Abstract
Sonoran Desert habitat in southern Arizona is increasingly altered by urban development near metropolitan areas. Understanding how reptiles respond in these impacted habitats is critical to conservation efforts to retain intact biotic communities, especially those with a high diversity of reptile species. We surveyed snakes at one impacted site on the northern edge of the Phoenix metropolitan area in desert/urban interface, and at another site in a desert/rural interface near Florence, Arizona. The site near Phoenix was lower in species richness (15 spp.), and evenness: two snake species accounted for 75 % of all snakes encountered (total = 420). The site near Florence was higher in species richness (19 spp.), and a more even community: no species accounted for more than 20 % of snakes encountered (total = 594). Sampling methodology had a strong influence on species richness and abundance of snakes at the respective sites: road riding, coverboards, and traps each provided evidence of unique species missed by other methods. These results were compared with inventories at three other sites in the central Sonoran Desert of Arizona, and are consistent with the view that the impacted site near Phoenix is uneven, potentially as a result of a single species reacting to shifts in prey availability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Organisms isolated in remnant habitat patches near metropolitan areas potentially experience a host of ecological shifts due to urbanization (Sullivan et al. 2013, 2014a). Recent studies of small vertebrates have documented reduction in gene flow and increased predation risk for species isolated in these islands of habitat (e.g., Kjoss and Litvaitis 2001; Sorace and Gustin 2009). Avian species occurring in Sonoran Desert preserves in the rapidly expanding Phoenix metropolitan region are impacted by preserve size and nearby land use; these responses vary by ecological guild among passerine birds (Litteral and Wu 2012). By contrast, Common Chuckwalla lizards in these same Phoenix area preserves persist in even very small patches of appropriate habitat in otherwise highly disturbed landscapes (Sullivan and Sullivan 2012). Other lizards, however, may be lost from the same preserves as a result of the extinction of a single prey species (Sullivan et al. 2014b). For many groups we lack critical baseline data from relatively undisturbed habitats to allow comparisons with preserves: snakes are a particularly neglected group of mesopredators in part because of the difficulty of documenting populations accurately (Sorace and Gustin 2009; Loughran et al. 2013).
The community ecology of squamate reptiles (snakes and lizards, almost 40 % of amniotes), has received considerable attention, with lizards having long served as model organisms for elucidating a variety of ecological principles (Pianka 1986; Wiens 2015). Snakes have been the subject of far fewer studies, in part because of the difficulty of surveying populations and obtaining data on species richness and abundance of these reclusive reptiles (Sullivan 1981a; Sullivan 2012; McKnight et al. 2015). Recent analyses of snake community structure from temperate and tropical biomes suggest that snakes may play important roles as mesopredators and warrant consideration from a conservation perspective (reviewed by Luiselli 2007; Burbrink and Myers 2015). In a study of a farmland and forest mosaic in New England, Kjoss and Litvaitis (2001) documented that large snakes, in part as a result of preference for larger habitat patches, affected abundance of smaller snake species directly through predation. Thus, interactions among snakes influencing community structure are complex, and complicate conservation efforts for these reptiles. In spite of these recent studies, snakes of arid environments remain little studied, especially with respect to widespread anthropogenic effects (Jones et al. 2011).
It is widely appreciated that snakes are difficult to survey, but somewhat ironically, since the 1920s it has been recognized that many snake species can be censused effectively by simply driving a motor vehicle slowly on a paved road during the early evening in spring and summer (see reviews in Sullivan 2000; Jones et al. 2011; Sullivan 2012; Andrews 2013). Snakes may be found on roadways by chance, or because they use paved road surfaces, which retain heat, for thermoregulation in the early evening during spring and summer (Sullivan 1981b; Bernardino and Dalrymple 1992). Recently, in reviewing a number of methods of sampling reptiles, McKnight et al. (2015) documented that road riding was a highly effective means of sampling snakes in comparison to other methods such as coverboards and pitfall traps.
