Abstract
Urban parks are an important constituent of cities; they harbour microbial diversity which plays a key role in soil ecosystem functioning. The bacterial diversity of many urban parks around the world has been reported; however, the role of fungi is often ignored. There are only one or two published reports of fungal community composition analysis from urban parks. In the present study, we investigated soil fungal diversity in 24 urban parks in Shanghai using Illumina MiSeq sequencing. At the taxonomic level, 540,657 effective fungal sequences were acquired from the soil from 24 urban parks. Other than some unclassified phyla, the operational taxonomic units (OTUs) collected from all 24 urban park soils were distributed into five fungal phyla; of these, Ascomycota was the most dominant phylum, followed by Zygomycota and Basidiomycota. Many fungi identified in the present study are known to play key roles in the environment, with particularly important roles in maintaining sustainability in the soil ecosystem. The identified mycorrhizal fungi may play a major role in the biogeochemical cycle, as these fungi obtain carbon from their host plants and allocate it to the soil ecosystem; they also participate in mineralisation of various inorganic substances, helping with the growth of plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increasing human population is driving people from rural to urban areas, leading to fast-paced urbanisation. In order to live in harmony with nature, governments must ensure that they provide enough green spaces for their citizens by planning for and providing urban parks. Urban parks provide space for entertainment, recreational activities, and health and well-being, and thus, are attractive places for people of all ages to spend quality time (Sarah and Zhevelev 2007; Pröbstl-Haider 2015).
Urban parks also function as green havens within the urban area as they provide a variety of ecological niches that maintain and preserve biological diversity (Li et al. 2006; Shwartz et al. 2008). Urban parks are also great reservoirs for soils that harbour microbial communities (Roesch et al. 2007) which play an essential role in all biogeochemical cycles and transformation of nutrients in the soil (Nacke et al. 2011). Maintenance of microbial diversity and composition can directly influence the soil ecosystem and nutrient cycle in urban parks; thus, it is important to study the microbial community structure and diversity in urban parks.
Urbanisation may have detrimental effects on soil ecosystems through pollution discharge and changes in urban climate (Civerolo et al. 2007; Ash et al. 2008); this ultimately impacts on microbial diversity. The vast body of literature focused on the ecology of urban systems describes generalised biodiversity patterns (McKinney 2006). Of the few studies of microbial diversity, most have focused on bacterial diversity of urban parks (Xu et al. 2014). Further, the structures and functions of soil bacterial communities are reported to be successful indicators of changes in soil properties and vegetation characteristics in urban reclamations (Zhang et al. 2007). The soil biota plays a key role in the functioning of terrestrial ecosystems; however, the biodiversity of soils remains largely uncharacterised (Ramirez et al. 2014). Moreover, there is a worrisome lack of knowledge regarding fungal diversity in urban parks.
Fungi are often overlooked in ecological surveys, and studies of urban fungi are rare (Newbound et al. 2010). In general, the presence of fungi in urban ecosystems is beneficial to the soil environment, playing a role in the formation of large stable soil aggregates (Rillig 2004). Fungi are also an important food source for many vertebrate and invertebrate animals that are present in and around urban parks. Urban parks are usually home to a variety of plants, and fungi can be helpful in providing mineral nutrients to their symbiotic plant partners. Any alteration to the composition of floral or faunal habitats due to urbanisation is likely to affect fungi (Newbound et al. 2010). Therefore, more studies are needed in this field. Specifically, future studies should examine the effects of urbanisation on fungal diversity and investigate whether fungi can be an indicator of urbanisation. However, before we can study the interaction between urbanisation and fungal diversity, the fungal diversity of urban parks must be documented. In the present study, we performed fungal community composition analysis of 24 urban parks in Shanghai, China. Given that Shanghai is one of the most urbanised cities in the world, the findings of this study will have implications for future studies of fungal diversity in urban parks. Illumina MiSeq sequencing was utilised to analyse fungal diversity in this study.
Methods
Sampling sites
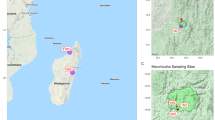
Soil was collected from 24 urban parks distributed across nine districts of Shanghai, China; namely, Putuo, Changning, Xuhui, Jingan, Huangpu, Hongkou, Yangpu, Minhang and Songjiang (Fig. 1, Table 1). A soil core was used to collect 100 g of soil from within 10 cm of the soil surface at each site.
