Abstract
The main objective of this study was to determine how stream salamander assemblages and species respond to varying levels of impervious surface cover within Maryland’s Piedmont physiographic province. We sampled stream salamanders in 21 first-order streams located in watersheds representing a range of impervious surface cover (0–24 %) across the northeastern part of Maryland’s Piedmont region. Habitat data, including stream substrate and riparian characteristics, were measured at each site. Eurycea bislineata was the predominant species of stream salamander encountered during this study (> 99 % of individuals) and most of these individuals were larvae (> 92 %). Consequently, statistical analysis was limited to E. bislineata larvae. We were unable to detect a difference in E. bislineata abundances or body mass index’s among varying levels of impervious surface cover % or any individual site habitat variables alone. These results suggest that in smaller watersheds within the Piedmont of Maryland, local habitat variables, in conjunction with land use variables, are necessary in determining the abundance and body mass index of E. bislineata larvae populations. This study makes a strong case for halting the over-simplification of the relationship between urbanization and the presence/abundance of stream salamanders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Severe declines in amphibian populations have been reported in many areas of the world (Lips 1999; Collins and Storfer 2003; Green 2003). These declines, while still poorly understood, are believed to have several causes including habitat destruction, modification, and fragmentation (Stuart 2004), disease (Laurance et al. 1996; Lips 1999), exploitation (Lannoo et al. 1994), pollution (Rouse et al. 1999), pesticide use (Davidson 2004), introduced species (Morgan and Buttemer 1996), climate change (Kiesecker et al. 2001), and increased UV-B radiation (McCallum 2007). While these individual causes have differing degrees of impact around the world, habitat destruction has been identified as the single greatest factor contributing to amphibian declines (Stuart 2004). Many amphibians have biphasic life histories. Therefore, loss of either aquatic or terrestrial habitat can diminish the ability of a population to persist in an area (Semlitsch and Bodie 2003). Habitat destruction in the form of agriculture and urbanization within the mid-Atlantic region of the United States is largely responsible for declines in amphibian populations (Todd et al. 2009).
Urbanization is currently one of the fastest growing types of land use change in the world (Paul and Meyer 2001). Increasing levels of urbanization within a watershed represent a threat to stream ecosystems due to physical and chemical changes. A central feature of urbanization is the increase in impervious surface cover (ISC) within the catchment area which, in turn, leads to decreased infiltration and increased runoff (Porcella and Sorensen 1980). Imperviousness has been shown to be an accurate predictor of urbanization within watersheds (McMahon and Cuffney 2000). While modification of the natural flow regime of streams and rivers worldwide drastically affects both aquatic and riparian species, ecological responses to that change depend upon the specific attributes of individual aquatic systems (Poff and Ward 1990). Stream form and function vary considerably among landscapes. Local geoclimatic characteristics influence the morphology, hydrology, and sediment composition of waterways (Poff et al. 1997). Due to such characteristics, the severity of geomorphic responses to land use change, such as urbanization, may depend on physiographic region (Poff et al. 2006; Utz et al. 2011). Consequently, ecological responses to land use change may also vary depending on physiographic region (Fitzpatrick and Knox 2000; Utz and Hilderbrand 2011). While a few studies have attempted to find general explanations and patterns linking hydrologic and geomorphic responses to land use change, there is still a great deal of uncertainty as to the vulnerability of different types of streams and their ecosystems to urbanization (Poff and Ward 1990; Poff et al. 2006).
An understanding of amphibian sensitivity to urbanization is crucial as watersheds continue to be altered. In smaller streams lacking fish, amphibians such as salamanders and anurans may assume the roles of top predators, strongly influencing ecosystem processes (Wyman 1998; Southerland et al. 2004). Amphibians are ideal environmental indicators because of their permeable skin, their longevity, and because their complex life cycles potentially expose them to both aquatic and terrestrial disturbances (Grover 2000; Blaustein and Johnson 2003). Amphibians, particularly stream salamanders, have been proposed for use in biological monitoring of stream health in Maryland. Preliminary research has demonstrated the usefulness of salamanders for assessing stream quality (Southerland et al. 2004).
