Abstract
Urbanization has tremendous impacts on most native species. This is especially true in land snails, which are surprisingly understudied organisms. Due to their low mobility and dispersal potential, land snails are valuable indicators of ecosystem disturbance. For this study, land snails were collected in 54 city parks along an urban gradient to understand impacts of urbanization on snail communities. Sampled parks include small extensively landscaped downtown parks, neighborhood and community parks, district parks, and large nature parks, each with variable vegetation, soil characteristics, disturbance regimes, and human activities. Sampling recovered 12,153 individual snails, representing 20 families, 43 genera, and 95 species. Seven new Tennessee state and 87 new county occurrences were recorded. Five non-native and one extra-limital non-native species were found, four of which are new Tennessee state records. Results show that urbanization greatly alters land snail community structure. Nature and district parks have significantly greater species richness, species diversity and species evenness than community, neighborhood, and downtown parks. Degradation of parks, distance from the park to the commercial city center and percent of coarse woody debris accounted for most of the variation between park types. Non-metric multidimensional scaling and pairwise Jaccard indices indicate that downtown snail communities are more similar whereas snail communities in nature parks are more distinct. This suggests that urbanization promotes homogenization among land snail communities. We also show that this homogenization is thus far driven mainly by synanthropic, broadly adapted native species rather than non-native snail species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urbanization of previously rural or natural land has led to dramatic changes in ecosystems all over the world. Globally, urbanization is now documented as a major threat to our planet’s biodiversity (Aronson et al. 2014). Total global urban area is predicted to triple between 2000 and 2030, with an additional 5.9 million km2 of non-urban land converted to urban land use (Seto et al. 2012).
Land snails are good subjects to study urbanization impacts because organisms with low dispersal capabilities, like snails, are very susceptible to anthropogenic activities (Ström et al. 2009). Microsnails (<5 mm in diameter), in particular, are often more vulnerable to disturbance because of their very limited mobility and dispersal, and their strong dependence on microhabitats (Baur and Baur 1988). For these reasons, land snail community composition, especially where microsnails are included, is a good indicator of the overall health of an ecosystem (Frest and Johannes 1995; Frest 2002).
Land snails are also important for biodiversity conservation reasons. As of 2004, 42% of the 693 documented animal extinctions have been mollusks (Lydeard et al. 2004). As of 2015, there were 1772 gastropods listed as threatened on the International Union for Conservation of Nature (IUCN) Red List. Even with the critical rate of imperilment, snails receive far less attention than their vertebrate counterparts, with relatively few researchers studying them (Lydeard et al. 2004).
Much previous work on human impacts on land snails has focused on islands and some European countries (e.g.,; Chiba 2010; Chiba and Roy 2011; Douglas et al. 2013; Horsák et al. 2009, 2013; Lososová et al. 2011). A review by Yanes (2012) concludes that three main anthropogenic factors are impacting land snails: habitat loss through urban and agricultural development, introduction of non-native predators, and introduction of non-native snail species. As cities are well known “hot spots” for the introduction of non-native species (Bergey and Figueroa 2016), this implies that urban areas are strongly impacting land snails via all three of these factors.
There are many ways to study urban impacts on biodiversity (Adler and Tanner 2013) but one of the most common methods is to compare biological communities along disturbance gradients, or different levels of urban disturbance. In a review, McKinney (2008) found at least 57 urban gradient studies on invertebrate species, but none examined land snails. Indeed, we know of only three studies, in European cities (Horsák et al. 2009, 2013; Lososová et al. 2011), that examine gradations of urban impacts on land snails in a systematic way.
This study analyzes the effects of urbanization on land snail communities in Tennessee across an urban disturbance gradient. Our null hypothesis is that urbanization will have no effect on land snail communities, regardless of degree of urbanized land disturbance. Expected outcomes, based on previous gradient studies of non-snail taxa are that communities of snails will differ among disturbance levels, and that some degree of homogenization will be seen in communities closer to the urban core (McKinney 2006; Horsák et al. 2013; Adler and Tanner 2013). We also expect to see a decrease in species richness and diversity in more urbanized habitats (McKinney 2008; Horsák et al. 2009; Lososová et al. 2011; Adler and Tanner 2013).
Methods
Study areas
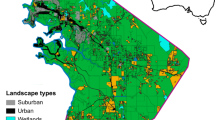
Snails were investigated in three Tennessee cities (Fig. 1). Nashville (764.6 km2; 644,014 population, settled in 1779) is located in the Central Basin of Middle Tennessee and is the second largest city in the state (US Census Bureau 2015). Nashville is situated on the Cumberland River, which runs east-west through the city. The topography of the area is rolling, with elevation ranging from 400 to 700 ft above sea level. The Central Basin is defined by the Hudson River and Trenton limestone groups composed of Lower Ordovician limestone and Lower Silurian, shale, and dolomite. Knoxville (255.1 km2; 184,281 population; settled in 1792) is located in eastern Tennessee in the Southern Appalachian Ridges and Valleys area (US Census Bureau 2015). Knoxville is established on the Tennessee River, which flows southwest through the city. The topography is defined as rolling, to hilly, with steep rugged ridges. The elevation ranges from 740 ft to 2128 ft. The bedrock is made of Cambrian, Ordovician, and some Silurian limestone, dolomite, shale, and siltstone. Chattanooga, (354.8 km2; 173,779 population; settled in 1819) in the southeastern part of Tennessee, lies in the Cumberland Plateau and the Southern Appalachian Ridges and Valleys (US Census Bureau 2015). The bedrock is made up of Cambrian, Ordovician, Mississippian, and Pennsylvanian rocks composed layers of sandstone, shale, limestone, chert, dolomite, claystone, and siltstone. Chattanooga is located on the Tennessee River, which enters the city from the northeast. The topography is similar to Knoxville, with rolling hills and rocky ridges. The elevation ranges from 640 ft in downtown to 2210 ft at Lookout Mountain .