Map of sampling points from 24 urban parks in Shanghai, China (reprinted with permission from Wang et al. 2018)
Total soil DNA extraction and Illumina MiSeq sequencing
Total soil DNA was extracted from 0.25 g of soil using the PowerSoil DNA isolation kit (MoBio, USA), according to the manufacturer’s instructions. Quantification of DNA was performed with a Qubit 2.0 Fluorometer (Invitrogen). A GeneAmp PCR System 9700 (Life Technologies) was used to perform initial PCR amplifications; then, Illumina Metagenomic Sequencing Library Preparation was carried out at Sangon Biotech Company, Shanghai.
Amplification via Illumina MiSeq was carried out in two steps. In the first step, the genomic regions of interest were amplified. In the second step, the sequencing adaptors were added to the samples. The internal transcribed spacer region was amplified with PCR using the Illumina ITS3F sequencing primer (GCATCGATGAAGAACGCAGC) and the ITS4 primer (TCCTCCGCTTATTGATATGC) (Nikolcheva et al. 2005).
Bioinformatic analysis
To determine differences in the taxonomic community composition across all 24 urban parks, QIIME v 1.8.0 was used to estimate pairwise dissimilarity between soil samples by calculating weighted UniFrac distances and unweighted UniFrac distances, and by performing Bray–Curtis analysis. The sequences were clustered at 97% identity and were aligned with PyNAST (Caporaso et al. 2010). Chimeras were detected using UCHIME v 4.2.40 software, and the high-quality sequences were grouped into operational taxonomic units (OTUs) (Edgar et al. 2011). The coverage across fungi was evaluated using the UNITE 97% identity ITS species hypothesis data set derived from all fungal ITS sequences in GenBank (Kõljalg et al. 2013), and was compared with the universal primers ITS3F and ITS4 using the SILVA LSU database (Pruesse et al. 2007). The richness index (Ace and Chao) and Shannon and Simpson indices for fungal diversity were determined with Mothur (V 1.30.1) software, as described by Schloss et al. (2009).
Data availability
Raw sequencing data for ITS 28S rRNA genes from the soils from 24 urban parks in Shanghai were deposited in the NCBI Sequence Read Archive (SRA) under submission ID SUB3120815 and BioProjectID PRJNA414019.
Results
General analysis of sequencing data of soils from urban parks
The fungal community compositions of collected soils were analysed by ITS3–ITS4 28S rRNA gene region Illumina MiSeq sequencing. After removal of chimeras, a total of 540,657 effective fungal sequences, with an average length of around 340 bp, were obtained from the soil samples; we identified 15,083 OTUs in the complete data set (Table 1). The number of sequences varied between 17,625 (sample 6, Hailli green park, Changning district) and 33,143 (sample 2, Changshou park, Putuo district).
The OTUs obtained from the soil from each urban park are shown in rarefaction curves (Fig. 2). The coverage of all samples from each park was 99%, showing the high richness of urban park soils. The Shannon index indicated that samples from Jiangpu park had the lowest fungal diversity. Most of the urban parks showed high fungal diversity, with Daning Tulip Park demonstrated the highest fungal diversity (Table 1).
Composition of fungal communities in urban park soils
Other than some unclassified phyla, the OTUs collected from all 24 urban park soils were primarily classified into five fungal phyla. Ascomycota was the most dominant phylum (with the exception of three urban parks), accounting for more than 50% of the sequences in the samples collected from six different urban parks (Sample ID 2, 4, 10, 16, 17 and 24) distributed in five districts of Shanghai, and accounting for 25–50% of the sequences in 14 of the urban parks located across all Shanghai districts studied (Fig. 3). It is noteworthy that Zygomycota dominated three parks, namely Jiangpu park (Yangpu district), Wenhua park and Mother park (both in the Minhang district, located in a sub-urban area of Shanghai) accounting for 95.9%, 40.9% and 36.3% of total sequences, respectively. In all other cases, Zygomycota, Basidiomycota, Chytridiomycota and Glomeromycota were the predominant phyla, accounting for 0.62–24.28%, 0.06–15.87%, 0.01–6.66% and 0.01–4.33% of the sequence counts, respectively, across all 24 urban park soils (Fig. 3). Further, Microsporidia and Incertae sedis phyla were rare, found only in Fuxing park (Huangpu district) and Zhongshan park (Changning district), respectively. It should be noted that a good number of visitors often use these two parks for regular recreational activities.