Several studies have demonstrated that salamander relative abundance is inversely proportional to the degree of urbanization (Orser and Shure 1972; Wilson and Dorcas 2003; Brannon and Purvis 2008), although responses tend to vary among species and developmental stage (Price et al. 2011). These studies have been conducted in a variety of geographic regions; however, there is very little research that investigates stream salamander responses to urbanization within the Piedmont physiographic province of Maryland. This is significant because development is occurring rapidly in this region (Maryland Department of Planning 2008). Recent assessments of fishes and macroinvertebrates at community (Morgan and Cushman 2005) and taxon-specific (Utz et al. 2009, 2010) scales have demonstrated that biological tolerance to urbanization can vary depending upon physiographic province.
The main objective of this study was to determine how stream salamander assemblages and species respond to varying levels of ISC within Maryland’s Piedmont physiographic province. Previous studies in other regions have shown that as urbanization increases, salamander species richness and abundances tend to decrease (Orser and Shure 1972; Price et al. 2012). Few studies, however, have identified the specific relationship between ISC and stream salamander presence/abundance. Specifically, we were interested in whether salamanders exhibited a threshold response to ISC, or whether changes are generally linear across a range of impervious surface covers. Our second objective was to determine if ISC in the riparian area, a measure of Effective Impervious Area (EIA), was a better predictor of salamander abundance than ISC across the entire watershed. The riparian zone is important habitat for organisms with biphasic life cycles. Although some studies have found that EIA adversely affects amphibian populations (Ficetola et al. 2008), others (Wilson and Dorcas 2003; Miller et al. 2007) have found EIA to be a poor predictor of salamander abundance. Our third objective was to determine whether site variables, such as physical stream properties, can be used to develop a habitat suitability model that can be related to stream salamander presence and abundance.
Body Mass Index (BMI; mass/snout-vent length) has historically been a reliable condition index used to assess the health of individual animals. Studies have shown that the BMI of many species, including salamanders, is generally negatively correlated with increased environmental degradation (Krause et al. 2011). The final objective of this study was to determine whether increased levels of ISC or EIA have a detrimental effect on the BMI of stream salamanders.
Methods
Site selection
Sites were selected using a targeted approach based on topographic and land cover features. Impervious surface cover (%) and Anderson 1st-level land use classes (Anderson et al. 1976) were quantified at the watershed scale by overlaying watershed boundaries with the 2006 National Land Cover Database (Fry et al. 2011) and extracting the relevant data. In addition to %ISC at the watershed level, we also quantified percent effective impervious surface area (% EIA) by extracting relevant data from a buffer zone of 60 m adjacent to each stream within the boundaries of the watershed. This approach differs slightly from some other determinations of % EIA in that it does not include drainage connections by pipes or lined channels (Hatt et al. 2004). Because of its impact on both hydrology (Poff et al. 2006) and amphibian habitat (Ficetola et al. 2008), % EIA was included as a variable in this study even though it is often correlated with %ISC.
Only watersheds that were between 1.0 to 2.5 km2 in area and that were entirely within the Piedmont physiographic province were included as candidates. Study sites were classified apriori into seven evenly-spaced groups based on watershed % ISC values (Table 1). ISC values ranged from 0 % in our least urbanized group to 24 % in our most urbanized group. Urbanization and ISC at our sites were highly correlated (r = 0.867). Three sites were selected within each group for a total of 21 sites. Groups were selected at small intervals across this ISC gradient in order to characterize the nature of the response (linear vs. threshold). We choose the range from 0 to 24 % ISC for several reasons. First, recent work with other aquatic taxa (fish and macroinvertebrates) has demonstrated that a number of species populations are impacted at low levels of ISC (e.g., <5.0 % ISC) (Hilderbrand et al. 2010; Utz et al. 2009, 2010). Second, similar ranges have been used for other studies examining the relationship between salamander assemblages and urbanization (Miller et al. 2007). Finally, watersheds examined in our study are small (1–2.5km2), and many of these smaller headwater streams are eliminated at higher levels of urbanization (Elmore and Kaushal 2007).
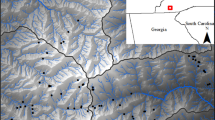
Sites were excluded if there was extensive agricultural (>25 %) cover. At each site the sampling unit was a 25 m stream reach. Sites selected for the study are shown in Fig. 1 and watershed attributes are provided in Table 1.
Salamander sampling
Sampling occurred in early April 2012. At each site, electrofishing was performed using a two-pass removal technique to obtain abundances for stream salamanders. Blocknets (8.5 mm mesh) were placed at both ends of the transect to prevent immigration or emigration of salamanders. Cover objects within the wetted perimeter of the channel were also searched during sampling. A two-person team was used to conduct the electrofishing passes. Rubber gloves and a modified Turkey baster were used to help assist in the capture of salamanders. The gloves allowed the searchers to capture salamanders more effectively while they were immobilized in the electric field. All salamanders captured were identified to species, weighed, measured for snout-vent length, and released. All individuals were held until both passes of electrofishing had been completed to prevent recapture. They were then released at the center of the transect.
