Abstract
Worldwide, cities are investing in greenspace to enhance urban quality of life and conserve biodiversity. Cities should ensure these investments do not unintentionally result in ecosystem disservices. Municipal management decisions regarding urban greenspaces, such as mowing frequency, could influence mosquito communities and public health. We examined how mowing, resultant vegetation characteristics, and landscape context influenced adult mosquito abundance in urban vacant lots. We sampled adult Culex and Aedes mosquitoes in a network of vacant lots within eight Cleveland, Ohio, USA neighborhoods in 2015 and 2016 using CO2-baited light traps and grass-infused gravid traps. For each lot, we quantified vegetation characteristics, including plant diversity, bloom area, and biomass, as well as the surrounding landscape composition at radii of 60 and 1000 m. We found that periodic mowing did not significantly affect mosquito abundances. However, vacant lots with more diverse plant communities were associated with a greater light trap capture of both Culex and Aedes. Both mosquito genera declined in light trap catches with increased impervious surface at 60 m. Similarly, Culex (gravid trap) declined with the amount of built infrastructure at 1000 m. In contrast, Aedes (light trap) increased with the concentration of buildings in the landscape at 1000 m. Our findings indicate that reducing the frequency of mowing within vacant lots will not necessarily increase adult mosquito abundance. Nonetheless, mosquito surveillance and management should be considered when planning conservation-focused greenspaces, as vegetation design choices and the landscape context of a site do influence vector abundance and potentially disease risk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cities are increasingly investing in urban conservation initiatives including the establishment and maintenance of urban greenspaces through a process referred to as urban greening (Goddard et al. 2010; Gardiner et al. 2013; Hicks et al. 2016). Urban greenspaces can be valuable in supporting biodiversity and supplying important ecosystem services and functions (Sandström et al. 2006; Gardiner et al. 2014; Braaker et al. 2014; Wolch et al. 2014; Riley et al. 2018a). Urban greening aimed at enhancing biodiversity often focuses on reducing habitat mowing to allow plants to flower and provide resources for species of concern, such as urban pollinators (Sivakoff et al. 2018). However, reduced greenspace management may unintentionally result in ecosystem disservices, such as increasing suitable habitats for vector species. Vector species, including disease-carrying arthropods such as the northern house mosquito, Culex pipiens, can negatively impact human health (Hamer et al. 2008) and reduce greenspace value (Shepard et al. 2014). Thus, it is essential to determine how urban greening practices influence vector abundances in order to accurately guide greenspace development.
Evaluating greenspace management practices is especially relevant to shrinking cities where economic decline has created extensive greenspace holdings in the form of vacant land. For example, the city of Detroit, Michigan, USA contains over 10,000 ha of vacant land (Burkholder 2012), and the European countries Poland and Romania contain 800,000 and 900,000 ha of urban vacant lots, respectively (Ferber and Schlappa 2016). In these areas, municipalities are responsible for the long-term management of greenspaces resulting from urban shrinkage and finding ways to positively utilize the spaces without contributing to ecosystem disservices. One common management approach is to seed vacant lots with turf grass and maintain them with periodic mowing (Gardiner et al. 2013). However, many shrinking cities are also investing in planting native vegetation, such as sunflowers (Lokman 2017) or urban prairies (Burkman and Gardiner 2015) as conservation habitat. As both growing and shrinking cities alike consider how best to manage urban greenspaces, it is critical to ascertain whether these management strategies may have unintended consequences.
Potential disservices from varying management strategies include negative financial, environmental, and social impacts (Lyytimäki and Sipilä 2009; Escobedo et al. 2011). Cost could be incurred by cities budgeting for consistent mowing. For instance, Cleveland spends 3 million USD annually to mow their 27,000+ vacant lots (Community Research Partners and Rebuild Ohio 2008; Delgado de la Flor et al. 2017). Likewise, mowing or trimming vegetation may cause ecosystem disservices and biodiversity losses by directly killing resident arthropods, disrupting habitats, or reducing floral availability for specialist pollinator species (Cizek et al. 2012; Wastian et al. 2016). However, while reducing mowing frequency or planting native wildflowers could lower greenspace management costs and increase a habitat’s value for biodiversity, this approach may also result in concerns from neighborhood residents (Turo and Gardiner 2019). Taller vegetation can raise aesthetic and safety concerns (Jansson 2013; Nassauer and Raskin 2014) or even create habitat for vector species, such as mosquitoes, that lead to higher prevalence of insect-borne diseases (Hamer et al. 2008).
Vector-borne diseases have become an increasing burden to public health due to globalization and urbanization (Gratz 1999; Norris 2004; Weaver 2013) and represent a significant ecosystem disservice. As cities increasingly invest in urban greenspaces or are tasked with managing newly created vacant lots, natural resource managers and urban planners must assess variable management strategies and their impacts on mosquito communities and public health (LaDeau et al. 2015). When conservation plans are developed, variables such as vegetation density and management of potential larval habitats (i.e. discarded containers) can influence mosquito abundance and taxonomic composition as well as interactions with potential hosts and predators (Freed and Leisnham 2014; Dowling et al. 2013; Gardner et al. 2013). The habitat characteristics associated with higher vector abundances can be complex; for instance, reduced vegetation was positively related to the abundance of juvenile Aedes albopictus except when abandoned infrastructure was common, in which case increased vegetation was positively related to vector abundance (Little et al. 2017a). Habitat management can also influence disease prevalence (Mackay et al. 2016); for example, mosquitoes collected from Chicago residential yards were more likely to be infected by West Nile virus (WNV) than those found in other urban greenspaces (e.g. parks and cemeteries) (Newman et al. 2017).
At larger scales, landscape composition, habitat connectivity, and the interweaving of land cover types may also influence mosquito communities and disease outbreaks (Pradier et al. 2008; Lambin et al. 2010; Deichmeister and Telang 2011; Ghosh 2011; Marcantonio et al. 2015). While urban areas often have reduced mosquito populations due to decreased resources and increased disturbance (Ferraguti et al. 2016), many taxa are highly adaptive to urban environments, e.g., Ae. albopictus, Ae. aegypti (Hemme et al. 2010; Ferraguti et al. 2016), and some Culex spp. (Cx. pipiens/ Cx. restuans/ Cx. quinquefasciatus) (Chaves et al. 2009; Deichmeister and Telang 2011). For example, when comparing exurban and suburban populations, urban sites have higher captures of Culex species (Pecoraro 2007; Deichmeister and Telang 2011) and a higher proportion of WNV positive mosquitoes (Deichmeister and Telang 2011). Within urban landscapes, mosquito populations have been positively correlated with landscape features including impervious surface, abandoned buildings, medium height trees (3–9 m), vacant lots, and residential habitats (Landau and Van Leeuwen 2012; Little et al. 2017a; Little et al. 2017b). Especially in the context of shrinking cities, abandonment or poverty at a landscape level is often associated with increased garbage or dumping, which is in turn associated with higher mosquito production (Little et al. 2017a; LaDeau et al. 2015). However, patterns can be variable and highly influenced by precipitation and temperature (Little et al. 2017a; Becker et al. 2014). For instance, while some studies have found greater mosquito abundance within a city block with a low number of abandoned buildings (Becker et al. 2014), others have found the opposite trend (Little et al. 2017a), and these relationships are mediated by seasonal variation.
