Abstract
Understanding the factors determining the occupancy and detection probability of birds in human dominated environments is important for their conservation. In this study we investigated various environmental variables believed to influence the site occupancy and detection probability of Trumpeter Hornbill (Bycanistes bucinator) in urban-forest mosaics of KwaZulu-Natal, South Africa. Presence/absence data were collected from a total of 50 point count stations established between September 2014 and March 2015 in urban-forest mosaics of Durban, Eshowe and Mtunzini. Mean occupancy rate of Trumpeter Hornbill was 0.40 ± 0.09 with a low detection probability of 0.28 ± 0.04. For Trumpeter Hornbills, large trees influenced their occupancy positively (sum AIC weight (ω i ) = 79%) while relative human abundance negatively influenced their occupancy (ω i = 91%). Model selection suggested that housing density had a strong negative influence on detection probability of Trumpeter Hornbills (ω i = 82 % ) and availability of fruiting trees influenced their detection positively (ω i = 29%). With continued changing land use in KwaZulu-Natal, these finding are important for conservation of Trumpeter Hornbills as we provide insight into landscape variables or features that influence Trumpeter Hornbill’s occupancy and detection in areas of urban-forest mosaics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Some of the leading causes of biodiversity lose are climate change, habitat fragmentation due to land use change and illegal international trade in flora and fauna species (Trail 2007; Vačkář et al. 2012; WWF 2016). The current Living Planet Report published by World Wide Fund for Nature in collaboration with Global Footprint Network and Zoological Society of London indicates that global vertebrate population may decline by 67% in the year 2020 as a result of human exploitation of natural resources (WWF 2016). As the world population continues to grow and is projected to reach 9 billion people by 2050 (UN 2015), natural landscapes are greatly being transformed by human encroachment and this has resulted in huge pressure being exerted on the environment (Foley et al. 2005; Péron and Altwegg 2015). These human induced land use patterns result in large-scale transformation of the environment as natural habitats are being converted to agricultural land, settlements, plantation forestry and livestock farming for the sole purpose of providing food, fibre, water and shelter for the growing global population (Foley et al. 2005). Living in these transformed environments, some birds will be favoured at the expense of others as a result of these land use changes (Hockey et al. 2011). For instance, large frugivorous birds such as, the Trumpeter Hornbills (Bycanistes bucinator), that persist in anthropogenic environments have the potential to move within fragmented landscapes and able to fly between forest patches (Lenz et al. 2011; Lenz et al. 2015). In addition, the disappearance of indigenous forests has resulted in some forest associated species, for example the Red-necked Spurfowl (Pternistis afer), to utilise commercial plantation forests in areas where indigenous forest patches covering a small part of the landscape have been extensively fragmented (Ramesh and Downs 2014). For some species such as, the Crested Guinea-fowl (Guttera edouardi), natural forests are important for their survival in landscapes modified for agroforestry (Maseko et al. 2016). These shifts in habitat use by many species of birds is not only a function of land use change but also climate change and that both factors may be acting simultaneously in influencing the dynamic range shifts by South African birds (Hockey et al. 2011). In South Africa, there are few studies on urban ecology (Cilliers and Siebert 2012). As the number of people living in urban areas is expected to increase in many areas globally (McPhearson et al. 2016; UN 2014), understanding the factors influencing the distribution and occupancy of wildlife species that persist and utilise the urban-forest environment is necessary for their management and conservation. This kind of information is lacking for the Trumpeter Hornbill in the urban environments of KwaZulu-Natal (KZN).
Hornbills and parrots are among the world’s most threatened group of birds (Marsden and Pilgrim 2003). Among the frugivorous birds of Africa and Asia, hornbills belong to the major seed dispersers of majority of fruiting trees (Kemp and Woodcock 1995; Kinnaird and O'Brien 2007; Kitamura 2011; Poonswad et al. 2013). The Trumpeter Hornbill is the largest obligate frugivore in South Africa and it is relatively common along the east coast of the country (Kemp and Woodcock 1995). Although the Trumpeter Hornbill is considered as “Least conservation concern” by International Union for Conservation of Nature (IUCN 2012), the species is threatened by habitat lose, international trade and possibly hunting (Trail 2007). The impacts of land use change on this forest dependent bird are relatively poorly known. Studies done on this species recently focused on its movement and seed dispersal patterns in fragmented landscapes dominated by agricultural activities in KZN (Mueller et al. 2014; Lenz et al. 2011, 2015). Other studies have highlighted on the aspects of its general biology, ecology, taxonomy and foraging behaviour (Kemp and Woodcock 1995; Viseshakul et al. 2011; Poonswad et al. 2013; Gonzalez et al. 2013). To our knowledge, little is known about the factors influencing the occupancy of Trumpeter Hornbill in urban-forest mosaics of KZN. We employed point count method to collect data on important environmental variables we predicted would influence their occupancy and detection probability in KZN urban-forest mosaics.