All parks were chosen from parks listed on each city’s Parks & Recreation Department website, with the exception of Prentice Cooper State Forest in Chattanooga. Parks were sorted into categories based on the cities’ description of the park and using Google Earth to determine forest cover. Categories were: nature parks (larger, mostly forested parks with minimal man-made alterations), district parks (mid-size parks with large open spaces with ample forested space), community parks (small mainly recreational parks with mostly open space, pavilions and sports fields, with some forested area), neighborhood parks (very small parks, situated in suburbs for human use), and downtown parks (located directly downtown, typically less than one acre, and very urban).
In each city, three nature parks, three district parks, three community parks, six neighborhood parks, and three downtown parks were chosen using a random number generator, for a total of 54 parks. Neighborhood parks made up the majority of every city’s park system so more neighborhood parks were sampled. Because Chattanooga lacks nature parks in its park directory, Prentice Cooper State Forest, was used as a proxy.
Snail sampling
Snails (living and empty shells) were collected between May and September, 2015 between the hours of 9 A.M. and 4 P.M. We did not collect slugs. We did collect one species of amphibious snail, Pomatiopsis lapidaria, which is often included in land snail studies (e.g., Douglas et al. 2013) as they often inhabit moist terrestrial habitats such as wet limestone exposures (Dourson 2010).
As snails are most active at night and after rainfall, surveys only occurred following a period of 48 h without rain to control for snail activity (Barker 2001). Two standard terrestrial gastropod sampling methods were used in this study: visual searches of plots and leaf litter/soil collection in the same plot. Visual surveying for land snails is very effective at locating macrosnails (>5 mm maximum width), but often misses microsnails (<5 mm maximum width) (Hotopp 2002). Therefore, litter/soil collection is recommended to account for the microsnail population at a given site (Clergeau et al. 2011). Clergeau et al. (2011) determined that using these two methods in urban gastropod sampling was effective in obtaining 55%–61.5% (visual search) and 92%–100% (litter/soil search) of snails.
The number of sampled plots per park was determined by the habitat and acreage of each park: more area and more habitats required more plots to be sample. Habitat variety was estimated via conversations with park officials, and visual walkthroughs when feasible. If a park had one habitat type, one plot was surveyed. In contrast, when a park had more than one habitat type, one plot was surveyed in each habitat. One plot was used for most downtown and neighborhood parks because they had one habitat type (typically non-native plant species, landscaping, or open lawn), and an average of four acres. The minimum number of plots sample was 1 (for small parks) and the maximum was 10 plots (for the largest and most habitat-diverse parks).
For the visual survey, at each habitat type within a park, two 9m2 plots within 30 m of each other were sampled in the same habitat type to account for microhabitat variation (the two plots are averaged to equal one plot for all analyses). Each plot was thoroughly searched for snails by two people for 30 min. Microhabitat searched within each plot included: leaf litter, under leaf litter, on vegetation, under rocks, on and under downed wood, tree crevices, and moss. Every living snail and intact shell encountered were collected. When possible (i.e., species identity was obvious), living snails were identified in the field and released.
To remove soft bodies of live snails for identification, snails were soaked in tap water overnight, boiled for one minute, and extracted with forceps. Shells were then placed in a 3% hydrogen peroxide solution for 24 h to clean both the inside and the outside of the shell and rinsed with water. No apparent dissolution or abrasion of the shell occurred from this process.
Soil and litter collection and processing
After the visual survey of a plot, one half liter of leaf litter/soil was collected to a depth of three cm in multiple locations throughout the plot and placed in a sealed plastic bag. Each bag was labeled and stored at room temperature until processed for microsnails. Traditional soil processing sometimes involves allowing the soil to dry for a 2–4 week period and then sorted using multiple soil sieves. All collected soil must then be visually searched under magnification for microsnails. This process can be tedious and time consuming. Instead, we used an alternative method of processing litter and soil that is sometimes used in paleontology to digest organic material in sediment (Rhodes 1998).