The goal of our study was to evaluate how site management and landscape context influence adult mosquito communities and potential risks of a mosquito-borne disease (i.e. WNV) within an urban ecosystem. To address this, we studied mosquito abundance within Cleveland, Ohio, USA, a shrinking city where economic decline has resulted in 1,500 ha of vacant land (Western Reserve Land Conservancy 2015). We investigated if decreased mowing frequency, a practice that would reduce management costs and potentially increase the conservation value of vacant land, would have unintended consequences such as increased mosquito abundances and disease transmissions. Specifically, we measured how periodic mowing activity, resultant vegetation characteristics, and landscape context in the inner-city of Cleveland influence adult Culex spp. and Aedes spp. abundance, and WNV-positive mosquito pools. We hypothesized that periodic mowing would reduce mosquito abundance and the number of WNV-positive mosquito pools because mowing is likely to disrupt adult resting sites and foraging resources. We also hypothesized that vacant lots embedded in landscapes with a higher proportion of greenspace would support a greater abundance of mosquitoes. These landscapes could aid mosquito dispersal into sampled patches and are likely to support a higher richness and abundance of hosts and nectar resources. Finally, we hypothesized that diverse, bloom rich habitats would exhibit greater mosquito abundances as more diverse habitat plantings with increased bloom area are likely to provide more nectar foraging options for adult mosquitoes.
Materials and methods
Study sites
This study was conducted in the city of Cleveland, Ohio, USA. A total of 16 vacant lots (each lot is approximately 30 m × 12 m in size) located in eight inner-city neighborhoods were selected for this study (Fig. 1). Two vacant lots were located within each neighborhood and were assigned to either a Control or Meadow treatment (Fig. 2). The Control treatment was managed following city guidelines, mown monthly to a height of approximately 10 cm (May–October). The Meadow treatment was mowed annually in October and remained unmanaged throughout the remainder of the growing season. To control for the effects of differential littering among sites on mosquito larval habitat, we removed trash twice per month so that garbage did not confound drivers of interest (mowing, local vegetation, landscape composition).
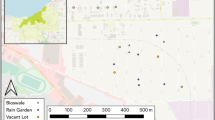
Map of mosquito collection sites in Cleveland, OH. Light gray shading indicates the eight neighborhoods where mosquitoes were studied: 1) Buckeye (BU), 2) Slavic Village (SV), 3) Central (CE), 4) Tremont (TR), 5) Detroit Shoreway (DS), 6) Fairfax (FA), 7) Glenville (GL) and 8) Hough (HO). Circles (Control) and squares (Meadow) indicate the location of each sampled vacant lot
Our vacant lot research sites were bordered on the roadside edge with fencing, signage, and bark mulch (a). All sites were cleaned of refuse twice per month. The Control (b) and Meadow (c) treatments were distinguished by mowing frequency. Control treatments were mown monthly and Meadow treatments were cut annually in October. This variation in management influenced vegetation characteristics such as plant diversity, biomass, and the availability of floral resources
Mosquito sampling
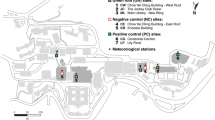
Adult mosquitoes were collected once every four weeks from July to August in 2015 (Jul 7, Aug 4, Aug 31). During 2016, mosquitoes were collected once in June and once every two weeks from July to August in 2016 (Jun 6, Jul 5, Jul 21, Aug 2, Aug 17, Aug 29). In order to treat collection time as a continuous rather than categorical variable, calendar dates were converted to Julian dates for statistical analyses (2015: 188, 216, 243; 2016: 158, 187, 203, 215, 230, 242). Two types of mosquito traps were used: A) a grass infusion-baited CDC gravid trap (GT) (Model 1712, John W. Hock Company, Gainesville, FL) placed at ground level in the center of each vacant lot, and B) a dry ice-baited CDC mini light trap (LT) with incandescent light (Model 2836BQ, BioQuip Products, Rancho Dominguez, CA) suspended from a tree branch at a height of approximately 1.5–2.0 m at the perimeter of each vacant lot. We elected to deploy gravid traps for their known effectiveness in trapping female Culex spp., the primary vector of WNV. Light traps were selected to attract a broad spectrum of mosquito species. Traps were set in the morning of each sampling date and retrieved approximately 24 h later. Captured mosquitoes were then transferred to a cooler with ice and transported to the Ohio Agricultural Research and Development Center (OARDC) in Wooster, OH where they were stored at −20 °C until further processing. All mosquitoes, except Culex females, were identified to species using a dissecting scope following the guide of Restifo (1982). Culex females were only identified to genus per recommendation from the Ohio Department of Health (ODH), as their standard traps can alter key identifiable features on the abdomen and all Culex in Cleveland are capable of transmitting WNV. After identification, Culex mosquitoes from each trap and site were pooled and stored at −80 °C until they were transferred to ODH for WNV detection using an established RT-PCR approach (Lanciotti et al. 2000). Two gravid traps collections were lost in 2015 and seven light trap and two gravid trap collections were lost in 2016 due to vandalism or theft.
Vegetation sampling
Local vegetation variables at each site were measured twice in 2015: early season (Jun. 16 – Jul. 3) and late season (Jul. 22 – Aug. 13), and three times in 2016, early season (Jun. 13 – Jun. 24), midseason (Jul. 11 – Jul. 22) and late season (Aug. 4 – Aug. 16). A 15 m × 7 m sampling grid, composed of 105 quadrats, was placed in the center of each site and 20 quadrats (1 m2) were randomly selected. Within the 20 selected quadrats we placed a 0.5 m2 PVC pipe square centrally and measured vegetation biomass and dominant plant species diversity.
Biomass was estimated with the comparative yield method which was developed to efficiently estimate plant biomass without removal of a significant amount of vegetation from a research site (Haydock and Shaw 1975). In order to compare biomass across the 20 randomly selected quadrats, five “standards” were initially selected to represent the range of biomass per quadrat within each lot. The standards ranged from 1 (lowest biomass) to 5 (highest biomass) and each “standard” consisted of 0.5 m2 area. After the standards were established, the comparative yields of twenty 0.5 m2 areas were estimated within random quadrats by comparing the average biomass to those five standards. Estimated scores ranged from 1 to 5 and allowed for quarter step (e.g. 4.25) measurements. After comparative yield scores were estimated, all vegetation within the 5 standards was harvested, dried, and weighed. The five dry weights were then used to form a linear regression equation and all 20 estimated yield scores were inserted into this equation to calculate biomass per quadrat. The calculated biomass of the 20 (0.5 m2) quadrats was then averaged and used to represent average site biomass in g/m2.
