (IFN-gamma, TNF-alpha, IL-10, and IL-13) by T cells in patients exhibiting a poor response to erythropoietin therapy. J Am Soc Nephrol 14:1776–1784
Yuen D, Richardson RM, Fenton SS, McGrath-Chong ME, Chan CT (2005) Quotidian nocturnal hemodialysis improves cytokine profile and enhances erythropoietin responsiveness. ASAIO J 51:236–241
Lin CL, Huang CC, Yu CC, Wu CH, Chang CT, Hsu PY, Yang CW (2002) Improved iron utilization and reduced erythropoietin resistance by on-line hemodiafiltration. Blood Purif 20:349–356
Gorman G, Furth S, Hwang W, Parekh R, Astor B, Fivush B, Frankenfield D, Neu A (2006) Clinical outcomes and dialysis adequacy in adolescent hemodialysis patients. Am J Kidney Dis 47:285–293
Chand DH, Brier M, Strife CF (2005) Comparison of vascular type in pediatric hemodialysis patients with respect to urea clearance, anemia management, and serum albumin concentration. Am J Kidney Dis 45:303–308
Roberts TL, Obrador GT, St Peter WL, Pereira BJ, Collins AJ (2004) Relationship among catheter insertions, vascular access infections, and anemia management in hemodialysis patients. Kidney Int 66:2429–2436
Movilli E, Brunori G, Camerini C, Vizzardi V, Gaggia P, Cassamali S, Scolari F, Parrinello G, Cancarini GC (2006) The kind of vascular access influences the baseline inflammatory status and epoietin response in chronic hemodialysis patients. Blood Purif 24:387–393
Sharples EJ, Varagunam M, Sinnott PJ, McCloskey DJ, Raftery MJ, Yaqoob MM (2006) The effect of proinflammatory cytokine gene and angiotensin-converting enzyme polymorphisms on erythropoietin requirements in patients on continuous ambulatory peritoneal dialysis. Perit Dial Int 16:64–68
Port RE, Kiepe D, Van Guilder M, Jellife RW, Mehls O (2004) Recombinant human erythropoietin for the treatment of renal anaemia in children: no justification for bodyweight-adjusted dosage. Clin Pharmacokinet 43:57–70
Besarab A (2006) Resolving the paradigm crisis in intravenous iron and erythropoietin management. Kidney Int Suppl 69:S13–S18
Chavers BM, Roberts TL, Herzog CA, Collins AJ, St Peter WL (2004) Prevalence of anemia in erythropoietin-treated pediatric as compared to adult chronic dialysis patients. Kidney Int 65:266–273
Kato A, Hamada M, Suzuki T, Maruyama Y, Hishida A (2001) Effect of weekly or successive iron supplementation on erythropoietin doses in patients receiving hemodialysis. Nephron 89:110–112
Axelsson J, Qureshi AR, Heimbϋger O, Lindholm B, Stenvikel P, Barany P (2005) Body fat mass and serum leptin levels influence epoietin sensitivity in patients with ESRD. Am J Kidney Dis 46:628–634
Nakamoto H, Kanno Y, Okada H, Suzuki H (2004) Erythropoietin resistance in patients on continuous ambulatory peritoneal dialysis. Adv Perit Dial 20:111–116
Odabas AR, Cetinkaya R, Selcuk Y, Keles S, Bilen H (2003) The effect of high dose losartan on erythropoietin resistance in patients undergoing hemodialysis. Panminerva Med 45:59–62
Schaefer F, Veldhuis JD, Robertson WR, Dunger D, Scharer K (1994) Immunoreactive and bioactive luteinizing hormone in pubertal patients with chronic renal failure. Cooperative Study Group on Pubertal Development in Chronic Renal Failure. Kidney Int 45:1465–1476
Di Iorio BR, Stellato D, De Santo NG, Cirillo M (2004) Association of gender and age with erythropoietin resistance in hemodialysis patients: role of menstrual status. Blood Purif 22:423–427
T’Sjoen GG, Beguin Y, Feyen E, Rubens R, Kaufman JM, Gooren L (2005) Influence of oestrogen or (anti-) androgen administration on soluble transferrin receptor in human plasma. J Endocrinol 186:61–67
Zeng SM, Yankowitz J, Widness JA, Strauss RG (2001) Etiology of differences in hematocrit between males and females: sequence-based polymorphisms in erythropoietin and its receptor. J Gend Specif Med 4:35–40
Hero M, Wickman S, Hanhijarvi R, Silmes MA, Dunkel L (2005) Pubertal upregulation of erythropoiesis in boys is determined primarily by androgen. J Pediatr 146:245–252
Le Petit-Thevenin J, Lerique B, Nobili O, Boyer J (1991) Estrogen modulates phospholipid acylation in red blood cells: relationship to cell aging. Am J Physiol 261:C423–C427
Paoletti E, Cannella G (2006) Update on erythropoietin treatment: should hemoglobin be normalized in patients with chronic kidney disease? J Am Soc Nephrol 17 [4 Suppl 2]:S74–S77
Acknowledgment
Supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Training Grant no. 9-526-3740