The study of bird abundances is commonly achieved by point counts sampling method (Marsden 1999; Diefenbach et al. 2003; Royle and Nichols 2003;MacKenzie and Royle 2005). At a slightly larger spatial scale, the use of a grid of points (spatial replications) without repeated visits or with fewer repeated visits (temporal replications) to study units is another method used to study avian community (Purcell et al. 2005; Sliwinski et al. 2015). Species presence or absence in a particular environment can be used as a surrogate for population size and abundance when monitoring populations (Mackenzie and Royle 2005). Point count survey is considered as a better method for surveying birds and in determining abundance, occupancy and habitat use (Ralph et al. 1995; Royle and Nichols 2003; Diefenbach et al. 2003; MacKenzie and Royle 2005; Purcell et al. 2005; MacKenzie et al. 2006). The method is cost-effective and its use for systematic detection and non-detection survey provides better assessment of the status of a species by detecting changes in their occupancy and probability estimates as a function of covariates (MacKenzie et al. 2006).
Urbanisation transforms and degrades natural habitats forcing animals to live in close proximity to humans (Marzluff et al. 2001; Chace and Walsh 2006; Bonier et al. 2007; McKinney 2002, 2008). In such degraded environments, many species withdraw into reduced ranges in response to spread of urban environments and anthropogenic climate change (Péron and Altwegg 2015). However, the Trumpeter Hornbill still persist and utilises the urban-forest mosaics of KZN. Little is known on how urban landscapes dominated by human activities influence the occupancy and distribution of this largest obligate frugivore. Here we estimated site occupancy and detection probabilities using presence/absence modelling framework (MacKenzie et al. 2002). We used point count data to evaluate Trumpeter Hornbill occupancy as a function of various land use covariates predicted to influence its occupancy and detection probability in the urban-forest mosaics of KZN. Our main objective was to examine the response of Trumpeter Hornbill to varying land use patterns and establish reliable estimates of occupancy and detection probabilities. Based on the species diet mainly consisting of fruits (Kemp and Woodcock 1995), we predicted that the presence of large trees and fruiting trees would positively influence the occupancy and detection probability of Trumpeter Hornbills in an urban-forest mosaic. Sampling points with large trees and with fruiting trees will be preferred as they provide better refuge and foraging opportunities in an urban-forest environment. We further predicted that human abundance and housing density would negatively influence the occupancy and detection probability of the study species.
Methods
Study area
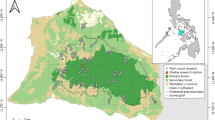
The study was conducted in urban-forest mosaics of KZN, South Africa. This province is situated on the east coast of South Africa and supports one sixth of the remaining South Africa’s indigenous forest which is the smallest biome represented in the country (Eeley et al. 1999; Mucina and Rutherford 2006). The two major forest types, Afromontane forest and Indian Ocean coastal belt forest, which differ in species composition are found in KZN (Mucina and Rutherford 2006). However, these forests have been severely altered by anthropogenic changes and are highly fragmented (Eeley et al. 1999). The urban environment of KZN is dominated by anthropogenic structures (for example, buildings and roads) and natural vegetation is continuously being converted to agricultural land and patches of plantation forests. Fragmented indigenous forests (protected or not protected) that are remaining in the province are unique as they support a significant proportion of the country’s diverse flora and fauna species (Eeley et al. 1999). Three towns in the province were selected for this study. These include Kloof-Durban (Site A), Eshowe (Site B) and Mtunzini (Site C) (Fig. 1). The choice of these towns was based on the fact that each one of them has one or more protected areas (Forests or Nature Reserves) surrounded by human settlements and resultant anthropogenic structures. Such urban or suburban areas provide a perfect scenario for studying the factors influencing the distribution pattern and occupancy of avian species that persist and utilise an urban-forest mosaic.