During long-term studies of urban impacts on amphibians and reptiles of Phoenix area preserves, we have gathered data on snakes at the largest and most disturbed preserve (~ 2100 ha) on the edge of the metropolitan region, Cave Buttes (“CB” hereafter; Sullivan et al. 2014a, b, c). Here we evaluate the hypothesis that the CB community is shifted as a result of urban impacts relative to less disturbed sites more distant from metropolitan areas. We compare five Sonoran Desert sites with regard to species richness and evenness of their respective snake communities.
Materials and methods
We employed multiple sampling methods, including road riding, coverboards and incidental encounters, to document species richness and abundance at the CB site over the past five years (2010–2015). For comparative purposes, we selected a similar but relatively unimpacted site to the southeast of the Phoenix metropolitan area, the Florence Military Reservation (“FMR” hereafter), Pinal Co., Arizona. Methods differed somewhat at these two sites, but nonetheless we obtained a sufficiently large number of snakes for evaluation; sites and variation in methodology are described in greater detail below. We also make use of data on the snake communities at three other sites in the central Sonoran Desert relatively distant from urban centers that have been surveyed and described in relative detail to date: Organ Pipe Cactus National Monument (Rosen and Lowe 1994; “SR 85” hereafter), the Mobile Road area south of Phoenix (Jones et al. 2011; “SR 238” hereafter), and the Sun Valley Parkway west of Phoenix (Jones et al. 2011; “SVP” hereafter).
Cave buttes
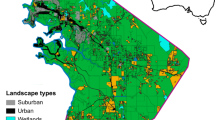
CB, northcentral Maricopa Co., Arizona, comprises the flood plain of Cave Creek, a xero-riparian corridor on the eastern edge of the Union Hills, a portion of the hills proper (Arizona Upland subdivision of the Sonoran Desert), and the open flats (Lower Colorado River subdivision of the Sonoran Desert) on either side of the typically dry creek bed (Sullivan et al. 2014a, b, c). CB is a significantly impacted site containing two large dams (250–750 m in length), three earthen dikes (0.5–2.0 km), and an extensive interconnecting series of maintained roadways (paved, gravel, and dirt) for flood control purposes, initiated almost 100 years ago (Fig. 1). It was heavily grazed over the past 100 years until 2010; the site was also heavily used by off-road vehicle enthusiasts (1978–2009) until air quality issues lead to closure of the site for those activities.
Left panel: Study sites for snake communities of the north-central Sonoran Desert for the present study (CB and FMR), and two previous studies Jones et al. (2011: SVP and SR 238) and Rosen and Lowe (1994: OPCNM). Right panel: Study sites with adjacent paved roads (green) within a five km buffer area as mapped for fragmentation analysis
The survey methods adopted at CB from 2010 to 2015 consisted of 1) road riding, 2) coverboards, and 3) incidental observations while hiking or driving through the site. First, dedicated road riding was conducted along a 4 km stretch of paved roadway during the early evening (1900–2200 h, April through October) from 2010 to 2015. In 2013, a second paved road was placed across the northern third of the CB site, a large, well shouldered public thoroughfare, 9.6 km long. During 2012–2013 we were afforded access to this route prior to opening for public use, and we combined road riding efforts along both roads for that year (once per month). During 2014–2015, we only sampled this second longer route during the late morning to make note of any road killed snakes. Because of traffic issues, we did not make use of this road for dedicated road riding after 2013.
A second survey method was the use of two coverboard arrays, which comprised roughly 1 m2 pieces of refuse plywood that we placed along a 100 m transect in an open flats. Each coverboard array constituted 8–10 separate boards, each of which was placed singly, 5 m from the nearest adjacent coverboard, under the canopy of a large palo verde tree so that it was shaded for most of the day. A total of 18–20 coverboards were sampled from 2010 through 2015, although uneven weathering and flooding reduced the number to 15 in two of the six periods.
Last, “incidental” observations of snakes were obtained while we walked through the site using visual encounter survey methods to sample desert tortoises or lizards (Sullivan et al. 2014a, b, c). From 2010 through 2015 we amassed 2250 person hours of incidental survey activity. These surveys included driving on dirt tracks between sampling areas, as well as hiking on steep slopes in upland habitats not typically adjacent to roadways, and difficult to sample using coverboard arrays.