In order to determine if sampling season influenced salamander assemblages, subsequent sampling occurred during follow-up visits in August and September 2012. During these subsequent visits we sampled not only the focal 25 m stream reach but adjacent riparian areas and sections of the stream channel above and below our study reach.
Habitat sampling
Habitat data were collected at each site in early August during low flow conditions using techniques based on Kayzak (1995) and Rosgen (1996). Bankfull width and wetted width were averaged from six measurements at 0 m, 5 m, 10 m, 15 m, 20 m, and 25 m locations of the transect. Average stream depth was determined by taking depth measurements every 20 cm across the wetted width of the stream at these six locations. Maximum stream depth within the 25-m transect was recorded. Canopy cover was estimated using a spherical densiometer at the downstream, mid-section, and upstream points of the transect. Riparian buffer width was measured on both sides of the stream at the downstream, mid-section, and upstream points of the transect. If the buffer width was greater than or equal to 50 m, 50 m was recorded. At the downstream, mid-section, and upstream points of the transect, the percent coverage of 8 substrate classes (Table 2) was visually estimated using a 1 m2 quadrate.
Statistical analysis
Principal components analysis (PCA) was used to graphically depict habitat and land use differences among sites. One-way analysis of variance (ANOVA) was used to test for stream and riparian habitat differences among treatment groups.
We used multiple regression to predict salamander abundances from physical habitat and land use/land cover attributes for each site. A pairwise correlation analysis of all predictor variables was conducted to identify any multicollinearity. The Bayesian information criterion (BIC) selection method was used to select significant explanatory variables and determine the best predictive multivariate model of salamander abundance. A maximum of seven variables was included in the model as a result of our data set being limited to 21 stream sites. Consequently, more predictive models were eliminated because of the inclusion of too many independent variables. Explainable variation due to each variable was assessed using the ‘lmg’ metric in the package ‘relaimpo’ (Groemping 2006) in the Program R (R Development Core Team 2010). The total amount of variation explained by each variable was calculated by multiplying the explainable variation for each variable by the total explainable variation for the entire model. A similar analysis was also conducted for the BMIs of salamanders. The residuals of the most predictive model were analyzed to confirm normality and constant variance. A Kruskal-Wallis test was used to determine whether there were any differences in BMI values among treatment groups. A multiple comparisons test was used in order to determine pairwise differences in BMI means among treatment groups. All models were run in the statistical program R (R Development Core Team 2010).
Results
The initial design of our study included sampling all stream salamanders that were present in Maryland’s Piedmont region; however, during preliminary sampling it became apparent that Pseudotriton montanus, Pseudotriton ruber, Desmognathus fuscus, Eurycea longicauda, and Eurycea bislineata adults were too rare or absent to analyze statistically. Eurycea bislineata larvae, however, were found in varying abundances and became the focus of our study (Fig. 2).
Habitat
A principal components analysis (PCA) of the 18 site habitat variables showed the first five principal components accounted for 72.4 % of the total variation in our habitat data (Fig. 3; Table 3). The first principal component explained 20.18 % of the variation in the dataset and was dominated by % ISC, % EIA, and % forest cover. The second principal component loaded most heavily on substrate variables; specifically % sand, % gravel, and % cobble. The third principal component was associated with % canopy cover, % boulder, and riparian buffer width. The fourth principal component described a gradient in course woody debris, maximum depth, and average depth. Variables loading most heavily on the fifth principal component were bankfull width, mud/silt, and riparian buffer width.
Principal components analysis of 18 site habitat variables from 21 stream sites. The first principal component explained 20.18 % of the variation in the dataset and was dominated by % ISC, % EIA, and % forest cover. The second principal component loaded most heavily on substrate variables; specifically % sand, % gravel, and % cobble (15.66 % of the variance). The third principal component (14.68 %) was associated with % canopy cover, % boulder, and riparian buffer width
We found no significant differences among urbanization categories for stream substrate composition (Fig. 4), stream channel characteristics (Fig. 5), or riparian habitat characteristics (Fig. 6).
Multicollinearity among habitat variables
Multicollinearity between the independent site habitat variables was analyzed by examining cross-correlations (Pearson correlation coefficient, r). No significant multicollinearity (i.e., r ≥ 0.9) existed between any of the site habitat variables.