The climate of KZN is generally described as warm and temperate and most rainfall occurs in summer (Mucina and Rutherford 2006). Summary information on climate and selected protected areas for the three towns considered in this study is presented together with information on altitude, coordinates and number of point count stations established in each site (Table 1).
Data collection and analysis
We established 50 point count stations to record the presence and absence of Trumpeter Hornbills in the three study areas in KZN (Fig. 1). The points were established systematically by selecting the first point at random and setting the remaining points in relation to the first point with inter point distance of approximately 1 km. The point count stations were established using a hand held GPSMAP 62sc (Garmin International, Kansas, USA). To reliably separate out occupancy from detection (i.e. where the species is versus where the species is found), repeated surveys are required. In view of this, each point count station and survey occasion was treated as independent and presence (1) and absence (0) data were collected by temporal replication by visiting the same point more than once. To avoid heterogeneity in detection probabilities resulting from multiple observers, presence/absence data were collected by a single observer. According to the law of diminishing returns, the number of visits suggested for studies using point counts is between two and five mostly based on forest bird studies (Grant et al. 2004; Field et al. 2005; Koper et al. 2009; Ralph et al. 1995). However, Sliwinski et al. (2015) argue that unless the species or all species in the community have detection probabilities of greater than 0.7, repeated visits of between two to five times may be insufficient sampling effort for detecting species or communities at single points with 90% confidence. They recommended at least seven visits to the same count location to be confident that the species are truly absent if not detected. In view of this, sampling points were each surveyed 10 times between September 2014 and March 2015. Data were collected from 6 h00 to 11 h00 and 20 min was spent at each sampling point. At each point, important site-specific covariates were also collected within a radius of 30 m. Each point was assessed with regards to the number of fruiting trees available, number of large trees, human abundance and elevation. Fruiting trees were defined as any tree bearing fruits (indigenous, alien or cultivated), and large trees were defined as any tree with diameter at breast height (DBH) of greater than 50 cm and were counted. The number of humans and vehicles counted/10 sampling occasions at each point count station was considered as relative abundance index (RAI) of human activity. By considering the mean daily distance covered by Trumpeter Hornbill (about 1 km) in an urban-forest mosaic (Chibesa et al. 2017), we extracted the housing density at each point count station within a 1 km square grid using ArcGIS 9.3.1 (ESRI, Redlands, CA, USA) from the 2005/6 housing mapping map for the eastern region of the country (GeoterraImage 2010). All covariates were standardised to z scores (Cooch and White 2005). Many factors could influence the occupancy and distribution of Trumpeter Hornbills in the environments they are found. In this study, we only considered those factors we thought would influence the occupancy and detection probability of the study species in an urban-forest environment (Table 2).
We used a single-season occupancy model to estimate the occupancy (ψ or psi) and detection probability (p) of Trumpeter Hornbill (MacKenzie et al. 2006). For each point count station, we generated detection history of Trumpeter Hornbill consisting of ‘1’ meaning species detected during the sampling occasion and ‘0’ indicating species not detected (Otis et al. 1978). PRESENCE 11.6 (Hines 2006) was used to model site occupancy and detection probability with its covariates. A global model that contained all potential covariates for occupancy was calculated. We then allowed detection probability (p) to vary by all covariates. A two-step procedure was followed, with detection probability (p) modelled first, then occupancy (ψ). Next we allowed the potential covariates for occupancy to vary singly or in combination, whereas detection was maintained either in the global model or kept constant (that is, ψ(covariate), p(covariate) or ψ(covariate),p(.)). For model selection, calculation of model weights and averaging of parameters, we followed the framework of Burnham and Anderson (2002). Using 10,000 parametric bootstrap in the final model, we tested model fit by estimating mean dispersion parameter (ĉ or c-hat) (White and Burnham 1999). Models with ĉ values of ~1 were better descriptors of data and models with ĉ ˃ 1 indicate that there was more dispersion or variation in observed data than anticipated (Burnham and Anderson 2002). Akaike’s information criterion (AIC ≤ 2) was used to rank the models (Burnham and Anderson 2002; Hines 2006). Occupancy and detection probability parameters were estimated from the best model that had the lowest AIC and ∆AIC values and high value of Akaike weights (AICwgt or ω i ). The variable strength on occupancy and detection probability was determined by calculating the Akaike weights. To determine the relative importance of each covariate on Trumpeter Hornbill occupancy and detection, model weights were summed over all models containing the particular covariate of interest.