This method used relies on hydrogen peroxide to reduce the volume of organic, non-mineralized materials, without affecting the mineralized snail shells. Soil was emptied into a container where rocks, twigs, leaves, and other large matter was removed. Soil was then placed into flexible plastic mesh screen with openings of 0.5 mm. This size was chosen because the smallest known terrestrial snail is 0.7 mm; most researchers agree that a 0.6 mm sieve will catch all adult snails (Fontaine et al. 2007; Vermeulen et al. 2015). Bags were secured with zip ties and repeatedly dunked in a 3% hydrogen peroxide solution to remove soil matter that was less than 0.5 mm. The bags were then thoroughly rinsed with tap water until the water coming out of the bag ran clear. Bags were placed in a sunny window where they were dried for 2–3 days. This process significantly reduces the amount of soil matter and therefore the amount of time it takes to sort through the remaining soil. Once dry, the soil was carefully examined under a stereo microscope or using a head-mounted optical magnifier. All snails were removed and placed into a labelled vial for later identification.
Snail identification
Nearly all specimens (95.5%) were identified to species level based on morphological features according to Burch (1962), Dourson (2010), and Pilsbry (1940, 1946, 1948). In addition, some difficult specimens were identified by consultation with snail taxonomists, Amy and Wayne Van Devender, Ron Caldwell, Tim Pierce, Daniel Douglas, and Franciso Borreo. Those remaining (4.5%) were identified to genus or not identified (juveniles and broken shells).
Habitat data collection
Abiotic and biotic factors that may influence land snails were recorded or collected at each plot. These factors included: percent of non-native plant species and percent coarse woody debris cover (CWD) in a plot, leaf litter depth, tree species present, and soil samples to be tested for pH and calcium (Ca). Before each plot was searched, dominant vegetation within the plot was identified and calculated into a percentage of area covered by non-native plant species. CWD, which includes rotting wood or bark was visually estimated in each plot as percent cover. Leaf litter depth was measured in three locations within the plot. A composite soil sample was taken from each plot and stored in a sealed plastic bag at 4° Celsius. Those samples were later analyzed by the Soil, Plant, and Pest Center at the University of Tennessee, Nashville TN, to determine pH and calcium content.
Degradation was estimated at each site using three explanatory variables based on similar work by Horsák et al. (2009). These three variables are: human alteration of the habitat, isolation of the park, and percent of non-native species present (Table 1). The three variables were combined to produce a degradation rank. As an example, Krutch Park is a small downtown park in Knoxville, TN. On the degradation scale it receives 3 out of 3 for human alteration of the habitat, because the entire park has been affected by humans through heavy landscaping and little forest. For the second variable, isolation of the park, Krutch Park receives a 2 of 2 for being completely surrounded by the city. For percent of non-native species present, Krutch Park receives a 3 of 3 because 100% of plant species in the plot(s) were non-native. These three parts combine to give Krutch Park a degradation rank of 8, the highest degradation rank.
Data analysis
To account for variation in number of plots searched in each park, plots were averaged together to determine species diversity, richness, abundance, and evenness. Shannon-Weiner species diversity and evenness indices were calculated for every plot and an average species diversity and evenness per plot for each park was produced based on number of plots searched. Snail abundance and richness for each plot were also averaged to together to produce one average number for each plot that accounted for variation in plot numbers. Differences in species diversity, richness, abundance, and evenness among park types in each city and among park types irrespective of city were tested using a factorial analysis of variance (ANOVA), (PROC MMAOV; Saxton 1998) in SAS ver. 9.3. Fisher’s least significant difference (LSD) mean separation at the 5% significance level was used to compare means.
Variable selection (PROC REG; Saxton 1998), performed in SAS, was used to determine the best regression model for species diversity, richness, abundance, and evenness for all three cities combined and separately. Model variables were chosen from: acreage, calcium, CWD, degradation, straight-line distance to commercial city center (using Google Earth), litter, and Best fits were chosen based on the R-squared, Akaike’s information criterion (AIC), and Mallow’s Cp in the following conditions: AIC measures the fit of the model, but penalizes for the number of parameters. Models within two AIC of each other are considered nearly equally good. All models chosen were within two AIC of the lowest AIC of all the models. Mallow’s Cp is used to measure fit and prediction, standard error, and bias/precision of the model. The best Cp is lower than how many variables are in the model, plus one.
Regressions were run in SAS (PROC REG; Saxton 1998) based on the variable selection. Final variables in the regression models are different than what was chosen in variable selection due to collinearity and low significance (P > 0.05) of some variables. All simple linear regressions were first run as a 4th degree polynomials and simplified based on the P-values for each degree.
A synanthropic species analysis was also performed. A synanthropic species is defined as a species that is commonly found in close association with human activities, and that thrives in or “exploits” urban habitats (Adler and Tanner 2013; Guette et al. 2017). This includes both native and non-native species that are common in highly modified habitats including gardens, lawns, and other urban areas. Here, we designated a snail species as synanthropic if it is commonly found in roadside habitats, residential areas, and other highly modified urban habitats as noted by Burch (1962), Dourson (2010), and Hotopp et al. (2013). The specific species that we designated as “synanthropic” are denoted in the Appendix Table 6.