Predictors of Eurycea bislineata larval abundance
A seven-variable model was the best at predicting E. bislineata abundance. Member variables were: % EIA, % agriculture, average depth, maximum depth, % mud/silt, % gravel, and % cobble; and they explained 71 % of the variation (Table 4). Positive relationships existed between E. bislineata abundance and % EIA, % agriculture, and maximum depth. Percent EIA accounted for 5 % of the total variation. Percent agriculture accounted for 16 % of the total variation. Maximum depth accounted for 13 % of the total variation (Table 4). Negative relationships existed between E. bislineata abundance and % mud/silt, % gravel, % cobble and average depth. Percent mud/silt accounted for 9 % of the total variation. Percent gravel accounted for 12 % of the total variation. Percent cobble accounted for 5 % of the total variation. Average depth accounted for 11 % of the total variation.
Predictors of Eurycea bislineata larval BMI
The best model to predict BMI contained % ISC, % EIA, average depth of stream, riparian buffer width, course woody debris (CWD), % sand, and % gravel, and had an R 2 of 0.72 (p = 0.0018; Table 5). Riparian buffer width, % EIA, % course woody debris, % sand, and % gravel had positive significant relationships with larval E. bislineata BMI. Riparian buffer width accounted for 10 % of the total variation. Percent EIA accounted for 10 % of the total variation. Percent course woody debris accounted for 18 % of the total variation. Percent sand accounted for 10 % of the total variation. Percent gravel accounted for 16 % of the total variation (Table 5). Average depth of stream and % ISC were negatively related with E. bislineata BMI. Average depth of stream accounted for 4 % of the total variation. Percent ISC accounted for 4 % of the total variation.
The Kruskal-Wallis test for BMI among the differing ISC categories was significant (p < 0.0001). Using the multiple comparisons test it was evident that treatment group 1 (0–1 % ISC) had significantly lower BMIs (p = 0.05) than all of the other treatment groups (Fig. 7). Treatment group 4 (10–12 % ISC) BMIs were also significantly lower than treatment groups 2, 3, 6, and 7 (3–5 % ISC, 6–8 % ISC, 18–20 % ISC, and 22–24 % ISC, respectively).
Discussion
Our results indicate that increased urbanization within small (≤ 2 km2) watersheds of Maryland’s Piedmont physiographic province may not result in detectable changes to stream geomorphology or riparian habitat. These results contradict some research demonstrating that urbanization tends to alter the geomorphology of streams and is often associated with less riparian forest cover (Paul and Meyer 2001). Our results suggest that smaller watersheds and headwater streams may be physically less altered by urbanization (at levels <25 %) than previously believed. Such a finding is not unexpected. Utz and Hilderbrand (2011) also found no differences in channel morphology for similarly sized Piedmont watersheds, although the D50 and D75 particle diameters were significantly larger in urban streams. Prior work demonstrates that hydrologic responses of streams to urbanization vary by position within the watershed (O’Driscoll et al. 2010). Moglen and Beighley (2002) found peak flows at the outlet of an urbanized Maryland stream were not indicative of peak flows in smaller subwatersheds nested within the larger watershed. Spatial variation in hydrology is likely to result in differences in biological responses of stream organisms to urbanization, with implications for biological monitoring.
In Maryland streams draining catchments of less than 120 ha, fish species diversity and abundance are too low to gain any significant monitoring information (Klauda et al. 1998). As a result, amphibians are currently being used to monitor stream health in these smaller catchments (Southerland et al. 2004). Our results demonstrate that stream channel characteristics, riparian buffer width and canopy cover, and stream salamander abundances and BMIs are not influenced by levels of ISC < 24 % in small Piedmont streams. Consequently, biological monitoring of these streams using salamander metrics is unlikely to provide useful information. The spatial scale (i.e., watershed area) at which urbanization begins to significantly modify salamander abundances or habitat characteristics in Piedmont needs to be established.
Additional considerations regarding the effective use of stream salamanders in biological monitoring of Piedmont streams are species richness and assemblage composition. The four metrics used by Southerland et al. (2004) to distinguish between degraded and reference streams were species number, abundance, number of intolerant salamanders, and number of adult salamanders. In our study streams individuals of the pollution-tolerant salamander, E. bislineata, comprised over 99 % of all salamanders captured – 93 % of E. bislineata individuals were larvae. Only one other species (P. ruber) was captured, representing less than one percent of all individuals encountered (3 individuals).