Results
The estimated site occupancy and detection probability of Trumpeter Hornbill based on the model with all parameters held constant (i.e. psi(.),p(.)) was 0.40 ± 0.07 and 0.28 ± 0.03 respectively. The difference between naive occupancy (0.38) and estimated site occupancy was minimal (Table 3). Four of the variables considered were substantially associated with Trumpeter Hornbill occurrence (High sum of AIC weight, Table 3). A test of goodness of fit for the global model suggested no lack of fit (ĉ = 1.1) and the best occurrence model (ΔAICc = 0) was ψ(HA + LTREES),p(HD) (Table 3, highest AIC weight = 0.42) indicated that the variables, number of large trees influenced occupancy positively (β = 0.86 ± 0.45, Table 4) and relative human abundance influenced occupancy negatively (β = −1.21 ± 0.68, Table 4; Fig. 2b). In the same model, the detection probability of Trumpeter Hornbill was 0.27 ± 0.04 and it was negatively influenced by housing density (β = −0.39 ± 0.15, Table 4). Of the two top ranked models (ΔAICc ≤ 2, Table 3), occupancy for both models was positively influenced by the presence of large trees while relative human abundance influenced occupancy negatively and detection was negatively influenced by housing density (Figs. 2a, b and 3b). In the second ranked model, detection was positively influenced by fruiting trees availability (Fig. 3a).
The overall summed model weights for the four variables in the top two models with respect to Trumpeter Hornbill occupancy were human abundance (91%) and number of large trees (79%). The influence of elevation on occupancy was negligible (5%). The variables that best predicted Trumpeter Hornbill detection probability across all models were housing density (ω i = 0.82 ; negatively) and fruit availability (ω i = 0.29 ; positively). The mean model occupancy (0.40 ± 0.09) and detection probability (0.28 ± 0.04) were chosen as final estimates. This corresponded to a difference of 5.2% from naive occupancy.
Discussion
Our study indicated the importance of various environmental factors that influence the occupancy and detection probability of Trumpeter Hornbills in an urban-forest mosaic. In such modified landscapes dominated by human activities the importance of these covariates is of relevance to Trumpeter Hornbills conservation and for the formulation of management strategies for the persistence of forest dependent species. Trumpeter Hornbills were only detected at 19 of the 50 point count stations (naive occupancy of 0.38). Often Trumpeter Hornbills were only detected in one or two of the repeated surveys, clearly indicating that detection probabilities are less than 1. There conceivably may be a number of points where the Trumpeter Hornbills were indeed present but simply never detected during the survey. Such low detection probabilities observed could be possibly attributed to their movement and flocking patterns as the presence/absence data were collected during the time that encompassed their breeding season. In the breeding period, majority of the females are sealed in their nests and only small groups of 3 to 5 individuals are observed as opposed to non-breeding period when large flocks of up to 100 individuals are observed often at a fruiting tree (Kemp and Woodcock 1995; pers. obs.). In addition, the abundance of large frugivorous hornbills is known to be associated with food availability and some species are also negatively related to habitat disturbance due to lower availability of food resources (Anggraini et al. 2000). This agrees with what we found as the detection probability of Trumpeter Hornbills in the urban-forest mosaics of KZN were positively influenced by availability of fruiting trees and negatively influenced by housing density. Both indigenous, alien and cultivated fruits were available in various urban gardens of KZN thus providing food resources for the Trumpeter Hornbills all year round as they do not all fruit at the same time (Bleher et al. 2003). Areas with high housing density tend to have fewer large trees and fruiting trees as most of the natural habitat is cleared for housing development and other anthropogenic structures such as access roads. Although a variety of cultivated fruits and isolated keystone species such as figs which are presumably preferred by frugivorous hornbills (Lambert and Marshall 1991; Kemp and Woodcock 1995; Kitamura 2011; Winarni and Jones 2012) may be found in high housing density areas, hornbills tend to avoid such landscapes as they have fewer or no larger trees in close proximity to a fruiting tree which are important for perching and providing cover when hornbills are disturb from the fruiting tree (pers. obs.). Another possible explanation for the low detection probabilities observed may be attributed to the scarcity of ripe fruits and fruiting trees during the period when presence and absence data were collected. Trumpeter Hornbills selectively feed on ripe fruits (Kemp and Woodcock 1995) and the peak periods of fruiting trees and ripe fruits in KZN have been reported to be during the end of August to early September and highest peak being between March and May (Bleher et al. 2003). It is highly likely that detection probabilities would have been higher than what we found during these periods of high fruit availability which is also a non-breeding season of the study species when large flocks are observed.