Plant diversity was measured from the same twenty, randomly selected, 0.5 m2 quadrats where biomass was estimated. In each quadrat, the top three most abundant plants were recorded, and species occurrences were summed by site. Dominant plant species diversity per site was then calculated with a Shannon-Wiener Index (H), \( \mathrm{H}=-{\sum}_{i=1}^R{P}_i\ln {P}_i \), where R is the species richness and Pi is the proportion of ith plant species of total number of plants.
Total bloom area was measured at each site from 6 additional, randomly selected, quadrats (1 m2). In each quadrat, a 0.5 m2 PVC square was placed centrally, and all flowering species were recorded. Bloom abundance was determined by counting all blooms per flowering species within the sub-quadrat. Then, five individual blooms of each plant species were measured (mm2) and averaged to determine the mean bloom size for each species. Total bloom area at a site was then calculated as the product of plant species abundance multiplied by each species’ mean bloom size. No vegetation data were obtained from the Meadow treatment of Detroit Shoreway for three vegetation samplings (late season 2015, early season 2016, late season 2016) due to accidental mowing by the City of Cleveland Land Bank.
Landscape variables
The Cleveland City Planning Commission provided landscape data for all sites at a 1 m2 resolution which were combined into the following land cover classes for analysis: Grass & Shrubs, Buildings, Impervious Surface (e.g. streets, highways, railroads), Tree Canopy over Vegetation, and Tree Canopy over Impervious Surface (buildings and other paved infrastructure). Water was not included in our analysis (despite the importance of water in mosquito biology) because the percentage of water was < 2% of any landscape. Landscape composition was quantified at 60 and 1000 m radii surrounding the central point of each vacant lot site.
Principal components analysis of landscape variables
To reduce the dimensions of the landscape variables, we performed a principal component analysis (PCA) using JMP version 14 (SAS Institute Inc., Cary, NC). Principal component axes were extracted using correlations among variables. A PCA was performed at two spatial scales, 60 m and 1000 m, which encompass a range of average flight distances for weak (Aedes) and strong (Culex) mosquito fliers. We restricted our analysis to the first two eigenvectors. The variation in landscape variables explained by principal components 1 and 2 ranged from 71.8% to 89.6%.
The interpretation of principal components 1 and 2 was dependent on the spatial scale of analysis (Fig. 3). At 60 m radii, the variables Buildings and Tree Canopy Over Impervious Surfaces loaded positively on PC1 while the variable Grass & Shrubs loaded negatively. Therefore, sites with positive values of PC1 suggest a landscape dominated by built infrastructure, whereas sites with negative values of PC1 suggest a landscape with a higher concentration of grass and shrubs. For PC2, sites with positive loadings were associated with more abundant Impervious Surface while sites with negative loadings were associated with more Tree Canopy Over Vegetation in the landscape (Fig. 3), suggesting sites with high PC2 values were embedded in landscapes with a high concentration of roadways, parking lots and railways whereas sites with low PC2 values were found in landscapes with a greater amount of tree-covered greenspaces.
At a 1000 m landscape radius, tree canopy variables and Grass and Shrubs loaded positively on PC1, and Impervious Surface loaded negatively, indicating that landscapes with high positive PC1 values had a greater green infrastructure whereas landscapes with negative PC1 values were dominated by roads and parking lots. For PC2, Buildings had the highest positive loading whereas Impervious Surface, Grass & Shrubs, and Tree Canopy Over Vegetation loaded most negatively (Fig. 3). Therefore, landscapes with high PC2 values were dominated by built structures whereas landscapes with low PC2 values had a greater concentration of greenspace and paved surfaces.
Statistical analyses
To determine if periodic mowing (i.e. a treatment effect) influenced mosquito abundance, we developed generalized linear mixed models (GLMMs) using the “lme4” package (Bates et al. 2015) in R (R Core Team 2014). Due to overdispersion all GLMM models used a negative binomial distribution (Lindén and Mäntyniemi 2011). All analyses were performed separately by trap type (light and gravid) and mosquito genus (Aedes and Culex). We examined three response variables: 1) Aedes abundance and 2) Culex abundance from the light trap collections, and 3) Culex abundance from the gravid trap collections. Predictor variables included Treatment (Control and Meadow), Julian date (as a proxy for seasonal variation in temperature and precipitation), the interaction between Treatment and Julian date, and Year. Random terms included Julian date as a random slope and Neighborhood (sites located in 8 inner-city neighborhoods) as a random intercept. The ‘Anova’ function in the “car” package (Fox and Weisberg 2011) was then used to perform a Type II analysis of variance that generated analysis of deviance tables from which likelihood-ratio test statistics were obtained. An alpha level of 0.05 was specified for all statistical tests.
To examine how mosquito abundance was influenced by landscape composition and local vegetation characteristics, we developed generalized linear models (GLMs) with a negative binomial distribution using the “MASS” package (Venables and Ripley 2002) in R. We again examined three response variables: 1) Aedes abundance and 2) Culex abundance from the light trap collections, and 3) Culex abundance from the gravid trap collections. Landscape composition variables included PC1 and PC2 at both the 1000 m and 60 m scales. Local vegetation variables included Biomass, Diversity, and Bloom area. Additionally, full models included the predictor variables Julian date and Year. Variance inflation factors were calculated and assessed for each predictor variable to ensure the absence of multicollinearity (VIF < 3). Backwards model selection was then performed until reduced models contained predictors significant at an alpha of 0.05.
Results
Mosquito abundance and West Nile virus testing
A total of 2,350 mosquitoes were collected across our 2015 and 2016 sampling periods. Culex spp. were most abundant and represented 64.6% and 82.2% of the total mosquitoes captured in 2015 and 2016, respectively. We collected five species of Aedes (Ae. japonicus, Ae. vexans, Ae. triseriatus, Ae. trivittatus and Ae. albopictus), Anopheles punctipennis, Orthopodomia signifera, Uranotaenia sapphirina and Coquillettidia perturbans (Table 1). In 2015 and 2016, Ae. japonicus and Ae. albopictus were respectively the most abundant Aedes species in our collections; both are invasive species in North America (Bonizzoni et al. 2013; Kaufman and Fonseca 2014). Notably, the abundance of Ae. albopictus increased in all traps from 2015 to 2016 (Table 1); this species was collected in four neighborhoods in 2015 (i.e. Central (Control), Glenville (Control and Meadow), Hough (Control) and Tremont (Meadow)), and all eight neighborhoods in 2016.
Of the 92 and 136 pools of Culex mosquitoes tested for WNV in 2015 and 2016, respectively, one pool was positive in 2015 (Tremont Control- 8/4) and 4 pools were positive in 2016 (Buckeye Control- 8/2, Slavic Village Meadow- 8/2, Hough Meadow- 8/2, and Hough Meadow- 8/17).