Nonmetric multidimensional scaling (NMDS) (Kruskal 1964) was performed using PC ORD 6.19 to obtain a graphical representation of snail community relationships among park types. NMS is an ordination method used for non-normal data common in ecological data sets. It is an iterative search that finds the best position of n entities on k dimensions, with the lowest stress of the k-dimensional configuration. Low stress for ecological community data is considered to be less than 15, while stress values approaching 20 are of concern (McCune and Grace 2002). Bray-Curtis dissimilarity was used for running the NMDS with 250 iterations. This analysis was conducted on all parks types using park data, on nature parks and downtown parks to determine if homogenization is occurring, and on degradation ranks using plot data. Degradation ranks were rounded to the nearest whole number to create degradation ranks 1–9.
Finally, to directly examine a possible trend toward biotic homogenization with urbanization intensity, we measured pairwise community similarity between intensively disturbed urban locations (downtown parks) and compared those to pairwise community similarity of least disturbed habitats (nature parks). We chose downtown versus nature parks as these represent two end-members of the disturbance spectrum we studied. To measure the similarity of species composition among localities we used the Jaccard index, which measures the proportion of shared species across the two, combined assemblages. There are many indices for measuring similarity of species composition between sites but most of these indices strongly covary (Koleff et al. 2003) and many studies on homogenization have used the Jaccard index (Olden and Rooney 2006).
In addition to the snail data collected herein, our analysis included the species list from Bergey and Figueroa (2016) which examined the snail fauna of 61 residential yards in Norman, Oklahoma, USA. They found 32 species including 4 species of slugs. As we did not collect slugs we included only the 28 land snails species that they found. We included their data because it is a high quality data set of urban snails in very disturbed habitats that provide a way to examine snail community similarity, and potentially biotic homogenization, with Tennessee urban habitats.
Results
Snail sampling recovered 12,153 individual snails, representing 20 families, 43 genera, and 95 species (Appendix Table 6). Seven new state and 87 new county occurrences were recorded for Davidson, Knox, Hamilton, and Marion counties. We also found five non-native species (from outside the USA): Cochlicopa lubricella, Cepaea nemoralis, Oxychilus cellarius, Paralaoma servilis, and Lamellaxis gracilis. We also found one extra-limital non-native species (native to US but located outside its native range): Polygyra cereolus. Four of these non-natives are new Tennessee state records (Appendix Table 6).
The most abundant snail family encountered was Zonitidae, which accounts for 64.0% of all snails found in this study, followed by Polygyridae (20.0%), Pupillidae (3.6%) and Discidae (2.1%). This was followed by: Glyphyalinia (10.8%), Triodopsis (7.8%), Inflectarius (4.8%), Zonitoides (3.5%) and Gastrocopta (3.0%). For species, Ventridens demissus (Binney, 1843), represented 21.2% of all snails, followed by Ventridens ligera (Say, 1821) (15.1%), Triodopsis hopetonensis (Shuttleworth, 1852) (7.3%), Ventridens percallosus (Pilsbry, 1898) (5.1%), and Inflectarious inflectus (Say, 1821)(4.2%).
Park comparisons
Significant differences were observed in Shannon-Weiner diversity among park types (Fig. 2, P = 0.001). Nature parks had the highest mean species diversity and were not different from district parks. Downtown parks had the lowest mean species diversity and were not different from community parks or neighborhood parks.
Cities did not differ in species richness, but differences were observed in species richness among park types (Fig. 3). District parks had the highest mean species richness and were not different from nature parks. Mean species richness in community, neighborhood, and downtown parks were not different. Community parks, neighborhood parks and downtown parks had similar mean Shannon-Weiner species evenness (Fig. 4). Nature parks and district parks had greater mean species evenness (Fig. 4).
Factors affecting snail communities
Degradation rank was the most common predictor variable chosen across the four metrics (species diversity, species richness, species abundance, and species eveness), in each city separate and combined, with eight models selecting with degradation as the most important model variable (Table 2). Park acreage was also a factor; it was found to be the most important factor in five models. Calcium was a common secondary factor in seven models, while CWD and distance were secondary factors in six models. Litter depth and pH were the least important variables, only appearing secondarily in four and three models respectively. The average pH for all three cities combined was 6.83, and ranged from a maximum of 8.11 to a minimum of 4.66.
A multiple regression model of species diversity in all parks indicated degradation, distance to commercial city center, and CWD as major variables (Table 3). In a multiple regression model of Nashville and a simple linear model for Knoxville, both park species diversity and distance to commercial city center were prominent. Nashville park species diversity was also strongly related to acreage. CWD and degradation were each related to Chattanooga park species diversity (Table 3).
The best fit multiple regression model of species richness in all parks indicated CWD, calcium, degradation, and distance to commercial city center as significant factors (Table 4). Chattanooga parks species richness was most related to CWD in a simple linear model. No significant correlations were found for Knoxville parks species richness.
The best fit multiple regression model of species evenness in all parks indicated degradation as the most dominant variable in a simple linear model (R-square = 0.31, P = 0.0001). While Chattanooga and Nashville parks species evenness was best fit to a simple linear model with degradation (R-square = 0.57, 0.23, P = 0.0003, 0.040, respectively), Knoxville parks species evenness fit best to a simple linear model with calcium (R-square = 0.27, P < 0.026).
A simple linear regression, with all parks, comparing degradation to species diversity, richness, evenness, and abundance, found all but abundance to be significantly related. Species diversity and evenness were found to be most related to degradation. Species richness was also related to degradation but to a lesser extent.