We also found that the pattern of occupancy by Trumpeter Hornbills in urban-forest mosaics of KZN were positively influenced by the presence of large trees and negatively affected by relative human abundance. Large trees provide suitable opportunities for nesting (Kemp and Woodcock 1995; Kinnaird and O'Brien 2007; Poonswad et al. 2005, 2013), although there is little evidence of Trumpeter Hornbills nesting in urban areas in the absence of a nearby protected natural forest, and its large trees within the canopy which may produce the larger fruit crops (Kinnaird and O'Brien 2007). The presence of protected natural forest in urban environments act as roosting, foraging and nesting sites for Trumpeter Hornbills. However, during periods of food resources scarcity, urban environments that maintain a health state of vegetation cover (low human abundance and low housing density) presents a suitable alternative for foraging opportunities and possibly nesting sites for Trumpeter Hornbills. The availability of large trees and the presence of a variety of fruiting trees attract Trumpeter Hornbills to such less modified urban settlements. The negative consequence is that the Trumpeter Hornbills might be dispersing the seeds of alien plants to natural forests by consuming fruits of alien plants from urban environments and transporting them to natural forests within KZN. As such, advising and encouraging people living in urban environment where the Trumpeter Hornbills are a come sighting to plant indigenous fruiting trees in their gardens will be a positive move in trying to halt the proliferation of alien plants in natural forests resulting from alien seeds possibly dispersed by Trumpeter Hornbills from urban gardens. The influence of elevation on occupancy was minimal possibly due to the fact that the difference in elevation for the three sites considered in this study was not significant.
Our occupancy modelling indicated a clear understanding of the factors determining the occupancy and detection probabilities of Trumpeter Hornbills in urban-forest mosaics of KZN. Four important environment covariates influenced occupancy and detection probabilities. Our results indicated that the distribution and occupancy of Trumpeter Hornbills is strongly influenced by the availability of large trees and relative human abundance and that detection is a function of fruit availability and housing density. However, we believe that there could be other factors that might influence the occupancy and detection probabilities of the study species that were not included in this study. Our findings have important conservation implications for managing the Trumpeter Hornbills in urban-forest mosaics of KZN. We provide insight into landscape variables or features that influence Trumpeter Hornbill’s occupancy and detection in an urban-forest mosaic. However, further research is required to determine whether this is typical throughout its range and how this varies with season.
References
Anggraini K, Kinnaird M, O'Brien T (2000) The effects of fruit availability and habitat disturbance on an assemblage of Sumatran hornbills. Bird Conserv Int 10:189–202
Bleher B, Potgieter CJ, Johnson DN, Böhning-Gaese K (2003) The importance of figs for frugivores in a South African coastal forest. J Trop Ecol 19:375–386
Bonier F, Martin PR, Wingfield JC (2007) Urban birds have broader environmental tolerance. Biol Lett 3:670–673
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Chace JF, Walsh JJ (2006) Urban effects on native avifauna: a review. Landsc Urban Plan 74:46–69
Chibesa M, Taylor B, Tharmalingam R, Downs CT (2017) Home range and habitat use of trumpeter hornbill in an urban-forest mosaic, Eshowe. South Africa, Ostrich In press
Cilliers SS, Siebert SJ (2012) Urban ecology in Cape Town: South African comparisons and reflections. Ecol Soc 17:33
Cooch E, White G (2005) Program mark: a gentle introduction. Available at: http://www.phidot.org/software/mark/docs/book. downloaded November, 2016
Diefenbach DR, Brauning DW, Mattice JA (2003) Variability in grassland bird counts related to observer differences and species detection rates. Auk 120:1168–1179