Mosquito abundance: Mowing frequency
Aedes and Culex mosquito abundances within CO2-baited light traps did not significantly differ between mowed Control and unmanaged Meadow treatments in either 2015 or 2016 (Aedes: χ2 = 1.06 (1, N = 127), P = 0.30; Culex: χ2 = 1.77 (1, N = 127), P = 0.18) (Table 2, Fig. 4a-d). Similarly, abundances of Culex adults caught by gravid traps did not significantly differ between treatments in either year (χ2 = 0.28 (1, N = 129), P = 0.60) (Fig. 4e-f). While mowing did not influence mosquito abundance, sampling period was a significant predictor; Julian date was positively associated with Aedes abundances from light traps and negatively associated with Culex abundances from gravid traps (Table 2). Light traps caught significantly more Culex adults in 2015 while gravid traps captured a greater number in 2016 (Table 2).
Adult Culex and Aedes species collected within Control versus Meadow treatment vacant lots using CO2-baited light traps and grass-infused gravid traps in 2015 and 2016. Light-trapped Aedes mosquito abundances (a and b) and light-trapped Culex mosquito abundances (c and d) from 2015 and 2016 are shown. We found no difference in Aedes or Culex abundance among our treatments. Gravid Culex mosquito abundances from 2015 and 2016 (e and f) are also shown. We also found no difference in gravid Culex abundance among our treatments
Mosquito abundance: Local vegetation and landscape variables
Aedes and Culex mosquito abundances were significantly influenced by both landscape composition and local vegetation variables. Greater vegetation diversity within a vacant lot was positively associated with increased Aedes and Culex catches from CO2-baited light traps (Table 3). Vegetation biomass also positively influenced Aedes abundances within light traps (Table 3). However, we did not find a significant relationship between bloom area and mosquito abundance. At the 60 m radius landscape scale we found a negative relationship between Aedes and Culex light trap captures and PC1, indicating that mosquitoes were collected more frequently in lots surrounded by a high proportion of grass and shrub habitat (Fig. 3). We also found a negative relationship between PC2 and Aedes light trap captures at 60 m (Table 3), indicating that these mosquitoes were collected more frequently in sites surrounded by increased urban tree canopy over vegetation versus impervious surface (Fig. 3). We found no significant relationship between gravid trap captures of Culex females and either PC1 or PC2 at 60 m (Table 3). At the 1000 m radius scale, we found a positive relationship between Aedes within CO2-baited light traps and PC2 (Table 3), indicating that a greater number of adult Aedes were found in sites surrounded by a high concentration of built infrastructure (Fig. 3). We found no significant relationship between Culex light trap captures and either PC1 or PC2 at 1000 m. Finally, we observed a negative relationship between PC2 at 1000 m and gravid trap captures of Culex (Table 3), which illustrated that females seeking oviposition sites were more common in landscapes with fewer buildings and a greater proportion of grass and shrub habitat and impervious surface (Fig. 3).
Discussion
Cleveland, OH has lost over 50% of its peak human population and currently maintains over 27,000 vacant lots with periodic mowing. Our study aimed to understand the impacts of mowing activity, resultant vegetation, and landscape composition on adult mosquito communities within inner-city vacant lots. While this overabundance of vacant land is unique to shrinking cities contexts, management through mowing is a common practice for urban greenspaces. Whether the target is spontaneous plant communities on vacant land or seeded turf grass in parks or cemeteries, mowing is viewed as a means to improve aesthetics and address nuisance species including mosquitoes (Heynen et al. 2006; McCormack et al. 2014; Riley et al. 2018b). However, mowing is a significant financial burden when considering the large area of vacancy in many cities and can reduce the conservation value provided by these reclaimed greenspaces (Cizek et al. 2012; van de Poel and Zehm 2014; Wastian et al. 2016). Many conservation-based management strategies for vacant land suggest reducing the intensity of site management to promote desired wildlife (i.e., Gardiner et al. 2013), however, these initiatives may have unintended consequences if they influence vector-host-disease relationships (Riley et al. 2018a). Importantly, we documented that reduced mowing did not result in higher Aedes or Culex abundance within vacant lots. However, we did find local plant diversity and biomass as well as surrounding landscape context shape the distribution of adult mosquitoes within vacant land, resulting in implications for conservation initiatives.
Heterogeneity in habitat persistence, size, and quality are known to influence vector survivorship and transmission potential (LaDeau et al. 2015). Therefore, we hypothesized that periodic mowing, representing a significant habitat disturbance, would result in localized reductions in mosquito populations. Mowing could negatively impact mosquito populations by causing direct mortality, reducing suitability of a patch for host populations (i.e. birds), or by removing floral resources utilized by adult mosquitoes (Swengel 2001; Cizek et al. 2012). Instead, our findings suggest that mowing, an economically and ecologically costly activity (Wastian et al. 2016; Community Research Partners and Rebuild Ohio 2008), is not necessarily helpful in mosquito control. This information is informative to vacant lot management as well as urban parks and open spaces, which employ strategies such as reduced mowing frequency or establishment of taller meadow plantings to promote conservation initiatives (Southon et al. 2017).
Importantly, we did not measure how mowing frequency might impact mosquito reproductive success, which is key to understanding how this shift in management could impact public health. In some instances, mowing has resulted in improved conditions for mosquito larvae (MacKay et al. 2016). For example, plant detritus resulting from mowing was found to enrich aquatic microhabitats for larval mosquitoes within dry retention basins (Mackay et al. 2016). Removing emergent vegetation from semi-aquatic habitats can also interrupt predator-prey interactions (Grieco et al. 2005), increase bacteria that facilitate larval growth (Walton and Jiannino 2005) and increase the attraction of female mosquitoes to sites for oviposition (Jiannino and Walton 2004). Conversely, larval development may also be enhanced in sites with reduced management or mowing. Sites that are considered unmanaged by passersby are at higher risk of dumping (Nassauer and Raskin 2014) and litter can serve as breeding sites for mosquitoes (Dowling et al. 2013; Becker et al. 2014; Little et al. 2017a). For instance, the abundance of water-holding containers littering a habitat has been found to be a key predictor of Ae. albopictus occurrence (Dowling et al. 2013). Further, shading from tall vegetation could slow evaporation from water-holding garbage during hot/dry periods. Within our sampled vacant lots, trash was removed twice per month from all sites, reducing potential larval habitats. However, within standard city-managed vacant lots, trash removal is not typical. We might have found different results had we left trash unmanaged as mowing is likely to destroy a proportion of water-holding refuse containers whereas unmown lots would have remained undisturbed. Thus, future research incorporating larval trends with adult populations would help disentangle these variable drivers at different timepoints in mosquito species’ life cycle. If conservation initiatives do prescribe reduced mowing, regular trash removal may also be helpful in avoiding unintentional mosquito increases (Dowling et al. 2013).