The fungal community composition did not differ significantly between the 24 urban parks. A suite of diverse taxa were obtained from all 24 urban park soils, with a total of 462 genera, including unclassified genera. A total of 48 fungal genera were present in the soil samples from all 24 urban parks (Fig. 4); their abundances are presented via heatmap analysis (Fig. 5).
Principal component analysis (PCA) revealed phylogenetic dissimilarity in the soils from several urban parks. Soil samples from Guangqi park, Luxun park, Jiangpu park and Mother park were phylogenetically dissimilar compared to other urban parks (Fig. 6). It should be noted that these parks attract less human or recreational activities, and have less vegetation compared to other parks. Overall, the urban park soils exhibited diverse fungal community compositions which could be helpful in supporting greenery of parks through their involvement in the growth of plants and ecosystem functioning.
Discussion
Traditional molecular methods such as denaturing gradient gel electrophoresis (DGGE), restriction fragment length polymorphism (RFLP), amplified ribosomal DNA restriction analysis (ARDRA), and single strand conformation polymorphism (SSCP) are limited by their resolution when studying microbial diversity; specifically, it is difficult to detect subtle changes in banding patterns or changes in closely related species using these methods, leading to underestimation of diversity by one to two orders of magnitude (Lee et al. 2011; Nacke et al. 2011). High-throughput sequencing has revolutionised the study of microbial diversity, and has offered deeper insight into fungal biodiversity (Geml et al. 2008; Jumpponen and Jones 2009; Taylor et al. 2014; Tedersoo et al. 2014).
Although most of the urban parks studied showed very high fungal diversity, the patterns in the Shannon index and Chao1 index were different to the patterns in the OTUs; while there was a correlation between the OTUs and Chao1 index, there was no correlation between the OTUs and Shannon index. Our findings are completely at odds with other reports on bacterial diversity of deciduous and evergreen forests in the Taihu Lake area, China (Wei et al. 2017), indicating that we cannot predict exact data pattern when studying fungal diversity.
Ascomycota and Basidiomycota, which we were dominant in our soil samples, have also been reported to be dominant in various other soil ecosystems; the dominance of these phylum benefits plants by increasing nutrient availability and contributing to the formation of a well-structured porous soil (Newbound et al. 2010; Sun et al. 2015; Zheng et al. 2017). It should also be noted that most of the urban parks in the present study contained aquatic habitats in the form of lakes, an environment suitable for providing habitat for most of fungi, in addition to dry land and grassland; this enables the growth of fungi of phylum Ascomycota (Table 2). Thus, our results indicate that the observed fungal diversity in Shanghai urban parks helps to maintain soil fertility and promote plant growth. Ascomycota was not the dominant phylum in Jiangpu park; this could be due to the nature of this park, being small with comparatively less greenery and less plants compared to the other studied urban parks.
At the class level, a total of 28 fungal classes were identified in the 24 urban park soils; however, the majority were unclassified. Sordariomycetes, Pezizomycetes, Saccharomycetes and Leotiomycetes were dominant classes in most of the samples, while Ustilaginomycetes, Wallemiomycetes, Exobasidiomycetes, Taphrinomycetes and Pucciniomycetes were rarely observed in our samples.
With the exception of Jiangpu park soil, the majority of our urban park soils contained unclassified fungi (average of 38.7%); these fungi must be identified as they could have an ecological impact. It is also noteworthy that Jiangpu park is not highly developed in terms of green landscape; instead, it is characterised by exposed garden soil. Lots of debris was also observed in this park (Table 2). Several fungal families belonging to the ectomycorrhizal fungi group, such as Pezizales, were present in all urban park soils; these fungi could play an important role in the growth of fauna in urban parks. However, many of these were unclassified. The results also stress the need to use culture-independent techniques together with culture-dependent ones (Liu et al. 2005).