Florence military reservation
The FMR site is located in Pinal County, Arizona, covers approximately 2288 ha of Sonoran Desert. The vegetation is characteristic of both the Lower Colorado River Valley and the Arizona Upland subdivisions of the Sonoran Desert. State Route 79 bisects the site east and west and the canal cuts across the southeastern corner. Most of the site contains gently sloping desert with deeply incised washes; steep, boulder strewn hills are present on the eastern border.
The survey methods adopted at FMR from 2008 to 2015 consisted of 1) road riding, 2) traps, and 3) visual encounter surveys while hiking through the site. Between 2008 and 2014, we conducted road riding surveys between the months April and June on SR 79 from Hunt Highway to the railroad crossing just south of Florence Junction (Leavitt et al. 2013). Between 2008 and 2014, we also sampled snakes using trapping arrays, hoping to sample small, secretive snakes similar to those seen in coverboard arrays at CB. Twenty-nine trapping locations were selected at random within the FMR primary training area as well as on State Trust leased lands to the north. Each trapping array was composed of a 30-m drift fence with four box traps following standard designs (Willson 2013). Each trapping array was randomly oriented with minor adjustments to avoid destruction of dense vegetation. Finally, we placed soil in and around the traps in order to keep them cool and further facilitate thermoregulation.
Visual encounter surveys were conducted on 85, randomly selected, 3-ha plots between 2011 and 2014 (Hoffman and Leavitt 2015). We conducted surveys between sunrise and 1200 and between 1500 and sunset, depending on the temperature. Surveyors walked parallel transects at approximately 30 m intervals to ensure complete coverage of each plot. All reptile species encountered on plot were recorded along with location data and photographs for identification. Each plot was surveyed 5 times throughout the summer monsoon season (July to October). Although systematic in execution, these were roughly analogous to the incidental survey methods used at CB (3400 person hours), clearly contrasting with road riding and traps or coverboards.
Data analysis
To calculate a measure of diversity between sites and using different methods we applied Fisher’s alpha, recognized as a sample-size, independent diversity metric and appropriate with data from different scales (Magurran 2004). To examine how methods influence the outcome of diversity estimates we compared values of diversity for each method with those for the total estimate per site. To measure the relative level of fragmentation (as one index of urban impacts) for each site we created a 5 km buffer around the entire site. Then, using the recent paved roads layer, we divided the total road length within the buffer area by the total site area for a fragmentation index. This fragmentation index can be interpreted as road density per site (km/ha). All landscape analysis was conducted in ArcGIS Desktop (ESRI 2011).
We used data obtained in our prior work, published in Jones et al. (2011), representing snake communities of two sites to the west and southwest of Phoenix; we also extracted data from Rosen and Lowe (1994). To visualize the differences in relative snake abundance between Sonoran Desert communities we combined data from CB and FMR with those from three additional surveys at Organ Pipe Cactus National Monument (SR 85; Rosen and Lowe 1994) and Sun Valley Parkway and Maricopa Road (SVP and SR 238, respectively; Jones et al. 2011) on a rank abundance plot (Whittaker 1965; Krebs 1999). In order to assess differences in dominance or evenness between these sites we compared the slopes of the rank abundance plots using analysis of covariance (ANCOVA). Because the rank abundance curves are not linear we log transformed raw abundance data (Magurran 2004). To compare methods used to capture snakes between CB and FMR we compared richness and diversity measures (above).
Results
Methodological differences
Overall, Florence Military Reservation (FMR) was more diverse than Cave Buttes (CB; Table 1); 19 species were detected at FMR (Fisher’s alpha =3.75), and only 15 species at CB (Fisher’s alpha =3.05). Sampling methods provided dramatically different pictures of the snake community at the two primary study sites (Table 1). For example, at FMR, road riding was the most effective method with respect to documentation of overall species richness, a total of 18 species (of 19 possible), with trapping arrays a close second (14 species), and incidental captures (7 species) were least effective. By contrast, at CB incidental captures made up the majority of snakes recorded and represented the highest species richness (11 species, of 15 possible), whereas road riding documented only half as many (7 species) and coverboards even fewer snakes (5 species). At FMR, incidental captures heavily underestimated overall species richness; yet the opposite was true for CB where lower species richness was estimated using road riding methods. Differences in incidental captures at the two sites were not the result of higher levels of survey activity as total survey hours at CB (2250 h) did not exceed those at FMR (3400 h).