As predictors of larval E. bislineata abundance, our site habitat variables fell into two explanatory categories: correlates of human disturbance, such as land use variables, and correlates of physical habitat features. Our results suggest that local habitat conditions in conjunction with land use variables are better predictors of larval E. bislineata abundance than land use variables alone. We were unable to detect a difference in E. bislineata abundances among varying levels of ISC or any individual site habitat variables alone. Other researchers have observed a decline in stream salamander abundance with increasing ISC (Orser and Shure 1972; Wilson and Dorcas 2003; Miller et al. 2007). Miller et al. (2007) found that E. cirrigera abundances decreased linearly as ISC increased within the watershed. A similar study, which was also conducted in the Piedmont of North Carolina, found that D. fuscus and E. cirrigera abundances were inversely proportional to the amount of disturbed habitat (% agriculture, % ISC, and residential area) within the watershed (Wilson and Dorcas 2003).
Most of the variables present in the best model predicting salamander abundance were those associated with physical habitat characteristics of the stream. This suggests that local attributes such as channel substrate and channel morphology may be more important to E. bislineata abundance than many watershed-level characteristics. Many studies of stream-associated salamanders have demonstrated the importance of local habitat features on distribution (Orser and Shure 1972; Miller et al. 2007). For example, Orser and Shure (1972) found that salamander densities increase with available cover. Other researchers have demonstrated that time since disturbance (e.g., road construction, building a house, etc.) may be another important influence. Miller et al. (2007) found that some of their sites that had less ISC but more recently disturbed sites had lower abundances of larval E. cirrigera than sites that had more ISC constructed many years prior (e.g., ~ 40–50 years ago). Some studies have shown that ecological responses in streams to land use change within a watershed depend on how the components of stream flow have changed relative to the natural flow regime for that particular stream (Poff and Ward 1990). These studies lend support to our findings, suggesting that ecological responses, such as salamander abundance, are determined by more than watershed-level characteristics alone.
Despite the importance of local variables, the best predictor of E. bislineata larval abundance was the percentage of agriculture cover within the watershed. Agriculture was positively related to E. bislineata larval abundance and explained 16 % of the variation in the multivariate model. Although this initially seems counterintuitive, historically, most of Piedmont Maryland was agricultural land. Within the last century, many of these agricultural lands have been developed into urban areas (Maryland Department of Planning 2008). Consequently, many of our stream sites that were not highly urbanized had higher percentages of agriculture cover within the watershed.
Stream channel complexity appears to be important to E. bislineata abundance; greater numbers were associated with sites having the greatest maximum depth, but more shallow depths on average. Similar relationships were found previously (Southerland et al. 2004) between stream depth and salamander abundances in Maryland streams west of the Coastal Plain (Piedmont, Blue Ridge, Valley and Ridge, and Appalachian Plateau physiographic provinces). Southerland and his colleagues included streams of a wide range of sizes (1st through 4th order streams) in their analysis. In a study conducted in the Piedmont of Maryland, female D. fuscus were found to locate nests adjacent to deeper portions of the stream. The researchers hypothesized that this likely was a result of deeper portions of the stream being the last to dry out during low flow conditions in the summer. By placing nests closer to these deeper portions, the larvae would persist through the low flow period and successfully metamorphose (Snodgrass et al. 2007).
Within Maryland, E. bislineata is the only species of stream salamander consistently found in degraded streams according to MBSS sampling data (Southerland et al. 2004). Eurycea bislineata is more tolerant of human disturbance than other species of stream salamander (Wilson and Dorcas 2003; Southerland et al. 2004); however, at severe levels of stream degradation their populations have been found to decline or disappear over time.
During April sampling, we expected to primarily encounter larval and juvenile/adult E. bislineata and juvenile/adult D. fuscus individuals. By sampling earlier in the year, we intended to exclude young-of-year larvae for both species. Eurycea bislineata was the predominant species of stream salamander encountered (> 99 % of individuals), and most of these individuals (> 92 %) were larvae. Three larval P. ruber individuals were also encountered during April sampling.