Dipodascus, Mortierella, Pichia, Trichosporon, Kazachstania, Fusarium and Anguillospora were the dominant genera present in most of the urban park soils, comprising 31.6%, 34.2%, 25.9%, 13.9%, 15.4%, 3.2% and 17.7% of the sequences, respectively. Staphylotrichum and Scytalidium were distributed in each urban park soil, except for Jiangpu park, and Caohejing Development and Fuxing parks; however, they accounted for only 0.04–1.53% and 0.02–5.74% of the sequence counts, respectively. The subtropical maritime monsoon climate of Shanghai may not be suitable for the growth of Staphylotrichum which requires a more dominant warmer climate, while Scytalidium could be associated with specific plants or fruit trees. Both genera are known to have a pathogenic nature. Aspergillus was present in all urban park soils, comprising 0.01–1.59% of the total sequence counts; this suggests that there are no significant adverse environmental impacts associated with this genus. Several species of Aspergillus have a pathogenic nature and can induce diseases in plants. Zygomycota was dominant in Jiangpu park, Wenhua park and Mother park; this could be because these parks contained more suitable habitats (exposed soil and decaying plants).
The wide distribution of ectomycorrhizal fungi identified in all urban park soils highlights their role in various biogeochemical cycles. The carbon, nitrogen, phosphorus and sulphur geochemical cycles are important in sustaining ecosystem function, including providing nutrients for plant growth in urban parks (Xue et al. 2013). The contribution of fungi to biogeochemical processes has largely been deduced through their involvement in the carbon and nitrogen cycles in the terrestrial ecosystem (Sterflinger 2000; Clipson and Gleeson 2012). The presence of many fungi in urban park soils also mediates solubilisation of rock phosphate and mineralisation of several metals (Achal et al. 2007; Gadd 2007). These fungi are also reported to participate in the immobilisation of heavy metals, and thus, could play an additional role in the remediation of metals arising in urban park soils due to human activities (Qian et al. 2017).
Many Ascobolaceae and Pezizales fungi in urban parks could be saprophobic and mycrorrhizal in nature, which is beneficial to the functioning of the soil ecosystem. It is noteworthy that Leotiomycetes were present in all urban park soils, except for Yangpu park, and many members of this class cause serious plant diseases; thus, there is a need to identify these fungi using culture-dependent techniques in order to understand their impact. Fusarium, known as phytopathogenic fungi, were also identified in all urban park soils, except for soil from Zhongshan park. Future studies are needed to investigate their direct impact on plant growth or human health.
Saprophytic fungi were widely spread among all urban park soils; these fungi can act as opportunistic pathogens for humans and animals (Stojanov et al. 2007). Dogs are commonly kept as pets in Shanghai, and regularly wander in the urban parks. Our findings should serve as a warning to dog owners to take necessary precautions so as to avoid health implication associated with these fungi. Research has shown that opportunistic pathogens such as Trichosporon and Fusarium can be present on the skin of dogs (Stojanov et al. 2007; Greene 2011). Metarhizium and Humicola, entomopathogenic fungi (Cloyd 1999; Ko et al. 2011), were present in soil from 16 of the studied urban parks. The presence of such fungi indicates a natural defence system against pests and various insects, providing biological control of plant diseases for the promotion of vegetation.
Conclusion
In summary, there is a consensus that urbanisation may have a negative impact on urban fungal communities. There is no historical or current data reporting on fungal communities in cities. Such research must be carried out at regular time intervals in order to identify soil eutrophication or pollution in urban parks caused by harmful pollutants from anthropogenic sources; this can be assessed by examining disturbances in fungal communities (Newbound et al. 2010). It is important to understand the effects of urbanisation on fungi given that they are a major constituent of the soil ecosystem; however, there is limited research on fungi in urban areas. This study describes the distribution of fungi in all major urban parks in Shanghai. This is the first such study from China, and one of few studies worldwide. Given the importance of fungi to humans and the functioning of the soil ecosystem, the current study highlights the importance of fungal research in urban areas in the context of fast-paced urbanisation. Shanghai is one of the most urbanised and populated cities in the world, and the current study is unique in its description of the fungal diversity in such an urbanised area. The results of this study can provide a benchmark to other researchers for future related comparative studies.