To date, urban conservation initiatives for vacant lot management have focused on altering existing weedy vegetation by creating habitats such as native wildflower plantings or urban farms (Burkman and Gardiner 2015; Delgado de la Flor et al. 2017; Sivakoff et al. 2018). To gauge how shifts in vegetation design might influence mosquito communities we also measured several vegetation variables and found that, as we had predicted, vegetation diversity and biomass were positively correlated with Aedes and Culex abundance in light traps. Species rich plant communities provide nectar and pollen resources (Foster 1995; Stone et al. 2012), and resting areas/refuge from predators (Gardner et al. 2013). As such, adult mosquitoes are often strongly associated with vegetation, which provides food, shade, and shelter for them (Zhou et al. 2007; Brown et al. 2008; Roiz et al. 2015). For instance, the abundance and condition of vegetation within an urban landscape (NDVI) as well as its internal water content (DNVI) have been positively related to mosquito abundance (Brown et al. 2008). Therefore, changing plant community composition and structural complexity can influence adult mosquito survival, biting rates, and vectorial capacity (Stone et al. 2012). This raises concern, as managing for a rich plant community is a common goal of conservation-minded plantings, focused on supporting beneficial arthropods and other wildlife (Burkman and Gardiner 2015; Hicks et al. 2016; Delgado de la Flor et al. 2017). However, our treatments were generally dominated by exotic and/or weedy species, such as chicory (Cichorium intybus L.), red clover (Trifolium pratense L.) and Queen Anne’s lace (Daucus carota L.) (Supplementary Table 1). Therefore, our findings may not be directly applicable to conservation efforts focused on establishing and maintaining native plants within greenspaces. Some mosquito predators, such as birds, may also recruit to more diverse plant communities that incorporate native vegetation (Burghardt et al. 2009) thereby mitigating plant diversity’s positive influence on mosquito abundances. Moreover, our results indicated that bloom area was not a significant predictor of mosquito abundance. This finding implies that adding more flowering species to an urban conservation site may ultimately have no net effect on mosquito abundances, while still supporting local conservation targets.
The distribution of mosquitoes across Cleveland’s vacant lots was also driven by landscape patterns. Following our hypothesis, we found partial support that mosquito abundances are higher in greener landscapes. At a localized scale of 60 m, we captured more mosquitoes in our light traps when landscapes had greater proportions of tree canopy over vegetation (Aedes) and grass and shrubs (both Aedes and Culex). Gravid Culex mosquitoes, however, did not follow any trends at a 60 m radius, potentially because Culex females tend to fly longer distances when seeking oviposition sites (Hamer et al. 2014). At a 1000 m radius scale, gravid Culex females captures declined as the land cover occupied by buildings increased. As Culex mosquitoes are known to utilize urban structures for oviposition, (e.g. drainage infrastructure, residential area) (Deichmeister and Telang 2011; Ferraguti et al. 2016), this result is somewhat surprising. Instead, gravid Culex females were more frequently captured from vacant lots surrounded by green land cover and impervious surface at 1000 m. Positive associations between mosquitoes and tree cover have also been detected previously (Landau and Van Leeuwen 2012). These patterns could be due to several variables, ranging from woody vegetation aiding adult dispersal (Lacroix et al. 2009), supporting increased vertebrate host abundance (Anderson et al. 2006; Molaei et al. 2006), and/or resulting in a higher number of both natural oviposition sites as well as tires and refuse commonly discarded in minimally-managed greenspaces (Kaufman et al. 2010; Bartlett-Healy et al. 2012; Gardner et al. 2013). Interestingly, at the 1000 m radius scale we found that landscapes with increasing concentrations of buildings and tree canopy over impervious surface resulted in higher Aedes captures in light traps. Variation in the response of Aedes could be due to a concentration effect at our larger landscape scale, wherein a greater proportion of the urban species pool relies on each individual habitat patch to provide critical resources when fewer sites are available (Veddeler et al. 2006, Sivakoff et al. 2018). A similar pattern has been documented for bees within vacant lots, where abundance was positively correlated with green landscapes locally and built infrastructure at larger landscape scales (Sivakoff et al. 2018).
Finally, temperature and precipitation can significantly influence mosquitoes and WNV prevalence (Chase and Knight 2003; Wang et al. 2010; Paaijmans et al. 2007; Ruiz et al. 2010; Little et al. 2017a). We observed significant annual variability for Culex abundances in both trap types, with fewer adults captured in light traps and more captured in gravid traps in 2016. Warmer temperatures have been shown to result in a higher light trap catch of Culex mosquitoes (DeGaetano 2005), yet we found a reduced abundance of Culex in 2016, when average daily temperatures recorded within Cleveland, OH were three degrees warmer during our sampling period (21.2 versus 24.2 °C in 2015 and 2016, respectively (NOAA 2018)). This counterintuitive finding might be due to precipitation, as drier conditions have been shown to reduce Culex catches within light traps (DeGaetano 2005), and precipitation was reduced during our 2016 study period (6.7 versus 11.0 cm of rainfall from June–August) (NOAA 2018). Furthermore, drier conditions may also have resulted in decreased habitat quality, which has been shown to result in greater attraction of females to artificial oviposition sites and a higher concentration of collected mosquitoes within gravid traps (O’Meara et al. 1989).
Conclusion
Managing urban greenspaces through periodic mowing can be very expensive and destructive to pollinators and other beneficial arthropod communities. However, reducing mowing intensity may also enhance arthropod vector abundances and harm public health. We demonstrated that periodic mowing did not affect adult mosquito abundances in urban vacant land, suggesting that less intensive management does not increase risks of mosquito-borne disease transmission. These findings provide further support for the potential of vacant land as a conservation space. However, additional research should clarify how reduced greenspace mowing influences mosquitoes’ larval development and their interactions with potential hosts and predators. Successful greenspace management must balance ecosystem functioning, cities’ financial resources, and residents’ opinions (Turo and Gardiner 2019). As urban greenspaces continue to grow in popularity and number, city planners and leaders need to consider how their greenspace designs and management strategies influence disease vectors and avoid unintended ecosystem disservices associated with mosquitoes and human health.