Our observations reveal that roughly 80 % of the species present at FMR (19 spp) were documented at CB (15 spp); the species absent from CB represent small secretive burrowing forms that reach their northeastern distributional boundaries in the Phoenix area. Hence, it is not surprising that they are absent from the CB site, which lacks historical records for these forms (and hence, they may have been absent prior to large scale habitat alterations). At CB we adopted coverboards in the hope of documenting the presence of such small secretive species, but were unsuccessful. The methods employed suggest that evenness was strongly influenced by sampling methodology. For example, the use of drift fences and traps led to a great increase in abundance of small, secretive forms at FMR, much higher than evidence provided by the use of coverboards at CB. Coverboards did provide critical evidence for two species at CB: Hypsiglena chlorophaea was not documented by any other method and Sonora semiannulata was documented at considerably higher numbers by use of coverboards.
Community differences
Snake community structure differed between our study sites (ANCOVA of rank abundance plots: F 5,83 = 157.10, P < 0.001). As compared to previously reported locations within the Sonoran Desert, FMR was equally if not more diverse whereas CB was less diverse (Table 2, Fig. 2). The higher, negative slope of the relative abundance distribution for CB indicates dominance of that community by a single species, and relatively uneven community overall (Table 2). There were 180 more captures of the most abundant species (Crotalus atrox) than the next most abundant species (Coluber flagellum; Table 1) at CB. Snake communities at all other Sonoran Desert sites (Fig. 2, Table 2) exhibited more evenness relative to CB. Both SVP and SR-238 were dominated by the same most abundant species (Crotalus cerastes; 36 % of all snakes observed) and there was a precipitous drop to the next most abundant species which contributed to nearly one fifth of all captures (SVP 24 %; SR-238 21 %). Conversely, both FMR and SR-85 had more evenness among their top three species (FMR: Coluber flagellum- 17.7 %, Chionactis occipitalis 16.8 %, Rhinocheilus lecontei 12.1 %; SR-85: Crotalus atrox 15.4 %, Rhinocheilus lecontei 12.9 %, Chionactis palarostis 12.6 %).
Our analysis of site characteristics in relation to urban impacts was limited by the small number of sites sampled (N = 5 different sites). Nonetheless, to the degree that road density within 5 km buffer areas around each site is an index of urbanization, CB has experienced a greater level of disturbance relative to all four of the other survey areas (Table 3). Thus, our GIS analysis supports the view that proximity to a metropolitan area is associated with increased disturbance and fragmentation (Table 3).
Discussion
A number of studies have documented impacts of urbanization on a variety of arthropods and avian forms (see reviews in Shochat et al. 2010; Davis et al. 2013; Sullivan et al. 2013). Fewer studies have assessed such impacts on reptiles (Sullivan et al. 2013), and even fewer have addressed urban impacts on snakes. Urbanization has been especially widespread in the arid southwestern USA over the past century, an area well-known for high diversity of reptiles, especially snakes and lizards. The resulting remnant habitats, often islands of desert in a sea of urban sprawl, represent a ready made laboratory for assessing how reptiles respond to urban impacts. Unfortunately, we lack detailed surveys of snake communities near urban centers.
Methodological comparisons
A number of investigators have evaluated the means by which we might increase our understanding of difficult to detect species, such as reclusive reptiles (McDiarmid et al. 2012; Erb et al. 2015). McKnight et al. (2015) found, similar to our results, that even though they are nonsystematic, incidental encounters often provide a relatively accurate depiction of diversity of snakes occupying a site. In fact, this method was most effective for sampling of many amphibians and reptiles. Our results for the primary study sites (CB, FMR) reveal that incidental encounters documented most species and most individuals at one but not the other site; coverboards were least effective, but nonetheless, documented species that otherwise would have gone undetected. Road riding continues to rank as an efficient and effective means by which surveyors can document the diversity and abundance of snakes, at least in the arid Southwest. Nonetheless, adopting coverboards and traps greatly increased sampling prospects for small, secretive forms less likely to be encountered on roadways as noted in prior reviews of road riding studies across a variety of habitats (see Dodd et al. 1989; Sullivan 2012). Our results confirm that adopting two or three sampling protocols is the optimal means of assessing the diversity and abundance of snakes, at least in arid environments.