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Appendix A. Objective malnutrition inflammation scores
-
(a)
Serum albumin: (1) ≥ 4 g/dl = 0, (2) 3.5–3.9 g/dl = 1, (3) 3.0–3.4 g/dl = 2, (4) < 3.0 g/dl = 3
-
(b)
Serum transferrin: (1) ≥ 250 mg/dl = 3, (2) 200–249 mg/dl = 2, (3) 150–199 mg/dl = 1, (4) < 150 mg/dl = 0
-
(c)
Body mass index: (1) ≥ 20 kg/m2 = 0, (2) 15–19.9 kg/m2 = 1, (3) 16–17.9 kg/m2 = 2, (4) < 16 kg/m2 = 3
-
(d)
Dialysis duration (years): (1) 0–1 = 0, (2) 1–3 = 1, (3) 3–5 = 2, (4) > 5 = 3
-
(e)
Infection: (1) none = 0, (2) low grade (e.g. line-induced bacteremia, upper respiratory tract infection = 0.5 × no. of events, (3) moderate (e.g. symptomatic line sepsis, pneumonia) = 1.0 × no. of events, (4) severe (infection warranting hospitalization) = 1.5 × no. of events
-
(f)
Dialysis adequacy: (1) URR ≥ 80% = 0, (2) 61–79% = 1, (3) 41–60% = 2, (4) < 40% = 3
-
(g)
Serum ferritin: (1) 0–500 mg/dl = 0, (2) 501–750 mg/dl = 1, (3) 751–1,000 mg/dl = 2, (4) > 1,001 mg/dl = 3
Appendix B. Clinical and laboratory definitions
EPO resistance index
ERI calculated as EPO dose (international units per kilogram per week) divided by a given value of hemoglobin concentration (grams per deciliter).
Folate deficiency
Because of an overlap in the diagnostic accuracy of the red blood cell (RBC) parameters [e.g. mean corpuscular volume (MCV) and red cell distribution width (RDW) were poorly specific], folate deficiency (FD) was defined as (a) Mean serum folate and/or RBC folate ≤ 2 standard deviation score (b) Mean MCV ≥ 95th percentile for age and gender and/or RDW ≥ 16 and (c) Normal serum vitamin B12.
Iron deficiency
Fe deficiency consists of (a) Mean Fe saturation < 20% (b) Mean serum ferritin < 100 mg/dl and (c) Mean MCV [and/or mean corpuscular hemoglobin (MCH)] < 5th percentile for age and gender.
Malnutrition inflammatory score
The objective malnutrition inflammatory score (OMIS), adapted from the comprehensive MIS [18], is a questionnaire designed to quantify nutritional deficiency and inflammatory burdens. Its components are primary renal disease, dialysis duration, dialysis adequacy, C-reactive protein, total iron binding capacity, serum ferritin, body mass index, serum albumin and transferrin.
Dialysis adequacy
Urea reduction ratio (URR) = pre-dialysis blood urea nitrogen (BUN) − post-dialysis BUN/pre-dialysis BUN
Kt/V was determined by computer analysis (formal urea kinetic modeling) of the variables K, extrapolated from the mass transfer area coefficient (KoA) of the dialyzer, V, estimated total body water, and G, urea generation rate [or normalized protein catabolic rate (nPCR)].
Pubertal status
Post-puberty was defined as (a) Tanner stage (breast and/or pubic hair) ≥ 2, (b) plasma levels of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) consistent with the stage of puberty (c) and normal plasma free-thyroxine (FT4) and thyroid-stimulating hormone (TSH). Plasma gonadotropins were measured by ultra-sensitive chemiluminometric assay. Tanner I, FSH > 0.65 ng/ml, LH > 0.75 ng/ml; Tanner II, FSH > 2.75 ng/ml, LH > 1.0 ng/ml; Tanner III, FSH > 2.25 ng/ml, LH > 1.0 ng/ml; Tanner IV, FSH > 2.5 ng/ml, LH > 2.5 ng/ml; Tanner V, FSH > 2.5 ng/ml, LH > 2.5 ng/ml.
Infection score
Severity of infection: mild (e.g. line-induced bacteremia, upper respiratory tract infection) = 1, moderate (e.g. symptomatic line sepsis, pneumonia) = 2, and severe (infection warranting hospitalization) = 3.
Grade of surgery
minor (e.g. vascular access placement or revision) = 1, major (e.g. nephrectomy, parathyroidectomy, appendectomy) = 3
Estimated blood loss
Amount of blood collected for all laboratory procedures was measured; blood loss during dialysis (e.g. clotted circuit) was subjectively quantified by experienced dialysis nurses, and surgical blood loss was estimated by the attending surgeons. Blood loss was corrected for blood volume (BV) as EBL/BV. Calculated blood volume = 87 ml/kg for 7–18 years, and 72 ml/kg for > 18 years.
Residual renal function
An estimated minimum daily urine output of 50 ml and 100 ml for study subjects below 10 years of age and above 10 years of age, respectively.
Rights and permissions
About this article
Cite this article
Bamgbola, O.F., Kaskel, F.J. & Coco, M. Analyses of age, gender and other risk factors of erythropoietin resistance in pediatric and adult dialysis cohorts. Pediatr Nephrol 24, 571–579 (2009). https://doi.org/10.1007/s00467-008-0954-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-008-0954-3