Synanthropic snail species accounted for a greater percentage of species in increasingly urbanized parks (Table 5). Nature parks have the lowest percentage of synanthropic species with downtown parks exhibiting 3 times that figure (Table 5). District parks, community parks and neighborhood parks were each 10–20% different in percentage of synanthropic snail species. Nature parks also have fewer synanthropic snail individuals as a measure of total abundance (34%). District and downtown parks contained similar amounts of synanthropic individuals (84%, 81% respectively), while neighborhood and community parks contained 10% more snyanthropic individuals (92%, 95%, respectively).
Nonmetric multidimensional scaling ordination
The NMDS ordination across all 54 parks indicated various differences in species composition across the five parks types (Fig. 5). A noticable difference in snail communities can be seen between nature and district parks and the three smaller, more urbanized community, neighborhood, and downtown parks. This indicates that the snail communities found in nature and district parks are more distinct from the other park types.
The NMDS ordination was performed by plots within parks because some degradation rank numbers are present only once (Fig. 6). NMDS requires at least two occurrences of the independent variable, as it cannot ordinate data based on one sample unit. Differences in snail communities across degradation rank vary in terms of separation. Degradation rank zero and two are grouped closely together, implying that species are separated based on their lack of tolerance for degradation. Rank three also shows some overlap with zero and two. One side of degradation rank seven also overlaps with zero, two, and three, implying some shared species among the ranks. Degradation rank four overlaps with several other ranks. All other degradation ranks overlap and show no noticeable community patterns.
The NMDS ordination for the nine nature and nine downtown parks indicates that downtown parks are clustered in dimensional space relative to nature parks (Fig. 7). This implies that species composition in downtown parks is more similar to each other than to nature parks. Data points for nature parks show relatively less overlap in species composition with each other, and much less with downtown parks (Fig. 7).
Pairwise similarity coefficients
The results of the Jaccard index comparison of snail community similarity were as follows (where K = Knoxville, C = Chattanooga, N = Nashville, for species lists of combined downtown parks vs combined nature parks for each city): K-C downtown = 0.193, K-C, nature = 0.159; K-N downtown = 0.194, K-N nature = 0.167; C-N downtown = 0.224, C-N nature = 0.165. These indicate that, for each of the three pairwise comparisons, downtown parks are more similar in composition than nature parks.
Comparison of all downtown parks (combined for all three cities) with the 28 species found in the 61 residential yards in Norman, Oklahoma, USA (Bergey and Figueroa 2016) found 10 shared species for a Jaccard index of 0.164 = 10/(10 + 28 + 23). Comparison of the Norman, Oklahoma residential data with the combined species list for all nature parks in our 3 cities found 14 shared species for a Jaccard index of 0.129 = 14/(14 + 28 + 66). This indicates that the residential snail communities of Norman are more similar to the downtown parks of the three Tennessee cities than to the nature parks.
Discussion
This study indicates that urbanization is affecting land snail community composition across cities in Tennessee. Park type correlated with acreage (Spearman correlation = 0.87) and degradation rank (Spearman correlation = 0.76). Increasing acreage was also correlated with decreased degradation (spearman correlation = 0.80). These two factors (degradation and acreage) were the most commonly identified in variable selection models as correlated to snail community metrics, and are considered to be the strongest factors affecting snail communities in this study. Additional factors that partially relate to snail community variation in a given plot include: soil pH, soil calcium, coarse woody debris present, the depth of leaf litter, distance to commercial city center, and percentage of non-native species. Many of these factors are directly related to the urbanization process.
Among the snail community indices, Shannon-Weiner species diversity was the most clearly divergent among park types. Nature and district parks (larger parks with greater tree coverage) had nearly twice the diversity of community, neighborhood and downtown parks (smaller, intensely used urban parks (Fig. 2). Species richness and species evenness were also found to be highly related to park type. For species richness, between 30% and 50% more species were found in nature and district parks compared to community, neighborhood and downtown parks (Fig. 3). On average, nature and district parks exhibited between 23% to 38% greater species evenness than community, neighborhood, and downtown parks (Fig. 4). It is apparent that decreasing size and increasing degradation of parks reduce diversity in snail communities. In particular, degradation of these parks accounted for over 30% of snail species diversity and evenness. In general, nature and district parks contain more woody plants and mature forested landscapes. They also tend to have less disturbance than the smaller parks, which are in high traffic urban centers. Lososová et al. (2011) also found that highly urbanized plots near urban centers had reduced land snail species richness.
The only community metric that did not align within this trend was mean snail abundance. No clear trend was evident and only neighborhood parks offered fewer individual snails per plot, an average of 37. All other park types, including downtown parks, had between 63 and 78 snails per 9 m2 on average. It may be that neighborhood parks are divergent in their habit availability for land snails as they have more open lawn space for human recreation, and are utilized daily for people and their companion pets.