Southwest snake communities
Our sites differ somewhat from the snake communities reviewed by Jones et al. (2011), communities that are only ~75 km to the west of CB and FMR sites. The sites in Jones et al. (2011) comprise a mosaic of Lower Colorado River and Upland subdivisions of the Sonoran Desert. One road (SVP) was situated along the bajada of a mountain range primarily in the Arizona Upland subdivision of the Sonoran Desert. The other road (SR 238) crossed a relatively broad valley, passing primarily through the Lower Colorado River Valley subdivision of the Sonoran Desert (Brown 1982). CB and FMR are similarly mosaics of these same two subdivisions of the Sonoran Desert. Nonetheless, at CB our work with desert tortoises afforded the opportunity of incidental observations of rocky, slope dwelling snakes such as Crotalus tigris, only likely to occur on a roadway in a rocky canyon (this species was not detected at either the SVP or SR 238 sites). CB is an area in which much of the site is associated with the historic floodplain of Cave Creek, a xero-riparian corridor that afforded opportunity for observations of mesic habitat forms, such as Thamnophis cyrtopsis, a form found near the sites surveyed by Jones et al. (2011).
In spite of these differences, overall there is not a dramatic shift in floral elements between our study sites, CB and FMR, and the sites SVP and SR 238 surveyed by Jones et al. (2011); hence what can account for the dramatic difference in community composition? One difference, though only 50–75 km apart, is the shift in squamate diversity as one travels from the southwest to the northeast across the northern Sonoran Desert (Sullivan et al. 2014a; Sullivan and Vernon 2015). A number of arid adapted species, commonly associated with open, sandy areas of the Sonoran Desert, such as C. cerastes and Dipsosaurus dorsalis, reach the northeastern edge of their distribution in the Phoenix metropolitan region (Brennan and Holycross 2005), and are absent from CB as a result of biogeographic aspects of their distribution rather than sampling or impacts related issues (see Sullivan et al. 2014b). For example, small burrowing forms such as Phyllornychus browni and P. decurtatus, may never have been present at the CB site, but represent two of the additional species documented at the FMR site. The community at FMR includes the diversity documented at the sites surveyed by Jones et al. (2011); the difference in eveness of the community at FMR is no doubt a result of the additional sampling methods adopted (traps/incidental surveys). We hypothesize that CB is depauperate for historical, biogeographic reasons concerning the distribution of snakes, while FMR is more even as a result of methodological differences relative to previously studied sites (Rosen and Lowe 1994; Jones et al. 2011).
With respect to more broad scale habitat comparisons, in habitats that are more mesic, such as grassland and oakwoodland–chaparral mosaics in California, road riding surveys indicate P. catenifer is by far the most abundant snake species (Klauber 1939; Sullivan 2000). In a road riding survey of a Great Basin Desert scrub habitat in southern Idaho, Jochimsen (2006) also found that P. catenifer was the most common snake detected on paved roads even though off road riding methods suggested Crotalus oreganus was the more common snake in the immediate vicinity. These results highlight the caution required in extending results from road riding studies to estimation of the absolute abundance of snakes in the biotic communities adjacent to roadways. Nonetheless, given that P. catenifer appear especially likely to be found on roadways (Jochimsen 2006), our results and those of Rosen and Lowe (1994) suggest that this species does not numerically dominate the Sonoran Desert biotic communities as it does in either grassland (Sullivan 2000) or Chihuahuan Desert (Price and LaPointe 1990) habitats of the American Southwest. Our results, in which P. catenifer account for only 6 % of snakes at CB, and those of Jones et al. (2011) in which they similarly constitute 5–7 % of snakes observed using road riding exclusively, support this general view. Rosen and Lowe (1994) also documented that P. catenifer constituted 6 % of their sample of Sonoran Desert snakes, a result showing remarkable consistency across five sites in the Sonoran Desert biotic community. In contrast to the grasslands of both California and New Mexico, the Sonoran Desert community does not support a high proportion of this generalized constrictor.