References
Achal V, Savant VV, Reddy MS (2007) Phosphate solubilization by a wild type strain and UV-induced mutants of Aspergillus tubingensis. Soil Biol Biochem 39:695–699
Ash C, Jasny B, Robert L, Stone R, Sugden A (2008) Reimagining cities. Science 319(5864):739
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336
Civerolo K, Hogrefe C, Lynn B, Rosenthal J, Ku J-Y, Solecki W, Cox J, Small C, Rosenzweig C, Goldberg R, Knowlton K, Kinney P (2007) Estimating the effects of increased urbanization on surface meteorology and ozone concentrations in the New York City metropolitan region. Atmos Environ 41:1803–1818
Clipson N, Gleeson DB (2012) Fungal biogeochemistry: a central role in the environmental fate of lead. Curr Biol 22:R82–R84
Cloyd RA (1999) The Entomopathogenic fungus Metarhizium anisopliae. Midwest Biological Control News VI(7)
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200
Gadd GM (2007) Geomycology: biogeochemical transformations of rocks, minerals, metals and radiounuclides by fungi, bioweathering and bioremediation. Mycol Res 111:3–49
Geml J, Laursen GA, Taylor DL (2008) Molecular diversity assessment of arctic and boreal Agaricus taxa. Mycologia 100:577–589
Greene C (2011) Infectious diseases of the dog and cat, 4th edn. St. Louis, Mo., Elsevier.
Jumpponen A, Jones KL (2009) Massively parallel 454 sequencing indicates hyperdiverse fungal communities in temperate Quercus macrocarpa phyllosphere. New Phytol 184:438–448
Ko W-H, Yang C-H, Lin M-J, Chen C-Y, Tsou Y-J (2011) Humicola phialophoroides sp. nov. from soil with potential for biological control of plant diseases. Bot Stud 52:197–202
Kõljalg U, Nilsson RH, Abarenkov K, Tedersoo L, Taylor AF, Bahram M, Bates ST, Bruns TD, Bengtsson-Palme J, Callaghan TM, Douglas B, Drenkhan T, Eberhardt U, Duenas M, Grebenc T, Griffith GW, Hartmann M, Kirk PM, Kohout P, Larsson E, Lindahl BD, Lucking R, Martin MP, Matheny PB, Nguyen NH, Niskanen T, Oja J, Peay KG, Peintner U, Peterson M, Poldmaa K, Saag L, Saar I, Schussler A, Scott JA, Senes C, Smith ME, Suija A, Taylor DL, Telleria MT, Weiss M, Larsson KH (2013) Towards a unified paradigm for sequence-based identification of fungi. Mol Ecol 22:5271–5277
Lee OO, Wang Y, Yang JK, Lafi FF, Al-Suwailem A, Qian PY (2011) Pyrosequencing reveals highly diverse and species-specific microbial communities in sponges from the Red Sea. ISME J 5:650–664
Li WF, Ouyang ZY, Meng XS, Wang XK (2006) Plant species composition in relation to green cover configuration and function of urban parks in Beijing, China. Ecol Res 21:221–237
Liu B, Zeng Q, Yan FM, Xu HG, Xu CR (2005) Effects of transgenic plants on soil microorganisms. Plant Soil 271:1–13
McKinney ML (2006) Urbanization as a major cause of biotic homogenization. Biol Conserv 127:247–260
Nacke H, Thurmer A, Wollherr A, Will C, Hodac L, Herold N, Schoning I, Schrumpf M, Daniel R (2011) Pyrosequencing-based assessment of bacterial community structure along different management types in German forest and grassland soils. PLoS ONE 6:e17000
Newbound M, Mccarthy MA, Lebel T (2010) Fungi and the urban environment: a review. Landsc Urban Plan 96:138–145
Nikolcheva LG, Bourque T, Bärlocher F (2005) Fungal diversity during initial stages of leaf decomposition in a stream. Mycol Res 109:246–253
Pröbstl-Haider U (2015) Cultural ecosystem services and their effects on human health and well-being – a cross-disciplinary methodological review. J. Outdoor Recreat. Tour. 10:1–13
Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J, Glockner FO (2007) SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res 35:7188–7196
Qian X, Fang C, Huang M, Achal V (2017) Characterization of fungal-mediated carbonate precipitation in biomineralization of chromate and lead from aqueous solution and soil. J Clean Prod 164:198–208
Ramirez KS, Leff JW, Barberan A, Bates ST, Betley J, Crowther TW, Kelly EF, Oldfield EE, Shaw A, Steenbock C, Bradford MA, Wall DH, Fierer N (2014) Biogeographic patterns in below-ground diversity in New York City’s Central Park are similar to those observed globally. Proc R Soc B 281:20141988