That abundance did not decrease, and may even increase, despite a diversity increase with urbanization has been found in many studies, including invertebrates (Faeth et al. 2011). It has been called the urban “density-diversity paradox” because diversity typically increases as the number of individuals increase in biological communities (Shochat et al. 2010). It has been suggested that increased abundance and decreased richness of animal species in cities occurs because synanthropic urban generalists increase in numbers due to their competitive superior abilities, human subsidized food resources, and lowered predation pressure in cities relative to wildlands, and thus decrease community evenness (Shochat et al. 2010).
Clear differences are apparent in terms of synanthropic snail types present at each park type (Table 5). Synanthropic snail species comprised increasingly greater percentages in more urbanized parks, ranging from 22% in nature parks to 67% in downtown parks. Changes in synanthropic species abundance can be seen between nature parks and all other parks types where 34% of individuals were classified as synanthropic in nature parks, and more than 80% of all snails found in community and neighborhood parks were synanthropic species. Increasing diversity and abundance of synanthropic species with urbanization intensity is a common pattern in many groups of organisms, likely driven by increasing alteration of abiotic and biotic factors with urban influence (Faeth et al. 2011).
The percentage of synanthropic snails in downtown parks is related to the relatively high abundance, and surprising appearances of two species not previously known to the urban habitat, Rabdotus dealbatus (Say, 1821) and Triodopsis alabamensis (Pilsbry, 1902). Rabdotus dealbatus is a calciphile that prefers meadow-like habitat, which may be mimicked in the open, grassy, downtown parks (Dourson 2010). Triodopsis alabamensis is typically found in pine-oak forests, but 50 individuals were in one downtown Chattanooga park (Hubricht 1985). These and the several other species found in heavily urbanized areas may have a higher tolerance for the degraded and fragmented habitat (which can resemble their known habitat preferences) found in city parks.
We found that local variables (calcium, pH, leaf litter depth, and CWD) were not major factors affecting urban land snail communities. This is the opposite of what is often seen in previous studies, where snails (and slugs) were found to be highly dependent on microhabitat conditions (Burch 1955; Beyer and Saari 1977). This disparity in findings may occur because these studies focused on natural habitats whereas much of our focus was on highly to moderately disturbed urban habitats where natural processes may be overwhelmed or at least strongly mitigated by anthropogenic factors (e.g., fertilizer, irrigation, and mowing). This displacement of natural abiotic factors by anthropogenic factors is a common pattern for many groups in urban habitats (Adler and Tanner 2013; Guette et al. 2017). For example, Coarse Woody Debris (CWD) is known to provide important refugia to land snails, as well as harbor uncommon species. According to Caldwell (1993), up to 25% of southeastern land snails depend on CWD. But on average, downtown parks only had CWD covering 0.003% of the plot, compared to neighborhood parks which had 16% or nature parks which had 23% CWD coverage. Leaving CWD in downtown parks could provide habitat for land snails, as well as other invertebrates.
The wide-ranging effects of anthropogenic activities are further seen in that degradation was the most prominent factor related to land snail community composition in urban environments. Increasing degradation was significantly correlated with decreased species diversity, richness, and evenness. Horsák et al. 2009 and Lososová et al. (2011) also found a decline in snail species richness with increased degradation. Degradation, for this study, includes many general landscape factors such as: amount of forest, lawn care, non-native plant species presence, and landscaping. It is also likely that unmeasured local variables associated with degradation (e.g., trampling, pedestrian traffic, pets) could be significantly affecting snail communities.
In a study analyzing land snail communities in old-growth and second-growth forests in Kentucky, Douglas et al. (2013) found that disturbance from human land use can alter land snail communities for decades. Their research was not able to determine which variables were most affecting snail communities, but came to the conclusion that the traditional variables most associated with land snails were not the most dominant. In addition, Douglas et al. (2013) found that 14 out of 18 snails identified as indicator species for old growth forests were microsnails, strengthening the argument that microsnails are more diverse in an undisturbed environment.
Our study also indicates that homogenization of snail communities is occurring in downtown parks, typically located nearest the city center. This is seen in the multivariate (NMDS) analysis and more directly in the pairwise Jaccard index which shows that, between cities, downtown parks have greater similarity than nature parks. Homogenization by urbanization is also supported by the greater similarity (Jaccard index) of snails communities in residential yard habitats of Norman, Oklahoma USA with snails in downtown parks than with the nature parks in our data set.
While it is relatively well documented that urbanization increases homogenization in birds and plants (McKinney 2006; Kühn and Klotz 2006; Murthy et al. 2016), there are far fewer studies of how urbanization affects community similarity among invertebrates. However, those few also show a similar pattern as ours, with homogenizing patterns seen in urban arthropods (Knop 2016) and urban land snails (Horsák et al. 2013). One potential explanation for urban homogenization is that, as cities are mainly built for the habitat needs of one species, humans, they produce a relatively similar physical environment inhabitable mainly by the relatively few species that can adapt to those urban habitats (McKinney 2006; Adler and Tanner 2013).