Is Crotalus atrox a weed?
Prior studies, including Jones et al. (2011); Mendelson and Jennings (1992), and Rosen and Lowe (1994), surveying a total of three sites in the Sonoran Desert and two sites in the Chihuahuan Desert and adjacent grassland, documented that C. atrox generally represents one of the top (numerically speaking) four snake species when road riding is the only survey method (three sites: 8 %, 8 % and 15 %; two non-Sonoran Desert sites: 16 % and 20 %). Nonetheless, these proportions pale by comparison to the dominance we observed at CB (60 %). The slightly greater dominance of a snake community by C. atrox documented in southeastern Arizona by Mendelson and Jennings (16 % and 20 %; 1992), one in a Chihuahuan Desert scrub habitat, and the other in a Plains Basin Grassland habitat, are of interest by comparison to our results at CB. Like Sullivan (2000), they used data from a prior study to assess any shifts in relative abundance of snakes. For these communities they documented that C. atrox and C. scutulatus were ranked either first, second or third in relative abundance, but more significantly, they suggested that C. atrox had expanded at the expense of C. scutulatus since the early 1960s due to the conversion of grassland to desert scrub habitat as a result of over-grazing by cattle, and other anthropogenic activities in recent decades. Even more interestingly, Price and LaPointe (1990) also documented that C. atrox was the dominant snake (46 %) in southcentral New Mexico, using road riding as a survey technique, along the Rio Grande in a heavily impacted area dominated by agricultural activity and considerable road traffic. These two studies are consistent with the hypothesis that disturbed communities may allow for the increased abundance of C. atrox.
We documented a higher density of paved roads surrounding the CB site. At first glance, this might be expected to negatively impact large, active snakes like C. atrox in two ways: indirectly due to fragmentation effects, and directly due to mortality when individuals are killed by vehicles while thermoregulating on the pavement (Sullivan 1981b). The high numbers of this species we observed at CB is not unlike the increase documented by Mendelson and Jennings (1992) described above, and is also similar to the increase in another rattlesnake, C. oreganus in California, documented by Sullivan (2000). Contrary to expectations of higher mortality of crotalines due to anthropogenic interactions, crotalines may be predisposed to resource utilization associated with biotic community shifts, such as an increase in lagomorphs, which some have suggested C. atrox is uniquely adapted to prey on (Loughran et al. 2013). The dramatic increases in lagomorphs in metropolitan regions of the Southwest is well documented, and Taylor and DeNardo (2005) showed that when well-fed, female C. atrox can reach reproductive maturity in roughly one year, growing at especially high rates. Hence, it might be especially well positioned to respond quickly to shifts in prey availability in a given community. Shochat et al. (2010) suggested that a minority of species might dominate the majority of the resources following changes due to urbanization; C. atrox may represent an example of this phenomenon.
Some have argued that preserves represent a reduced sample of successional stages of floral communities, and that this in turn, can reduce the diversity of various taxa, including birds, mammals and reptiles (Kjoss and Litvaitis 2001). In more arid environments, such as the deserts and grasslands of southwestern North America, others have suggested that the overall diversity of habitats initially contained within the preserved region is critical (reviewed in Sullivan et al. 2014c). Fine scale evaluation of species richness and abundance of snake communities, allowing documentation of truly sympatric congeners, and shifts in diversity across habitats, is vital to the kinds of community level analyses undertaken to elucidate broad scale phylogenetic and ecological impacts on community structure (e.g., Burbrink and Myers 2015). Many such surveys have relied on field guide level distribution maps to delineate reptilian community composition. By contrast, our results from five intensively surveyed sites less than 150 km apart in roughly comparable habitats provide considerably more robust estimates of community structure. Similar datasets will be vital to future efforts to understand how snake communities may shift in response to large scale biotic impacts due to urbanization.
References
Andrews KM (2013) Road cruising. In: Graeter GJ, Buhlmann KA, Wilkerson LR, Gibbons JW (eds) Inventory and monitoring: recommended techniques for reptiles and amphibians, Partners in Amphibian and Reptile Conservation Technical Publication, vol IM-1, pp. 94–98
Bernardino FS, Dalrymple GH (1992) Seasonal activity and road mortality of the snakes of the Pa-hay-okee wetlands of Everglades National Park, USA. Biol Conserv 62:71–75
Brennan TC, Holycross AT (2005) Amphibians and reptiles of Maricopa County. Arizona Game and Fish Department, Phoenix, Arizona, USA 68 pp.