Rillig MC (2004) Arbuscular mycorrhizae and terrestrial ecosystem processes. Ecol Lett 7:740–754
Roesch LF, Fulthorpe RR, Riva A, Casella G, Hadwin AKM, Kent AD, Daroub SH, Camargo FAO, Farmerie WG, Triplett EW (2007) Pyrosequencing enumerates and contrasts soil microbial diversity. ISME J 1:283–290
Sarah P, Zhevelev H (2007) Effect of visitors’ pressure on soil and vegetation in several different micro-environments in urban parks in Tel Aviv. Landsc Urban Plan 83:284–293
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, van Horn DJ, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541
Shwartz A, Shirley S, Kark S (2008) How do habitat variability and management regime shape the spatial heterogeneity of birds within a large Mediterranean urban park? Landsc Urban Plan 84:219–229
Sterflinger K (2000) Fungi as geologic agents. Geomicrobiol J 17:97–124
Stojanov IM, Jaksic SM, Prodanov JZ (2007) Presence and importance of saprophyte fungal organisms on dog skin. Proc Nat Sci, Matica Srpska Novi Sad 113:261–265
Sun H, Santalahti M, Pumpanen J, Köster K, Berninger F, Raffaello T, Jumpponen A, Asiegbu FO, Heinonsalo J (2015) Fungal community shifts in structure and function across a boreal forest fire chronosequence. Appl Environ Microbiol 81:7869–7880
Taylor DL, Hollingsworth TN, McFarland JW, Lennon NJ, Nusbaum C, Ruess RW (2014) A first comprehensive census of fungi in soil reveals both hyperdiversity and fine-scale niche partitioning. Ecol Monogr 84:3–20
Tedersoo L, Bahram M, Põlme S, Kõljalg U, Yorou NS, Wijesundera R, Ruiz LV, Vasco-Palacios AM, Thu PQ, Suija A, Smith ME, Sharp C, Saluveer E, Saitta A, Rosas M, Riit T, Ratkowsky D, Pritsch K, Poldmaa K, Piepenbring M, Phosri C, Peterson M, Parts K, Partel K, Otsing E, Nouhra E, Njounonkou AL, Nilsson RH, Morgado LN, Mayor J, May TW, Majuakim L, Lodge DJ, Lee SS, Larsson K-H, Kohout P, Hosaka K, Hiiesalu I, Henkel TW, Harend H, Guo L, Greslebin A, Grelet G, Geml J, Gates G, Dunstan W, Dunk C, Drenkhan R, Dearnaley J, De Kesel A et al (2014) Global diversity and geography of soil fungi. Science 346:1256688
Wang X, Wu J, Kumari D (2018) Composition and functional genes analysis of bacterial communities from urban parks of Shanghai, China and their role in ecosystem functionality. Landsc Urban Plan 177:83–91
Wei Z, Hu X, Li X, Zhang Y, Jiang L, Li J, Guan Z, Cai Y, Liao X (2017) The rhizospheric microbial community structure and diversity of deciduous and evergreen forests in Taihu Lake area, China. PLoS ONE 12:e0174411
Xu H-J, Li S, Su J-Q, Nie S, Gibson V, Li H, Zhu Y-G (2014) Does urbanization shape bacterial community composition in urban park soils? A case study in 16 representative Chinese cities based on the pyrosequencing method. FEMS Microbiol Ecol 87:182–192
Xue K, Wu L, Deng Y, He Z, Van Nostrand J, Robertson PG, Schmidt TM, Zhou J (2013) Functional gene differences in soil microbial communities from conventional, low-input, and organic farmlands. Appl Environ Microbiol 79:1284–1292
Zhang CB, Huang LN, Shu WS, Qiu JW, Zhang JT, Lan CY (2007) Structural and functional diversity of a culturable bacterial community during the early stages of revegetation near a Pb/Zn smelter in Guangdong, PR China. Ecol Eng 30:16–26
Zheng Y, Hu H-W, Guo L-D, Anderson IC, Powell JR (2017) Dryland forest management alters fungal community composition and decouples assembly of root- and soil-associated fungal communities. Soil Biol Biochem 109:14–22
Acknowledgements
This research has received funding from Key Projects of the National Social Science Fund 17AZD011 and Research Programs of Shanghai Science and Technology Commission Foundation 17DZ1202100.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Zhang, J., Wang, X., Wu, J. et al. Fungal community composition analysis of 24 different urban parks in Shanghai, China. Urban Ecosyst 22, 855–863 (2019). https://doi.org/10.1007/s11252-019-00867-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-019-00867-5