Eeley HAC, Lawes MJ, Piper SE (1999) The influence of climate change on the distribution of indigenous forest in KwaZulu-Natal, South Africa. J Biogeogr 26:595–617
Field SA, Tyre AJ, Possingham HP, Lubow BC (2005) Optimizing allocation of monitoring effort under economic and observational constraints. J Wildl Manag 69:473–482
Foley JA, DeFries R, Asner GP, Barford C, Bonan G, Carpenter SR, Chapin FS, Coe MT, Daily GC, Gibbs HK, Helkowski JH, Holloway T, Howard EA, Kucharik CJ, Monfreda C, Patz JA, Prentice IC, Ramankutty N, Snyder PK (2005) Global consequences of land use. Science 309:570
GeoterraImage (2010) 2005/6 ESKOM Dwelling mapping layer. (from SPOT2.5m resolution natural colour satellite imagery) Prepared for ESKOM, South Africa
Gonzalez JCT, Sheldon BC, Collar NJ, Tobias JA (2013) A comprehensive molecular phylogeny for the hornbills (Aves: Bucerotidae). Mol Phylogenet Evol 67:468–483
Grant TA, Madden E, Berkey GB (2004) Tree and shrub invasion in northern mixed-grass prairie: implications for breeding grassland birds. Wildl Soc Bull 32:807–818
Hines JE (2006) PRESENCE-Software to estimate patch occupancy and related parameters.USGS-PWRC. http://www.mbr-pwrc.usgs.gov/software/presence.html
Hockey PAR, Sirami C, Ridley AR, Midgley GF, Babiker HA (2011) Interrogating recent range changes in South African birds: confounding signals from land use and climate change present a challenge for attribution. Divers and Distrib 17:254–261
IUCN (2012) IUCN Red List of Threatened Species (ver. 2012.1). Available at: http://www.iucnredlist.org. downloaded June 2012
Kemp AC, Woodcock M (1995) The hornbills: Bucerotiformes. Oxford University Press, UK
Kinnaird MF, O'Brien TG (2007) The ecology and conservation of Asian hornbills: farmers of the forest. The University of Chicago Press, Chicago
Kitamura S (2011) Frugivory and seed dispersal by hornbills (Bucerotidae) in tropical forests. Acta Oecol 37:531–541
Koper N, Walker DJ, Champagne J (2009) Nonlinear effects of distance to habitat edge on Sprague’s pipits in southern Alberta, Canada. Landsc Ecol 24:1287–1297
Lambert FR, Marshall AG (1991) Keystone characteristics of bird-dispersed ficus in a Malaysian lowland rain forest. J Ecol 79:793–809
Lenz J, Fiedler W, Caprano T, Friedrichs W, Gaese BH, Wikelski M, Böhning-Gaese K (2011) Seed-dispersal distributions by trumpeter hornbills in fragmented landscapes. P Roy Soc B-Biol Sci 278:2257–2264
Lenz J, Böhning-Gaese K, Fiedler W, Mueller T (2015) Nomadism and seasonal range expansion in a large frugivorous bird. Ecography 38:54–62
MacKenzie DI, Royle JA (2005) Designing occupancy studies: general advice and allocating survey effort. J Appl Ecol 42:1105–1114
MacKenzie DI, Nichols JD, Gideon BL, Droege S, Royle JA, Langtimm CA (2002) Estimating site occupancy rates when detection probabilities are less than one. Ecology 83:2248–2255
MacKenzie DI, Nichols JD, Royle JA, Pollock KH, Bailey LL, Hines JE (2006) Occupancy estimation and modeling: inferring patterns and dynamics of species occurrence. Elsevier, Burlington
Marsden SJ (1999) Estimation of parrot and hornbill densities using a point count distance sampling method. Ibis 141:327–390
Marsden SJ, Pilgrim JD (2003) Factors influencing the abundance of parrots and hornbills in pristine and disturbed forests on New Britain, PNG. Ibis 145:45–53
Marzluff J, Bowman R, Donnelly R (2001) A historical perspective on urban bird research: trends, terms, and approaches. In: Marzluff J, Bowman R, Donnelly RD (eds) Avian ecology and conservation in an urbanizing world. Springer, US, pp 1–17
Maseko MST, Ramesh T, Kalle R, Downs CT (2016) Response of crested Guinea-fowl (Guttera edouardi), a forest specialist, to spatial variation in land use in iSimangaliso Wetland Park. South Africa J Ornithol. doi:10.1007/s10336-016-1406-7
McKinney ML (2002) Urbanization, biodiversity, and conservation. Bioscience 52:883–890
McKinney M (2008) Effects of urbanization on species richness: a review of plants and animals. Urban Ecosyst 11:161–176
McPhearson T, Pickett STA, Grimm NB, Niemelä J, Alberti M, Elmqvist T, Weber C, Haase D, Breuste J, Qureshi S (2016) Advancing urban ecology toward a science of cities. BioScience. doi:10.10993/biosci/biw002