References
Anderson JF, Andreadis TG, Main AJ, Ferrandino FJ, Vossbrinck CR (2006) West Nile virus from female and male mosquitoes (Diptera: Culicidae) in subterranean, ground, and canopy habitats in Connecticut. J Med Entomol 43:1010–1019. https://doi.org/10.1603/0022-2585
Bartlett-Healy K, Unlu I, Obenauer P, Hughes T, Healy S, Crepeau T, Farajollahi A, Kesavaraju B, Fonseca D, Schoeler G, Gaugler R, Strickman D (2012) Larval mosquito habitat utilization and community dynamics of Aedes albopictus and Aedes japonicus (Diptera: Culicidae). J Med Entomol 49:813–824. https://doi.org/10.1603/ME11031
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Becker B, Leisnham PT, LaDeau SL (2014) A tale of two city blocks: differences in immature and adult mosquito abundances between socioeconomically different urban blocks in Baltimore (Maryland, USA). Int J Environ Res Public Health 11:3256–3270. https://doi.org/10.3390/ijerph110303256
Bonizzoni M, Gasperi G, Chen X, James AA (2013) The invasive mosquito species Aedes albopictus: current knowledge and future perspectives. Trends Parasitol 29:460–468. https://doi.org/10.1016/j.pt.2013.07.003
Braaker S, Ghazoul J, Obrist MK, Moretti M (2014) Habitat connectivity shapes urban arthropod communities: the key role of green roofs. Ecology 95:1010–1021
Brown H, Diuk-Wasser M, Andreadis T, Fish D (2008) Remotely-sensed vegetation indices identify mosquito clusters of West Nile virus vectors in an urban landscape in the northeastern United States. Vector-Borne Zoonotic Dis 8:197–206. https://doi.org/10.1089/vbz.2007.0154
Burghardt KT, Tallamy DW, Shriver WG (2009) Impact of native plants on bird and butterfly biodiversity in suburban landscapes. Conserv Biol 23:219–224. https://doi.org/10.1111/j.1523-1739.2008.01076.x
Burkholder S (2012) The new ecology of vacancy: rethinking land use in shrinking cities. Sustainability 4:1154–1172. https://doi.org/10.3390/su4061154
Burkman CE, Gardiner MM (2015) Spider assemblages within greenspaces of a deindustrialized urban landscape. Urban Ecosyst 18:793–818. https://doi.org/10.1007/s11252-014-0430-8
Chase JM, Knight TM (2003) Drought-induced mosquito outbreaks in wetlands. Ecol Lett 6:1017–1024. https://doi.org/10.1046/j.1461-0248.2003.00533.x
Chaves LF, Keogh CL, Vazquez-Prokopec GM, Kitron UD (2009) Combined sewage overflow enhances oviposition of Culex quinquefasciatus (Diptera: Culicidae) in urban areas. J Med Entomol 46:220–226. https://doi.org/10.1603/033.046.0206
Cizek O, Zamecnik J, Tropek R, Kocarek P, Konvicka M (2012) Diversification of mowing regime increases arthropods diversity in species-poor cultural hay meadows. J Insect Conserv 16:215–226. https://doi.org/10.1007/s10841-011-9407-6
Community Research Partners, Rebuild Ohio (2008) $60 Million and counting: The cost of vacant and abandoned properties to eight Ohio cities
DeGaetano AT (2005) Meteorological effects on adult mosquito (Culex) populations in metropolitan New Jersey. Int J Biometeorol 49:345–353. https://doi.org/10.1007/s00484-004-0242-2
Deichmeister JM, Telang A (2011) Abundance of West Nile virus mosquito vectors in relation to climate and landscape variables. J Vector Ecol 36:75–85. https://doi.org/10.1111/j.1948-7134.2011.00143.x
Delgado de la Flor YA, Burkman CE, Eldredge TK, Gardiner MM (2017) Patch and landscape-scale variables influence the taxonomic and functional composition of beetles in urban greenspaces. Ecosphere. 8. https://doi.org/10.1002/ecs2.2007
Dowling Z, Ladeau SL, Armbruster P, Biehler D, Leisnham PT (2013) Socioeconomic status affects mosquito (Diptera: Culicidae) larval habitat type availability and infestation level. J Med Entomol 50:764–772. https://doi.org/10.1603/ME12250
Escobedo FJ, Kroeger T, Wagner JE (2011) Urban forests and pollution mitigation: analyzing ecosystem services and disservices. Environ Pollut 159:2078–2087. https://doi.org/10.1016/j.envpol.2011.01.010
Ferber U, Schlappa H (2016) Managing brownfield land in stagnant land markets. In: Schlappa H, Neill WJV (eds) Future directions for the European shrinking city. Routledge, New York, pp 138–154
Ferraguti M, Martínez-De La Puente J, Roiz D et al (2016) Effects of landscape anthropization on mosquito community composition and abundance. Sci Rep 6:1–9. https://doi.org/10.1038/srep29002
Foster WA (1995) Mosquito sugar feeding and reproductive energetics. Annu Rev Entomol 40:443–474
Fox J, Weisberg S (2011) An {R} companion to applied regression, 2nd edn. Sage, Thousand Oaks
Freed TZ, Leisnham PT (2014) Roles of spatial partitioning, competition, and predation in the north American invasion of an exotic mosquito. Oecologia 175:601–611. https://doi.org/10.1007/s00442-014-2909-7
Gardiner MM, Burkman CE, Prajzner SP (2013) The value of urban vacant land to support arthropod biodiversity and ecosystem services. Environ Entomol 42:1123–1136. https://doi.org/10.1603/EN12275
Gardiner MM, Prajzner SP, Burkman CE, Albro S, Grewal PS (2014) Vacant land conversion to community gardens: influences on generalist arthropod predators and biocontrol services in urban greenspaces. Urban Ecosyst 17:101–122. https://doi.org/10.1007/s11252-013-0303-6
Gardner AM, Anderson TK, Hamer GL, Johnson DE, Varela KE, Walker ED, Ruiz MO (2013) Terrestrial vegetation and aquatic chemistry influence larval mosquito abundance in catch basins, Chicago, USA. Parasites and Vectors 6:1–11. https://doi.org/10.1186/1756-3305-6-9
Ghosh D (2011) Geospatial analysis of West Nile virus (WNV) incidences in a heterogeneous urban environment: a case study in the twin cities metropolitan area of Minnesota. In: Maantay JA, McLafferty S (eds) Geospatial analysis of environmental health. Springer Netherlands, Dordrecht, pp 153–169
Goddard MA, Dougill AJ, Benton TG (2010) Scaling up from gardens: biodiversity conservation in urban environments. Trends Ecol Evol 25:90–98. https://doi.org/10.1016/j.tree.2009.07.016
Gratz NG (1999) Emerging and resurging vector-borne diseases. Annu Rev Entomol 44:51–75. https://doi.org/10.1146/annurev.ento.44.1.51
Grieco JP, Vogtsberger RC, Achee NL, Vanzie E, Andre RG, Roberts DR, Rejmankova E (2005) Evaluation of habitat management strategies for the reduction of malaria vectors in northern Belize. J Vector Ecol 30:235–243
Hamer GL, Kitron UD, Brawn JD, Loss SR, Ruiz MO, Goldberg TL, Walker ED (2008) Culex pipiens (Diptera: Culicidae): a bridge vector of West Nile virus to humans. J Med Entomol 45:125–128
