Abstract
The present trial investigated the feeding effect of B. subtilis spores on growth performance, blood metabolites, antioxidative status, and digestive enzyme activities in growing quails. A total of 750 1-day-old Japanese quail chicks were randomly allotted equally into five experimental groups: control (BS0) fed a maize-soybean basal diet with no additives, the others were supplemented with: B. subtilis spores with the levels of 1 × 103 (BS3), 1 × 105 (BS5), 1 × 107 (BS7), and 1 × 109 (BS9)/kg diet. Quails fed on B. subtilis diets exhibited linearly increasing live body weight and body weight gain and decreased feed-to-gain ratio compared with the control group. Daily feed intake was not significantly altered. Increasing levels of B. subtilis led to a linear increase in serum total protein and albumin levels, and a linear decrease in concentrations of glucose, creatinine, urea-N, aspartate aminotransferase, and alanine aminotransferase. Hypolipidemic impact of feeding B. subtilis spores was greatly observed and enhanced by increasing its dietary inclusion level. Triiodothyronine and thyroxine activities were significantly elevated in treated groups. Glutathione content and catalase activities were linearly increased in groups BS7, BS9, and BS5, while lipid peroxidation was decreased in all treatment groups. Duodenal proteolytic, lipolytic, and amylolytic activities as well as nutrient digestibility were linearly increased in treated groups. In conclusion, dietary supplementation of B. subtilis spores almost at all studied levels was able to promote the antioxidative status and digestive enzymes activities, while only the high concentrations (BS7 and BS9) could improve the nutrient digestion and growth performance of growing Japanese quail.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The excessive and indiscriminate use of antibiotics in poultry production industry poses a major threat to human health due to the high incidence of antibiotic-resistant bacteria. The growing global concerns about food security had led to the ban of antibiotics use as growth promoter in European Union countries. Probiotic, as a kind of green feed additive, has the ability to improve poultry production, intestinal health, morphology and microflora, and immune response (Abd El-Moneim and Sabic 2019, Abd El-Moneim et al. 2019, Ebeid et al. 2019, Fathi et al. 2018, Fathi et al. 2017). However, the probiotic sensitivity to environmental changes, particularly during the formulation and granulation of the diet, would reduce the amount of viable bacteria capable of reaching to the digestive tract of birds which in turn will reduce its beneficial effect (Abd El-Moneim et al. 2019, Leser et al. 2008). In adverse environmental condition, Bacillus subtilis can form spores that can grow fast with highly resistance to acid, alkali, heat, UV radiation, vacuum pressure, and chemicals (Hooge 2003). Thus, these spores can still grow and multiply in the intestinal tract upon arrival even after exposure to diets processing conditions and the acute acidic environment of proventriculus.
It is well known that B. subtilis is an aerobic bacterium that consumes large amounts of free oxygen in the digestive tract while proliferating. Therefore, it can inhibit aerobic pathogens growth and promote the growth of anaerobic probiotic such as bifidobacterium, yeasts, and Lactobacillus (Ebeid et al. 2019, Wang et al. 2006). Moreover, B. subtilis plays a vital role in the development of gastrointestinal tract and gut-associated lymphoid tissue as well as enhancing the innate and adaptive immunity of the host (Fathi et al. 2017, Huang et al. 2008). In brief, B. subtilis spores may exert their favorable effects in poultry diets and bodies by one or more of these mechanisms: (1) competitive exclusion in lowering the count of pathogenic bacteria (Abd El-Moneim et al. 2019, Ebeid et al. 2019); (2) oxygen consumption as mentioned above (La Ragione and Woodward 2003); (3) producing exogenous digestive enzymes (Abd El-Moneim and Sabic 2019, Li et al. 2014); (4) enhance immune response (Abd El-Moneim 2017, Huang et al. 2008, Yurong et al. 2005); and (5) promotion of intestinal function and development (Abd El-Moneim 2017, Abd El-Moneim et al. 2019, Yurong et al. 2005). These features make B. subtilis of particular interest for poultry producers to be used as an alternative supplement to antibiotics via various supplementation routes, especially in the diet. However, the optimal concentration for probiotic administration may be strain dependent and increasing the inclusion rate did not always perform better (Huang et al. 2004, Saleh 2014). Therefore, the present study was performed to evaluate the effect of dietary inclusion of serial doses of B. subtilis on growth performance, blood biochemical indices, oxidative status, and digestive enzyme activities in growing Japanese quail birds.