Desmognathus fuscus larvae typically hatch from late summer to early fall (Juterbock 1987). Thus, sites were revisited in August and September to determine whether spring sampling had excluded the species. During these subsequent visits, D. fuscus and E. longicauda were encountered, although only two individuals of each species were found across all 21 sites. Historically, large populations of D. fuscus have been found in less developed areas within the Maryland Piedmont, with abundances increasing headward in watersheds (Snodgrass et al. 2007; Southerland and Stranko 2008). However, we were unable to find more than a single D. fuscus at any of the 21 sites – including the reference sites. Our data suggest that D. fuscus distribution and abundances have decreased or are patchier throughout its range than previously believed. There may be a minimum habitat requirement, including watershed size and headwater position, which was not met for D. fuscus. We were unable to compare salamander assemblages among varying levels of ISC as a result of the low number of salamander species found.
Similar to the abundance model, most predictor variables used in the best BMI model were associated with stream physical habitat characteristics. The two best predictor variables in the model were % composition of coarse woody debris and gravel. Both variables were positively related to E. bislineata BMI and explained 18 % and 16 % of the variation in the multivariate model, respectively. The positive association of these variables to E. bislineata larval BMIs is possibly related to the fact that these two variables have been found to be positively related to increased abundances and diversity of aquatic macroinvertebrates (Wallace et al. 1996) which are major food items for stream salamanders (Petranka 1984; Cecala et al. 2007).
The BMI of many species, including salamanders, is negatively correlated with increased environmental degradation due to limited or suboptimal resource availability (Welsh et al. 2008; Krause et al. 2011). In contrast, larval E. bislineata BMIs in our lowest ISC group (0–1 % ISC) were significantly lower than groups with higher ISC. The body condition (variation from the expected mass of an individual) of many species has been shown to be influenced by factors other than environmental degradation. There is an extensive fisheries management literature showing negative relationships between body mass and population density (Jenkins et al. 1999; Lorenzen and Enberg 2001). This trend is hypothesized to be the byproduct of community interactions, such as competition. This tendency was not observed in our study; reference sites with lower salamander BMIs did not show increased abundances.
Our results make a strong case for re-examining the relationship between urbanization and stream salamanders across a range of watershed sizes. Our results suggest that, in smaller watersheds of Maryland’s Piedmont, local habitat variables in conjunction with land use variables are necessary in determining the abundance and BMI of E. bislineata larvae. We found no trend between urbanization and the degradation of stream habitat and failed to show a relationship between ISC % and E. bislineata larval abundance or BMI.
In future studies, it would be helpful to focus on a model which would predict the presence/absence of stream salamander species for smaller watersheds. Many studies have asserted that at lower levels of urbanization less pollution-tolerant species of salamanders should persist alongside more tolerant species. This conclusion may hold true in some areas; however, at the scale of our study, this was not found. One potential explanation for this finding is that there may be scaling discrepancies between various studies which would not account for species which have patchy distributions.
References
Anderson JR, Hardy EE, Roach JT, Witmer RE (1976) A land use and land cover classification scheme for use with remote sensor data. Professional Paper 964, US Geological Survey, Reston, VA.
Blaustein AR, Johnson PT (2003) The complexity of deformed amphibians. Front Ecol Environ 1:87–94
Brannon MP, Purvis BA (2008) Effects of sedimentation on the diversity of salamanders in a southern Appalachian headwater stream. J North Carolina Acad Scie 124:18–22
Cecala KK, Price SJ, Dorcas ME (2007) Diet of larval red salamanders (psuedotriton ruber) examined using a nonlethal technique. J Herpetol 41:741–745
Collins JP, Storfer A (2003) Global amphibian declines: sorting the hypotheses. Divers Distrib 9:89–98
Davidson C (2004) Declining downwind: amphibian population declines in California and historical pesticide use. Ecol Appl 14:1892–1902
Elmore AJ, Kaushal SS (2007) Disappearing headwaters: patterns of stream burial due to urbanization. Front Ecol Environ 6:308–312
Ficetola G, Padoa-Schippa E, De Bernardi F (2008) Influence of landscape elements in riparian buffers on conservation of semiaquatic amphibians. Conserv Biol 23:114–123