Mucina L, Rutherford MC (2006) The vegetation of South Africa, Lesotho and Swaziland, Strelitzia 19. South African National Biodiversity Institute, Pretoria
Mueller T, Lenz J, Caprano T, Fiedler W, Böhning-Gaese K (2014) Large frugivorous birds facilitate functional connectivity of fragmented landscapes. J Appl Ecol 51:684–692
Otis DL, Burnham KP, White GC, Anderson DR (1978) Statistical inference from capture data on closed animal populations. Wildl Monogr 3:135
Péron G, Altwegg R (2015) Twenty-five years of change in southern African passerine diversity: nonclimatic factors of change. Glob Chang Biol 21:3347–3355
Poonswad P, Sukkasem C, Phataramata S, Hayeemuida S, Plongmai K, Chuailua P, Thiensongrusame P, Jirawatkavi N (2005) Comparison of cavity modification and community involvement as strategies for hornbill conservation in Thailand. Biol Conserv 122:385–393
Poonswad P, Kemp A, Strange M, Laman T (2013) Hornbills of the world: a photographic guide. Draco Publishing and Distribution, Singapore
Purcell KL, Mori SR, Chase MK (2005) Design considerations for examining trends in avian abundance using point counts: examples from oak woodlands. Condor 107:305–320
Ralph CJ, Droege S, Sauer JR (1995) Managing and monitoring birds using point counts: Standards and applications. USDA Forest Service. Technical Report. PSW-GTR-149
Ramesh T, Downs CT (2014) Land use factors determining occurrence of red-necked Spurfowl (Pternistis afer) in the Drakensberg midlands, South Africa. J Ornithol 155:471–480
Royle JA, Nichols JD (2003) Estimating abundance from repeated presence-absence data or point counts. Ecology 84:777–790
Sliwinski M, Powell L, Koper N, Giovanni M, Schacht W (2015) Research design considerations to ensure detection of all species in an avian community. Methods Ecol Evol 7:456–462
Trail PW (2007) African hornbills: keystone species threatened by habitat loss, hunting and international trade. Ostrich 78:609–613
UN (2014) World urbanisation prospects: working paper no. ST/ESA/SER.A/352. United Nation Department of Economic and Social Affairs, Population Division, New York
UN (2015) World population prospects: working paper no. ESA/P/WP. 241. United Nation Department of Economic and Social Affairs, Population Division, New York
Vačkář D, Brink ten B, Loh J, Baillie JEM, Reyers B (2012) Review of multispecies indices for monitoring human impacts on biodiversity. Ecol Indic 17:58–67
Viseshakul N, Charoennitikul W, Kitamura S, Kemp A, Thong-Aree S, Surapunpitak Y, Poonswad P, Ponglikitmongkol M (2011) A phylogeny of frugivorous hornbills linked to the evolution of Indian plants within Asian rainforests. J Evol Biol 24:1533–1545
White GC, Burnham KP (1999) Program MARK: survival estimation from populations of marked animals. Bird Study 46:S120–S139
Winarni NL, Jones M (2012) Effect of anthropogenic disturbance on the abundance and habitat occupancy of two endemic hornbill species in Buton island, Sulawesi. Bird Conserv Int 22:222–233
WWF (2016) Living Planet Report (2014). Risk and resilience in a new era. WWF International, Switzerland
Acknowledgements
We thank the National Research Foundation (ZA), University of KwaZulu-Natal (South Africa) and the Copperbelt University (Zambia) for the financial assistance provided to the first author. We thank EKZN Wildlife for granting us permission to conduct research in their nature reserves. We thank all nature conservancies in KZN for assisting with information on Trumpeter Hornbill sightings. Last but not the least, thanks to all graduate and post-graduate students that assisted with data collection with regards to point count station establishment and recording data on large trees, fruiting trees and relative human abundance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chibesa, M., Downs, C.T. Factors determining the occupancy of Trumpeter Hornbills in urban-forest mosaics of KwaZulu-Natal, South Africa. Urban Ecosyst 20, 1027–1034 (2017). https://doi.org/10.1007/s11252-017-0656-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-017-0656-3