Materials and methods
Birds housing and experimental design
The present trial was conducted at the experimental poultry farm of the Poultry Research Unit, Biological Application Department, Radioisotopes Applications Division, Nuclear Research Center, Egyptian Atomic Energy Authority at Inshas, Egypt. All the procedures used in this trial were approved by Local Experimental Animals Care Committee, and approved by the Institutional Ethics Committee. The birds were cared using husbandry guidelines derived from Egyptian Atomic Energy Authority standard operating procedures. A total of 750 1-day-old Japanese quail chicks were randomly allotted equally into five experimental groups with five replicates (30 chicks each), to evaluate the effects of different concentrations of B. subtilis spores on growth performance, blood biochemical indices, antioxidant status, and digestive enzyme activities of fattening Japanese quails. Birds were caged in wire battery brooder cages equipped with stainless steel nipple drinker, which was supplying water ad libitum, and were kept under the same managerial, hygienic, and environmental conditions. House temperature was controlled during the first 14 days of the experiment ranged from 33 to 36 °C and then quails were reared until the end of the experimental period under the natural ambient temperature of summer condition. During the last period, ambient temperature was recorded from 1.00 a.m. to 9.00 p.m. (Fig. 1) and was ranged from 27 to 38 °C. Artificial light schedule was similar to the commercial condition (24 h light until the 4th day of age then followed by 23 h of light throughout the experimental period). The control group (BS0) received a maize-soybean mash basal diet without any additives. The basal diet was formulated to meet the nutrient requirements for growing Japanese quail from 1 to 6 weeks of age according to NRC (1994). The remaining experimental groups were fed the basal diet supplemented with B. subtilis spores with the levels of 1 × 103 (BS3), 1 × 105 (BS5), 1 × 107 (BS7), and 1 × 109 (BS9)/kg diet. The B. subtilis ATCC 35854 spores were obtained from Shandong Baolai-leelai Biotech Co., Ltd., Tai’an, China. The composition and the calculated analysis of the experimental diets are shown in Table 1.
Growth performance
Individual live body weight (BW) per replicate was recorded in early morning at 2, 4, and 6 weeks of age. Daily body weight gain (DBWG) was calculated during the experimental periods. Daily feed intake (DFI) was recorded weekly on replication basis to estimate feed conversion ratio (FCR) as g feed/g gain. Birds were monitored twice a day for mortality.
Blood sampling and biochemical analysis
At the end of the experimental period, five quails from each group were randomly chosen and blood samples were collected during slaughtering in a serum-separating tube. Blood samples were immediately centrifuged at 4500 r.p.m. for 15 min., and the serum was frozen at − 80 °C until analysis. Serum total protein (TP), albumin (ALB), globulin (GLO), glucose (GLU), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP), uric acid (UA), urea-N, creatinine (CR), total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL), very low-density lipoprotein (VLDL), and high-density lipoprotein (HDL) concentrations were analyzed using spectrophotometer (Spectronic 1201, Milton Roy, Ivyland, PA, USA) using commercial kits (Spinreact Co., Girona, Spain) according to the manufacturer’s instructions. Serum concentrations of triiodothyronine (T3) and thyroxine (T4) were measured in all blood samples using radioimmunoassay (RIA) kits. Serum contents of malondialdehyde (MDA) and reduced glutathione (GSH), and catalase (CAT) activity were analyzed using commercial kits (Cell Biolabs Inc., San Diego, CA, USA).
Enzyme activity assay
At the end of the experiment, five quails were randomly collected from each experimental group and slaughtered by severing the jugular vein, and then duodenum samples were immediately collected after birds’ evisceration. A crude mixture of homogenous duodenum content was obtained by massaging the tract from both ends following the operating on ice method of Jin et al. (2000). Based on the sample weight, samples were diluted 10× with ice-cold phosphate-buffered saline (PBS, pH 7.0), subsequently homogenized using a hand-held glass homogenizer and centrifuged at 5000g for 20 min at 4 °C. The supernatants were separated and stored at 4 °C until analysis. All enzymatic assays were performed within 24 h after extraction. Amylase, lipase, and protease activities were determined using the methods of Coles (1986), Boutwell (1962), and Lowry et al. (1951), respectively.
Nutrients digestibility coefficients
For each experimental group, eight male quails were weighed before and after the collection period and housed in metabolic cages individually. During the collection period (4 days), all birds had free access to food and water and a 24-h period had elapsed, as adaptation period, before the commencement of the excreta collection period. The proximate analysis of diets and dried excreta was carried out according to AOAC (2003) for determination of dry matter (DM) (#930.15), organic matter (OM) (#942.05), crude protein (CP) (#954.01), crude fiber (CF) (#978.10), ether extract (EE) (#920.29), and ash (#923.03). Fecal nitrogen and urinary OM were estimated using trichloroacetic acid procedure (Jacobsen et al. 1960) and the equation of Abou-Raya and Galal (1971), respectively.