Fitzpatrick FA, Knox JC (2000) Spatial and temporal sensitivity of hydrogeomorphic response and recovery to deforestation, agriculture, and floods. Phys Geogr 21:89–108
Fry J, Xian G, Jin S, Dewitz J, Homer C, Yang L, Barnes C, Herold N, Wickham J (2011) Completion of the 2006 national land cover database for the conterminous United States. Photogramm Eng Remote Sens 77:858–864
Green DM (2003) The ecology of extinction: population fluctuation and decline in amphibians. Biol. Cons. 111:331–343
Groemping U (2006) Relative importance for linear regression in R: The package relaimpo. Journal of Statistical Software. 17(1). http://www.jstatsoft.org/v17/i01
Grover MC (2000) Determinants of salamander distribution along moisture gradients. Copeia 2000:156–168
Hatt BE, Fletcher TD, Walsh CJ, Taylor SL (2004) The influence of urban density and drainage infrastructure on the concentrations and loads of pollutants in small streams. Environ Manag 34:112–124
Hilderbrand RH, Utz S, Stranko SA, Raesly RL (2010) Applying thresholds to forecast potential biodiversity loss from human development. J North Am Benthological Soc 29(1):1009–1016
Jenkins TM, Diehl S, Kratz KW, Cooper SD (1999) Effects of population density on individual growth of brown trout in streams. Ecology 80:941–956
Juterbock JE (1987) The nesting behavior of the dusky salamander. Desmognathus Fuscus II Nest Site Tenacity Disturbance Herpetol 43:361–368
Kayzak PF (1995) Maryland biological stream survey sampling manual. Maryland department of natural resources. Chesapeake Bay Research and Monitoring Division, Annapolis, MD
Kiesecker JM, Blaustein AR, Belden LK (2001) Complex causes of amphibian declines. Nature 410:681–684
Klauda R, Kayzak P, Stranko S, Southerland M, Roth N, Chaillou J (1998) Maryland biological stream survey: a state agency program to assess the impact of anthropogenic stresses on stream habitat quality and biota. Environ Monit Assess 51:299–316
Krause ET, Steinfartz S, Caspers BA (2011) Poor nutritional conditions during the early larval stage reduce risk-taking activities of fire salamander larvae (salamandra salamandra). J Ethol 117:416–421
Lannoo MJ, Lang K, Waltz T, Phillips GS (1994) An altered amphibian assemblage: Dickinson county, Iowa, 70 years after frank Blanchard’s survey. Am Midl Nat 131:311–319
Laurance WF, McDonald KR, Speare R (1996) Epidemic disease and the catastrophic decline of Australian rain forest frogs. Cons. Biol. 10:406–413
Lips KR (1999) Mass mortality and population declines of anurans at an upland site in western Panama. Cons. Biol. 13:117–125
Lorenzen K, Enberg K (2001) Density-dependent growth as a key mechanism in the regulation of fish populations: evidence from among-population comparisons. Proc. R. Soc. Lond. Series B 269:49–54
Maryland Department of Planning (2008) Where do we grow from here? Maryland Department of Planning, Annapolis Available: http://www.mdp.state.md.us/PDF/Yourpart/773/773TaskForceReport.pdf [accessed 07 June 2012]
McCallum ML (2007) Amphibian decline or extinction? Current decline dwarfs background extinction rate. J Herpetol 41:483–491
McMahon G, Cuffney (2000) Quantifying urban intensity in drainage basins for assessing stream ecological conditions. J Am Water Resour Assoc 36:1247–1262
Miller JE, Hess GR, Moorman CE (2007) Southern two-lined salamanders in urbanizing watersheds. Urban Ecosyst 10:73–85
Moglen GE, Beighley RE (2002) Spatially explicit hydrologic modeling of land use change. J Amer Wat Res Assoc 38:241–253
Morgan LA, Buttemer X (1996) Predation by the non-native fish gambusia holbrooki on small litoria aurea and L. dentata tadpoles. Aust Zool 30:143–149
Morgan RP, Cushman SE (2005) Urbanization effects on stream fish assemblages in Maryland. USA J N Am Benthol Soc 24:643–655
O’Driscoll M, Clinton S, Jefferson A, Manda A, McMillan S (2010) Urbanization effects on watershed hydrology and in-stream processes in the southern United States. Water 2010:605–648
Orser PN, Shure DJ (1972) Effects of urbanization on the salamander desmognathus fuscus fuscus. Ecology 53:1148–1154
Paul MJ, Meyer JL (2001) Streams in the urban landscape. Annu Rev Ecol Syst 32:333–365
Petranka JW (1984) Ontogeny of the diet and feeding behavior of Eurycea bislineata larvae. J Herpetol 18:48–55
Poff NL, Ward JV (1990) Physical habitat template of lotic systems: recovery in the context of historical patterns of spatiotemporal heterogeneity. Environ Manag 14:629–645
Poff NL, Allan JD, Bain MB, Karr JR, Prestegaard KL, Richter BD, Sparks RE, Stromberg JC (1997) The natural flow regime, a paradigm for river conservation and restoration. Bioscience 47:769–784
Poff NL, Bledsoe BP, Cuhaciyan CO (2006) Hydrologic variation with land use across the contiguous United States: geomorphic and ecological consequences for stream ecosystems. Geomorphology 79:264–285
Porcella DB, Sorensen DL (1980) Characteristics of urban runoff in the Maryland suburbs of Washington, D.C. EPA-600/3–80-032. U.S. Environmental Protection Agency, Washington, D.C.