Brown DE (ed) (1982) Biotic communities of the American southwest, United States and Mexico. University of Arizona Press, Tucson, 342 pp
Burbrink FT, Myers EA (2015) Both traits and phylogenetic history influence community structure in snakes over steep environmental gradients. Ecography 38:1036–1048
Davis RA, Gole C, Roberts JD (2013) Impacts of urbanization on the native avifauna of Perth, Western Australia. Urban Ecosystems 16:427–452
Dodd CK, Enge KM, Stuart JN (1989) Reptiles on highways in north-central Alabama, USA. J Herpetol 23:197–200
Erb LA, Willey LL, Johnson LM, Hines JE, Cook RP (2015) Detecting long-term population trends for an elusive reptile species. J Wildl Manag 79:1062–1071
ESRI (2011) ArcGIS desktop: release 10. Environmental Systems Research Institute, Redlands
Hoffman HA, Leavitt DJ (2015) Sonoran Desert Tortoise (Gopherus morafkai) occupancy monitoring on the Arizona Army National Guard Florence Military Reservation: 2014 Report. Arizona Game and Fish Department, Phoenix. doi:10.13140/RG.2.1.4706.5681
Jochimsen DM (2006) Factors influencing the road mortality of snakes on the Upper Snake River Plain, Idaho. Pp. 351–365 In Proceedings of the 2005 International Conference on Ecology and Transportation. Irwin, C.L., P. Garrett, and K.P. McDermott (Eds.). Center for Transportation and the Environment, North Carolina State University, Raleigh, NC, USA.
Jones TR, Babb RD, Hensley F, LiWanPo C, Sullivan BK (2011) Sonoran Desert snake communities at two sites: concordance and effect of increased road traffic. Herpetol Conserv Biol 6:61–71
Kjoss VA, Litvaitis JA (2001) Community structure of snakes in a human-dominated landscape. Biol Conserv 98:285–292
Klauber LM (1939) Studies of reptile life in the arid southwest. Bulletins of the Zoological Society of San Diego 14:1–100
Krebs CJ (1999) Ecological methodology, 2nd edn. Benjamin/Cummings, Menlo Park
Leavitt DJ, Sturla DP, Abbate D (2013) Tucson Shovel-nosed Snake (Chionactis occipitalis klauberi) surveys on the Arizona Army National Guard Florence Military Reservation. Arizona Game and Fish Department, Phoenix. doi:10.13140/2.1.4973.3128
Litteral J, Wu J (2012) Urban landscape matrix affects avian diversity in remnant vegetation fragments: evidence from the phoenix metropolitan region, USA. Urban Ecosystems 15:939–959
Loughran CL, Nowak EM, Schofer JX, Sullivan KO, Sullivan BK (2013) Lagomorph prey of western diamondbacked rattlesnakes (Crotalus atrox) in Arizona. Southwest Nat 58(4):506–509
Luiselli F (2007) Community ecology of African reptiles: historical perspective and a meta-analysis using null models. African Journal Science 46:384–394
Magurran AE (2004) Measuring biological diversity. Blackwell Publishing, Malden
McDiarmid RW, Foster MS, Guyer C, Gibbons JW, Chernoff N (eds) (2012) Reptile biodiversity: standard methods for inventory and monitoring. U.C. Press, Berkeley, 424 pp
McKnight DT, Harmon JR, McKnight JL, Ligon DB (2015) Taxonomic biases of seven methods used to survey a diverse herpetofaunal community. Herpetol Conserv Biol 10:666–678
Mendelson JR, Jennings WB (1992) Shifts in the relative abundance of snakes in a desert grassland. J Herpetol 26:38–45
Pianka ER (1986) Ecology and natural history of desert lizards. Princeton University Press, Princeton
Price AH, LaPointe JL (1990) Activity patterns of a Chihuahuan desert snake community. Ann Carnegie Museum 59:15–23
Rosen PC, Lowe CH (1994) Highway mortality of snakes in the Sonoran Desert of southern Arizona. Biol Conserv 68:143–148