Regarding exactly which species are “urban exploiters” and “urban adapters” (McKinney 2002; Adler and Tanner 2013), there are only six species that occur in downtown parks of all three Tennessee cities: Ventridens demissus, Triodopsis hopetonensis, Zonitoides arboreus. Gastrocopta contracta, Hawaiia minuscula, Glyphyalinia indentata. These six species are also among the most local abundant species by far (Appendix Table 6). Furthermore, these species (with the exception of Gastrocopta contracta) are the five most widespread species, being found in the highest percentage of residential yards in Norman, Oklalahoma (Bergey and Figueroa 2016). Given that non-native species are often identified as urban biotic homogenizers (McKinney 2002; Adler and Tanner 2013), it is interesting that none of these species widespread species shared among all the downtown habitats of Tennessee and the residential yards of Oklahoma include the six non-native species in our data set. We can only speculate but perhaps this is an “invasion debt” (Rouget et al. 2016) related to the slow dispersal of snails, and that given time these non-native species will become more widespread.
A major goal of future research on urbanization impacts on snails will no doubt include the mechanisms by which synanthropic snails are adapting to urban habitats. There is a substantial literature documenting many behavioral and physiological urban adaptations of birds, mammals and other well-studied organisms, including changes in diet, life history, reproduction, and social structure (Adler and Tanner 2013). But we know relatively little about the behavioral and physiological mechanisms allowing specific snail species to adapt to cities. In general, it seems clear that synanthropic urban snails tend to be generalists adapted to a wide variety of habitats. For example, the six species noted above (Ventridens demissus, Triodopsis hopetonensis, Zonitoides arboreus. Gastrocopta contracta, Hawaiia minuscula, Glyphyalinia indentata) are geographically widespread and found in several kinds of habitats (Dourson 2010). Indeed, Dourson (2010, p. 117) notes that Zonitoides arboreus is the “most common and wide-ranging land snail in North America”. Aside from that, little is known about the specific mechanisms of urban adaptation, other than a few interesting but anecdotal observations. Triodopsis hopetonensis, for example, is a “species of waste places, roadsides also found in and around scrap piles of construction lumbers” that are a mode of anthropogenic dispersal to new urban locations (Dourson 2010, p. 265).
References
Adler F, Tanner CJ (2013) Urban ecosystems. Cambridge University press, Cambridge, 382 pp
Aronson MF, La Sorte FA, Nilon CH, Goddard MA (2014) A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc R Soc B 281:20133330
Barker GM (2001) Chapter 1: gastropods on land: phylogeny, diversity, and adaptive morphology. In: Barker GM (ed) The biology of terrestrial mollusks. CABI Publishing, New York, pp 1–146
Baur A, Baur B (1988) Individual movement patterns of the minute land snail Punctum pygmaeum (Draparnaud)(Pulmonata: Endodontidae). Veliger 30:372–376
Beyer WN, Saari DM (1977) Effect of tree species on the distribution of slugs. J Anim Ecol 46:697–702
Bergey EA, Figueroa LL (2016) Residential yards as designer ecosystems: effects of yard management on land snail species composition. Ecol Appl 26:2538–2547. https://doi.org/10.1002/eap.1378/full
Burch JB (1955) Some ecological factors of the soil affecting the distribution and abundance of land snails in eastern Virginia. The Nautilus 69:62–69
Burch JB (1962) How to know the eastern land snail. In: William C (ed) Brown Company Publishers, Dubuque, 214 pp
Caldwell RS (1993) Biodiversity and coarse woody debris in southern forests. Proceedings of the workshop on coarse woody debris in southern forests: effects on biodiversity. In: McMinn J, Crossley DA (eds) Macroinvertebrates and their relationship to coarse woody debris: with special reference to land snails. Southern Research Station, Athens, pp 49, 156 pp–54
Chiba S (2010) Invasive non-native species’ provision of refugia for endangered native species. Conserv Biol 24:1141–1147
Chiba S, Roy K (2011) Selectivity of terrestrial gastropod extinctions on an oceanic archipelago and insights into the anthropogenic extinction process. Proc Natl Acad Sci 108:9496–9501
Clergeau P, Noelie T, Benoit F (2011) A simplified method for conducting ecological studies of land snail communities in urban landscapes. Ecol Res 26:515–521
Douglas DD, Brown DR, Pederson N (2013) Land snail diversity can reflect degrees of anthropogenic disturbance. Ecosphere 4:1–14
Dourson DC (2010) Kentucky’s land snails and their ecological communities. Goatslug Publications, Bakersville, NC, 298 pp
Faeth SH, Bang C, Saari S (2011) Urban biodiversity: patterns and mechanisms. Ann N Y Acad Sci 1223:69–81
Fontaine B, Olivier G, Eike N (2007) Priority sites for conservation of land snails in Gabon: testing the umbrella species concept. Divers Distrib 13:725–734