Hamer GL, Anderson TK, Donovan DJ, Brawn JD, Krebs BL, Gardner AM, Ruiz MO, Brown WM, Kitron UD, Newman CM, Goldberg TL, Walker ED (2014) Dispersal of adult Culex mosquitoes in an urban West Nile virus hotspot: a mark-capture study incorporating stable isotope enrichment of natural larval habitats. PLoS Negl Trop Dis 8:6–12. https://doi.org/10.1371/journal.pntd.0002768
Haydock KP, Shaw NH (1975) The comparative yield method for estimating dry matter yield of pasture. Aust J Exp Agric Anim Husb 15:663–670
Hemme RR, Thomas CL, Chadee DD, & Severson DW (2010) Influence of urban landscapes on population dynamics in a short-distance migrant mosquito: Evidence for the dengue vector Aedes aegypti. PLoS Negl Trop Dis 4:e634. https://doi.org/10.1371/journal.pntd.0000634
Heynen N, Perkins HA, Roy P (2006) The political ecology of uneven urban green space: the impact of political economy on race and ethnicity in producing environmental inequality in Milwaukee. Urban Aff Rev 42:3–25. https://doi.org/10.1016/0041-624X(81)90014-7
Hicks DM, Ouvrard P, Baldock KCR, Baude M, Goddard MA, Kunin WE, Mitschunas N, Memmott J, Morse H, Nikolitsi M, Osgathorpe LM, Potts SG, Robertson KM, Scott AV, Sinclair F, Westbury DB, Stone GN (2016) Food for pollinators: quantifying the nectar and pollen resources of urban flower meadows. PLoS One 11:1–37. https://doi.org/10.1371/journal.pone.0158117
Jansson Å (2013) Reaching for a sustainable, resilient urban future using the lens of ecosystem services. Ecol Econ 86:285–291. https://doi.org/10.1016/j.ecolecon.2012.06.013
Jiannino JA, Walton WE (2004) Evaluation of vegetation management strategies for controlling mosquitoes in a southern California constructed wetland. J Am Mosq Control Assoc 20:18–26
Kaufman MG, Fonseca DM (2014) Invasion biology of Aedes japonicus japonicus (Diptera: Culicidae). Annu Rev Entomol 59:31–49. https://doi.org/10.1146/annurev-ento-011613-162012
Kaufman MG, Pelz-stelinski KS, Yee DA et al (2010) Stable isotope analysis reveals detrital resource base sources of the tree hole mosquito, Aedes triseriatus. Ecol Entomol 35:586–593. https://doi.org/10.1111/j.1365-2311.2010.01217.x.Stable
Lacroix R, Delatte H, Hue T, Reiter P (2009) Dispersal and survival of male and female Aedes albopictus (Diptera: Culicidae) on Reunion Island. J Med Entomol 46:1117–1124
LaDeau SL, Allan BF, Leisnham PT, Levy MZ (2015) The ecological foundations of transmission potential and vector-borne disease in urban landscapes. Funct Ecol 29:889–901. https://doi.org/10.1111/1365-2435.12487
Lambin EF, Tran A, Vanwambeke SO, Linard C, Soti V (2010) Pathogenic landscapes: interactions between land, people, disease vectors, and their animal hosts. Int J Health Geogr 9:1–13. https://doi.org/10.1186/1476-072X-9-54
Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, Komar N, Panella NA, Allen BC, Volpe KE, Davis BS, Roehrig JT (2000) Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol 38:4066–4071
Landau KI, Van Leeuwen WJD (2012) Fine scale spatial urban land cover factors associated with adult mosquito abundance and risk in Tucson, Arizona. J Vector Ecol 37:407–418
Lindén A, Mäntyniemi S (2011) Using the negative binomial distribution to model overdispersion in ecological count data. Ecology 92:1414–1421
Little E, Bajwa W, Shaman J (2017a) Local environmental and meteorological conditions influencing the invasive mosquito Ae. albopictus and arbovirus transmission risk in new York City. PLoS Negl Trop Dis 11:1–19. https://doi.org/10.1371/journal.pntd.0005828
Little E, Biehler D, Leisnham PT, Jordan R, Wilson S, LaDeau SL (2017b) Socio-ecological mechanisms supporting high densities of Aedes albopictus (Diptera: Culicidae) in Baltimore, MD. J Med Entomol 54:1183–1192. https://doi.org/10.1093/jme/tjx103
Lokman K (2017) Vacancy as a laboratory: design criteria for reimagining social-ecological systems on vacant urban lands. Landsc Res 42:728–746. https://doi.org/10.1080/01426397.2017.1355446
Lyytimäki J, Sipilä M (2009) Hopping on one leg- the challenge of ecosystem disservices for urban green management. Urban For Urban Green 8:309–315. https://doi.org/10.1016/j.ufug.2009.09.003
MacKay AJ, Muturi EJ, Ward MP, Allan BF (2016) Cascade of ecological consequences for West Nile virus transmission when aquatic macrophytes invade stormwater habitats. Ecol Appl 26:219–232. https://doi.org/10.1890/15-0050/suppinfo
Marcantonio M, Rizzoli A, Metz M, Rosà R, Marini G, Chadwick E, Neteler M (2015) Identifying the environmental conditions favouring West Nile virus outbreaks in Europe. PLoS One 10:1–18. https://doi.org/10.1371/journal.pone.0121158
McCormack GR, Rock M, Swanson K et al (2014) Physical activity patterns in urban neighbourhood parks: insights from a multiple case study. BMC Public Health 14:962–2208. https://doi.org/10.1039/c8bm00492g
Molaei G, Andreadis TG, Armstrong PM, Anderson JF, Vossbrinck CR (2006) Host feeding patterns of Culex mosquitoes and West Nile virus transmission, northeastern United States. Emerg Infect Dis 12:468–474. https://doi.org/10.3201/eid1203.051004
Nassauer JI, Raskin J (2014) Urban vacancy and land use legacies: a frontier for urban ecological research, design, and planning. Landsc Urban Plan 125:245–253. https://doi.org/10.1016/j.landurbplan.2013.10.008
Newman CM, Krebs BL, Anderson TK, Hamer GL, Ruiz MO, Brawn JD, Brown WM, Kitron UD, Goldberg TL (2017) Culex flavivirus during West Nile virus epidemic and interepidemic years in Chicago, United States. Vector-Borne Zoonotic Dis 17:567–575. https://doi.org/10.1089/vbz.2017.2124
NOAA (2018) National Oceanic and Atmospheric Administration and National Weather Service’s forecast records for Cleveland, Ohio. https://w2.weather.gov/climate/xmacis.php?wfo=cle. Accessed 26 November 2018
Norris DE (2004) Mosquito-borne diseases as a consequence of land use change. Ecohealth 1:19–24. https://doi.org/10.1007/s10393-004-0008-7
O’Meara GF, Vose FE, Carlson DB (1989) Environmental factors influencing oviposition by Culex (Culex) (Diptera: Culicidae) in two types of traps. J Med Entomol 26:528–534. https://doi.org/10.1093/jmedent/26.6.528
Paaijmans KP, Wandago MO, Githeko AK, Takken W (2007) Unexpected high losses of Anopheles gambiae larvae due to rainfall. PLoS One 2:e1146. https://doi.org/10.1371/journal.pone.0001146