Price SJ, Cecala KK, Browne RA, Dorcas ME (2011) Effects of urbanization on occupancy of stream salamanders. Cons. Biol. 25:547–555
Price SJ, Browne RA, Dorcas ME (2012) Evaluating the effects of urbanization on salamander abundances using a before-after control-impact design. Freshw Biol 57:193–203
R Development Core Team (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org.
Rosgen D (1996) Applied river morphology, 2nd edn. Pagosa Springs, CO., Wildland Hydrology
Rouse JD, Bishop CA, Struger J (1999) Nitrogen pollution: an assessment of its threat to amphibian survival. Environ Health Perspect 107:779–803
Semlitsch RD, Bodie JR (2003) Biological criteria for buffer zones around wetlands and riparian habitats for amphibians and reptiles. Cons. Biol. 17:1219–1228
Snodgrass JW, Forester DC, Lehti M, Lehman E (2007) Dusky salamander (desmognathus fuscus) nest-site selection over multiple spatial scales. Herpetologica 63:441–449
Southerland MT, Stranko SA (2008) Fragmentation of riparian amphibian distributions by urban sprawl in Maryland, USA. In: Mitchell JC, Jung Brown RE, Bartholomew B (eds) Urban herpetology: a special publication from the J. Herpetol. Salt Lake City, UT, pp. 423–433
Southerland MT, Jung RE, Baxter DP, Chellman IC, Mercurio G, Volstad JH (2004) Stream salamanders as indicators of stream quality in Maryland. USA Appl Herpetol 2:23–46
Stuart SN (2004) Status and trends of amphibian declines and extinctions worldwide. Science 306:1783–1786
Todd BD, Luhring TM, Rothermel BB, Gibbons JW (2009) Effects of forest removal on amphibian migrations: implications for habitat and landscape connectivity. J Appl Ecol 46:554–561
Utz RM, Hilderbrand RH (2011) Interregional variation in urbanization-induced geomorphic change and macroinvertebrate habitat colonization in headwater streams. J N Am Benthol Soc 31:25–37
Utz RM, Hilderbrand RH, Boward DM (2009) Identifying regional differences in threshold responses of aquatic invertebrates to land cover gradients. Ecol Indic 9:556–567
Utz RM, Hilderbrand RH, Raesly RL (2010) Regional differences in patterns of fish species loss with changing land use. Biol. Cons. 143:688–699
Utz RM, Eshleman KN, Hilderbrand RH (2011) Variation in hydrological, chemical, and thermal responses to urbanization in streams between two physiographic regions of the mid-Atlantic United States. Ecol Appl 21:402–215
Wallace JB, Grubaugh JW, Whiles MR (1996) Influences of coarse woody debris on stream habitats and invertebrate diversity. Pages 119–129 In: J. W. McMinn and D. A. Crossley, Jr. (Eds.). Biodiversity and coarse woody debris in southern forests. USDA Forest Service, General Technical Report SE-94, Asheville, NC.
Welsh HH, Pope KL, Wheeler CA (2008) Using multiple metrics to assess the effects of forest succession on population status: a comparative study of two terrestrial salamanders in the US Pacific northwest. Biol Cons 141:1149–1160
Wilson JD, Dorcas ME (2003) Effects of habitat disturbance on stream salamanders: implications for buffer zones and watershed management. Cons Biol 17:763–771
Wyman RL (1998) Experimental assessment of salamanders as predators of detrital food webs: effects on invertebrates, decomposition and the carbon cycle. Biodivers Conserv 7:641–650
Acknowledgments
The authors wish to thank Dr. Don Forester and Dr. Mark Southerland for their suggestions regarding study design.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rizzo, A.A., Raesly, R.L. & Hilderbrand, R.R. Stream salamander responses to varying degrees of urbanization within Maryland’s piedmont physiographic province. Urban Ecosyst 19, 397–413 (2016). https://doi.org/10.1007/s11252-015-0504-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-015-0504-2