Frest TJ, Johannes EJ (1995) Interior Columbia Basin mollusk species of special concern. Final report: Interior Columbia Basin Ecosystem Management Project, Walla Walla, WA. Deixis Consultants, Seattle, WA. Contract #43-0E00-4-9112. 274 pp
Frest, T.J. (2002). Native snails: indicators of ecosystem health. In: Wuerthner G & Matteson, M. (eds) Welfare ranching. Island Press, Sausalito pp. 211-215
Guette A, Gaüzère P, Devictor V, Jiguet F, Godet F (2017) Measuring the synanthropy of species and communities to monitor the effects of urbanization on biodiversity. Ecol Indic 79:139–154
Horsák M, Juřičková L, Kintrová K, Hájek O (2009) Patterns of land snail diversity over a gradient of habitat degradation: a comparison of three Czech cities. Biodivers Conserv 18:3453–3466
Horsák M, Lososová Z, Čejka T, Juřičková L, Chytrý M (2013) Diversity and biotic homogenization of urban land-snail faunas in relation to habitat types and macroclimate in 32 central European cities. PLoS One 8:e71783
Hotopp KP (2002) Land snails and soil calcium in central Appalachian Mountain Forest. Southeast Nat 1:27–44
Hotopp KP, Pearce T, Nekola JC, Slapcinsky J, Dourson D, Winslow M, Kimber G, Watson B (2013) Land snails and slugs of the mid-Atlantic and northeastern United States. Online Resource: http://www.carnegiemnh.org/science/mollusks/index.html
Hubricht L (1985) The distributions of the native land mollusks of the Eastern United States. Fieldiana, Zoology New Ser: 24, 191 pp
Koleff P, Gaston KJ, Lennon JJ (2003) Measuring beta diversity for presence–absence data. J Anim Ecol 72:367–382
Knop E (2016) Biotic homogenization of three insect groups due to urbanization. Glob Chang Biol 22:228–236
Kruskal J (1964) Nonmetric multidimensional scaling: a numerical method. Psychometrika 29:115–129
Kühn I, Klotz S (2006) Urbanization and homogenization – comparing the floras of urban and rural areas in Germany. Biol Conserv 127:292–300
Lososová Z, Horsák M, Chytrý M, Čejka T, Danihelka J, Fajmon K, Hájek O, Juřičková L, Kintrová K, Láníková D, Otýpková Z (2011) Diversity of Central European urban biota: effects of human-made habitat types on plants and land snails. J Biogeogr 38:1152–1163
Lydeard C, Cowie RH, Ponder WF, Bogan AE, Bouchet P, Clark SA, Cummings KS, Frest TJ, Gargominy O, Herbert DG, Hershler R, Perez KE, Roth B, Seddon M, Strong EE, Thompson FG (2004) The global decline of nonmarine mollusks. Bioscience 54:321–330
McCune B, Grace JB (2002) Analysis of ecological communities. MjM Software Design, Glenden Beach
McKinney ML (2002) Urbanization, biodiversity, and conservation. Bioscience 52:883–890
McKinney ML (2006) Urbanization as a major cause of biotic homogenization. Biol Conserv 127:247–260
McKinney ML (2008) Effects of urbanization on species richness: a review of plants and animals. Urban Ecosyst 11:161–176
Murthy AC, Fristoe TS, Burger JR (2016) Homogenizing effects of cities on North American winter bird diversity. Ecosphere 7:e01216
Olden JD, Rooney TP (2006) On defining and quantifying biotic homogenization. Glob Ecol Biogeogr 15:113–120
Pilsbry HA (1940) Land mollusca of north America (north of Mexico). Volume I, Part II. The Academy of Natural Science of Philadelphia, Philadelphia. pp 575–994
Pilsbry HA (1946) Land mollusca of north America (north of Mexico). Volume II, Part I. The Academy of Natural Science of Philadelphia, Philadelphia, pp 1–520
Pilsbry HA (1948) Land mollusca of north America (north of Mexico). Volume II, Part II. The Academy of Natural Sciences of Philadelphia, Philadelphia, pp 521–1113
Rhodes AM (1998) Method for the preparation and quantification of microscopic charcoal from terrestrial and lacustrine sediment cores. The Holocene 8:113–117
Rouget M, Robertson MP, Wilson JRU, Hui C, Essl F, Renteria JL, Richardson DM (2016) Invasion debt - quantifying future biological invasions. Divers Distrib 22:445–456
Saxton AM (1998) A macro for converting mean separation output to letter groupings in Proc Mixed. In Proc. 23rd SAS Users Group International, SAS Institute, Cary, NC, pp 1243–1246
Seto KC, Guneralp B, Hutyra LR (2012) Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. PNAS 109:16083–16088
Shochat E, Lerman SB, Anderies JM, Warren PS, Faeth SH, Nilon CH (2010) Invasion, competition, and biodiversity loss in Urban Ecosyst. Bioscience 60:199–208
Ström L, Hylander K, Dynesius M (2009) Different long-term and short-term responses of land snails to clear-cutting of boreal stream-side forests. Biol Conserv 142:1580–1587
US Census Bureau (2015) www.census.gov. Accessed 11 Feb. 2016
Vermeulen JJ, Liew TS, Schilthuizen M (2015) Additions to the knowledge of the land snails of Sabah (Malaysia, Borneo), including 48 new species. ZooKeys 531:1–139
Yanes Y (2012) Anthropogenic effect recorded in the live-dead fidelity of land snail assemblages from San Salvador Island (Bahamas). Biodivers Conserv 21:3445–3466
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Hodges, M.N., McKinney, M.L. Urbanization impacts on land snail community composition. Urban Ecosyst 21, 721–735 (2018). https://doi.org/10.1007/s11252-018-0746-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-018-0746-x