Pecoraro HL, Day HL, Reineke R, Stevens N, Withey JC, Marzluff JM, Meschke JS (2007) Climatic and landscape correlates for potential West Nile virus mosquito vectors in the Seattle region. J Vector Ecol 32:22–28. https://doi.org/10.3376/1081-1710(2007)32[22:CALCFP]2.0.CO;2
Pradier S, Leblond A, Durand B (2008) Land cover, landscape structure, and West Nile virus circulation in southern France. Vector-Borne Zoonotic Dis 8:253–264. https://doi.org/10.1089/vbz.2007.0178
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Restifo RA (1982) Illustrated key to the mosquitoes of Ohio: Adapted from C.J. Stojanovich's keys to the common mosquitoes of eastern United States. College of Biological Sciences, The Ohio State University In cooperation with Vector-borne Disease Unit, Ohio Department of Health
Riley CB, Herms DA, Gardiner MM (2018b) Exotic trees contribute to urban forest diversity and ecosystem services in inner-city Cleveland, OH. Urban For Urban Green 29:367–376
Riley CB, Perry KI, Ard K, Gardiner MM (2018a) Asset or liability? Ecological and sociological tradeoffs of urban spontaneous vegetation on vacant land in shrinking cities. Sustainability 10:2139. https://doi.org/10.3390/su10072139
Roiz D, Ruiz S, Soriguer R, Figuerola J (2015) Landscape effects on the presence, abundance and diversity of mosquitoes in mediterranean wetlands. PLoS One 10:1–17. https://doi.org/10.1371/journal.pone.0128112
Ruiz MO, Chaves LF, Hamer GL, Sun T, Brown WM, Walker ED, Haramis L, Goldberg TL, Kitron UD (2010) Local impact of temperature and precipitation on West Nile virus infection in Culex species mosquitoes in Northeast Illinois, USA. Parasites and Vectors 3:1–16. https://doi.org/10.1186/1756-3305-3-19
Sandström UG, Angelstam P, Mikusiński G (2006) Ecological diversity of birds in relation to the structure of urban green space. Landsc Urban Plan 77:39–53. https://doi.org/10.1016/j.landurbplan.2005.01.004
Shepard DS, Halasa YA, Fonseca DM, Farajollahi A, Healy SP, Gaugler R, Bartlett-Healy K, Strickman DA, Clark GG (2014) Economic evaluation of an area-wide integrated pest management program to control the Asian tiger mosquito in New Jersey. PLoS One 9:e111014. https://doi.org/10.1371/journal.pone.0111014
Sivakoff FS, Prajzner SP, Gardiner MM (2018) Unique bee communities within vacant lots and urban farms result from variation in surrounding urbanization intensity. Sustainability 10:1926–1943. https://doi.org/10.3390/su10061926
Southon GE, Jorgensen A, Dunnett N, Hoyle H, Evans KL (2017) Biodiverse perennial meadows have aesthetic value and increase residents’ perceptions of site quality in urban green-space. Landsc Urban Plan 158:105–118
Stone CM, Jackson BT, Foster WA (2012) Effects of plant-community composition on the vectorial capacity and fitness of the malaria mosquito Anopheles gambiae. Am J Trop Med Hyg 87:727–736. https://doi.org/10.4269/ajtmh.2012.12-0123
Swengel AB (2001) A literature review of insect responses to fire, compared to other conservation managements of open habitat. Biodivers Conserv 10:1141–1169. https://doi.org/10.1023/A:1016683807033
Turo KJ, Gardiner MM (2019) From potential to practical: conserving bees in urban public green spaces. Front Ecol Environ 17:167–175. https://doi.org/10.1002/fee.2015
van de Poel D, Zehm A (2014) Die wirkung des mähens auf die fauna der wiesen – Eine literaturauswertung für den naturschutz. ANLiegen Natur 36:36–51. https://doi.org/10.1002/9783527678471.hbnl2015001
Veddeler D, Klein AM, Tscharntke T (2006) Contrasting responses of bee communities to coffee flowering at different spatial scales. Oikos 112:594–601. https://doi.org/10.1111/j.0030-1299.2006.14111.x
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York
Walton WE, Jiannino JA (2005) Vegetation management to stimulate denitrification increases mosquito abundance in multipurpose constructed treatment wetlands. J Am Mosq Control Assoc 21:22–27
Wang G, Minnis RB, Belant JL, Wax CL (2010) Dry weather induces outbreaks of human West Nile virus infections. BMC Infect Dis 10. https://doi.org/10.1186/1471-2334-10-38
Wastian L, Unterweger PA, Betz O (2016) Influence of the reduction of urban lawn mowing on wild bee diversity (hymenoptera, Apoidea). J Hymenopt Res 49:51–63. https://doi.org/10.3897/JHR.49.7929
Weaver SC (2013) Urbanization and geographic expansion of zoonotic arboviral diseases: mechanisms and potential strategies for prevention. Trends Microbiol 21:360–363. https://doi.org/10.1016/j.tim.2013.03.003
Western Reserve Land Conservancy (2015) Cleveland property inventory 2015: Cleveland neighborhoods by the numbers. CreateSpace Independent Publishing Platform
Wolch JR, Byrne J, Newell JP (2014) Urban green space, public health, and environmental justice: the challenge of making cities “just green enough”. Landsc Urban Plan 125:234–244. https://doi.org/10.1016/j.landurbplan.2014.01.017
Zhou G, Munga S, Minakawa N et al (2007) Spatial relationship between adult malaria vector abundance and environmental factors in western Kenya highlands. Am J Trop Med Hyg 77:29–35
Acknowledgements
We thank Dr. Richard Gary and the Ohio Department of Health for sharing their expertise on mosquito trapping and species identification, providing mosquito traps, and performing the molecular detection of West Nile virus in field-collected Culex spp. mosquitoes. We thank Dan Meaney, GISP (Cuyahoga County Planning Commission) for providing land cover raw data, and Emily Trejo Sypolt for ArcGIS help in creating Fig. 1. We thank Dr. Frances Sivakoff and Yvan Delgado de la Flor for providing inputs on statistical analyses, and Yvan Delgado de la Flor and Denisha Parker for assisting vegetation collection and data entry. We also thank the Cuyahoga County Board of Health for providing mosquito trapping equipment. The research was funded in part by Ohio Environmental Protection Agency Mosquito Control Grant SFY 2016, NSF CAREER 1253197 grant, and NSF DGE-1343012 fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 47 kb)
Rights and permissions
About this article
Cite this article
Yang, L., Turo, K.J., Riley, C.B. et al. Can urban greening increase vector abundance in cities? The impact of mowing, local vegetation, and landscape composition on adult mosquito populations. Urban Ecosyst 22, 827–839 (2019). https://doi.org/10.1007/s11252-019-00857-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-019-00857-7