Shochat E, Lerman SB, Anderies JM, Warren PS, Faeth SH, Nilon CH (2010) Invasion, competition, and biodiversity loss in urban ecosystems. Bioscience 60:199–208
Sorace A, Gustin M (2009) Distribution of generalist and specialist predators along urban gradients. Landscape Urban Planning 90:111–118
Sullivan BK (1981a) Distribution and relative abundance of snakes along a transect in California. J Herpetol 15:247–248
Sullivan BK (1981b) Observed differences in body temperature and associated behavior of four snake species. J Herpetol 15:245–246
Sullivan BK (2000) Long-term shifts in snake populations: a California site revisited. Biol Conserv 94:321–325
Sullivan BK (2012) Road riding. In: McDiarmid RW, Foster MS, Guyer C, Gibbons JW, Chernoff N (eds) Reptile Biodiversity: Standard Methods for Inventory and Monitoring. U.C. Press, Berkeley 424 pp, pp. 215–218
Sullivan BK, Vernon JM (2015) Dipsosaurus dorsalis (desert iguana). Urban habitats. Herpetological Review 46:90–91
Sullivan BK, Vanhaverbeke R, Chambers C (2013) Wildlife and anthropogenic changes in the arid southwest. In: Malloy R, Brock J, Floyd A, Livingston M, Webb RH (eds) Design with the Desert: Conservation and Sustainable Development. CRC press, Boca Raton, pp. 169–191
Sullivan BK, Averill-Murray RC, Sullivan KO, Sullivan JR, Sullivan EA, Riedle JD (2014a) Winter activity of Sonoran Desert tortoise (Gopherus morafkai) in Central Arizona. Chelonian Conservation and Biology 13:114–119
Sullivan BK, Sullivan KO, Vardukyan DE, Suminski TS (2014b) Persistence of horned lizards (Phrynosoma spp.) in urban preserves of Central Arizona. Urban Ecosystems 17:707–717
Sullivan BK, Vardukyan DE, Sullivan KO (2014c) Historic and current composition of lizard communities in urban preserves of Central Arizona. Urban Naturalist 2014(2):1–18
Taylor EN, DeNardo DF (2005) Sexual size dimorphism and growth plasticity in snakes: an experiment on the western diamond-backed rattlesnake (Crotalus atrox). J of Exp Zoology 303A:598–607
Whittaker RH (1965) Dominance and diversity in land plant communities: numerical relations of species express the importance of competition in community function and evolution. Science 147:250–260
Wiens JJ (2015) Explaining large-scale patterns of vertebrate diversity. Biology Lettters 11:20150506. doi:10.1098/rsbl.2015.0506
Willson JD (2013) Aquatic and terrestrial funnel trapping. In: Graeter GJ, Buhlmann KA, Wilkerson LR, Gibbons JW (eds) Inventory and monitoring: recommended techniques for reptiles and amphibians, Partners in Amphibian and Reptile Conservation Technical Publication, vol IM-1, pp. 109–113
Acknowledgments
D. Abbate, J. Alcock, C. Beach, R. Bowker, W. Carroll, R. Don, H. Hoffman, E. Makings, A. Owens, M. Olsen, C. Rubke, A. Scuderi, D. Sturla, D., E. and J. Sullivan assisted with some field observations. Maricopa County Flood Control personnel, especially Lisa Amos, Erik Arntz, Shelby Brown, Charlie Klenner, Bill Leal, Theresa Pinto, and Diana Stuart provided considerable assistance at the CB site; Rob Patterson and Andy Long of the Phoenix Parks Department, and Roger Moncayo also provided assistance with site security. All research was permitted under Arizona Game and Fish Department Scientific Collecting Permits (2010-2015) and ASU IACUC approval for the CB site.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sullivan, B.K., Leavitt, D.J. & Sullivan, K.O. Snake communities on the urban fringe in the Sonoran Desert: influences on species richness and abundance. Urban Ecosyst 20, 199–206 (2017). https://doi.org/10.1007/s11252-016-0577-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-016-0577-6