Statistical analysis
Data were analyzed by one-way analysis of variance using the general linear models (GLM) using SPSS software, Version 18.0. The experimental unit for growth and productive performance traits was a cage, whereas for the rest of the parameters it was the individuals’ data. Homogeneity of variance and normality of distribution were tested using Levene and Shapiro-Wilk tests, respectively. Tukey’s multiple range tests were performed to detect differences among dietary treatments at a significance level of P < 0.05.
Results
Growth performance
As presented in Table 2, live body weight showed a linear (P < 0.01) increase to B. subtilis supplementation at all studied ages. Moreover, daily body weight gain also observed a linear (P < 0.01) increase during the starter period (1–14 days of age) and the overall one (1–42 days of age). Daily feed intake was not affected by the feeding treatments for all periods considered. No mortality was recorded in all experimental groups throughout the trail period. Feed conversion ratio was linearly (P < 0.05) decreased with increasing dietary B. subtilis concentration during the starter and overall fattening periods. The highest levels of B. subtilis (BS7 and BS9 groups) recorded the best values of body weight, body weight gain, and feed conversion ratio.
Blood biochemical indices
Data presented in Table 3 indicate that dietary inclusion levels of B. subtilis significantly affect total serum protein, albumin, urea-N, and creatinine concentrations as well as liver enzymes activities. Increasing levels of B. subtilis led to a linear (P < 0.05) increase in serum concentrations of total protein and albumin, and a linear decrease in creatinine (P < 0.01), urea-N (P < 0.05 or P < 0.05 quadratically), ALT (P < 0.01), and AST (P < 0.01 or P < 0.01 quadratically) values. However, serum levels of globulin, uric acid, and ALP were not linearly or quadratically affected by dietary levels of B. subtilis.
Table 4 reports the concentrations of serum TC, TG, HDL, LDL, and VLDL as influenced by dietary inclusion of B. subtilis spores. Feeding treatments linearly reduced serum levels of TC and TG in groups BS7 and BS9, and BS9, respectively. Furthermore, serum concentration of VLDL was linearly decreased in groups BS5, BS7, and BS9 while LDL was insignificantly affected. HDL values were linearly elevated in experimental groups BS5, BS7, and BS9. The hypolipidemic impact of feeding B. subtilis spores was greatly observed and enhanced by increasing its inclusion level in quail diet.
Thyroid hormones activities
All dietary supplemental doses of B. subtilis were linearly and quadratically (P < 0.01) lowered serum glucose level compared with the unsupplemented group (Table 4), while T3 and T4 activities showed linear (P < 0.01) and quadratic (P < 0.05, for T4 only) elevation in treated groups compared with the control one. Groups BS7 and BS9 recorded the highest levels of T3 and T4 and the lowest level of glucose.
Antioxidative status and lipid peroxidation
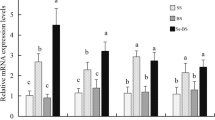
Serum MDA (as an index of lipid peroxidation in blood serum) and GSH levels and CAT activity as influenced by dietary levels of B. subtilis are depicted in Fig. 2. Antioxidative status of growing Japanese quails fed diets containing B. subtilis spores was greatly enhanced. All examined dietary levels of B. subtilis linearly and quadratically (P < 0.01) decreased serum content of MDA compared with the control group, while serum content of GSH and CAT activity were linearly (P < 0.01) increased in groups BS7, BS9, and BS5.
Effect of dietary supplementation of Bacillus subtilis on oxidative status (A) MDA, (B) GSH, and (C) CAT in the serum of Japanese quail birds. Treatment groups: BS0—corn-based diet, BS3—1 × 103B. subtilis spores/kg, BS5—1 × 105B. subtilis spores/kg, BS7—1 × 107B. subtilis spores/kg, and BS9—1 × 109B. subtilis spores/kg. Data presented as mean values with their standard errors. Values with different superscript letters are statistically different (P < 0.05)
Digestive enzyme activities
Data illustrated in Fig. 3 revealed a linear (P < 0.01) increase in the activities of protease, lipase, and amylase due to dietary supplementation of B. subtilis. The highest activity of the aforementioned enzymes was observed in BS9 group followed by BS7.
Effect of dietary supplementation of Bacillus subtilis on digestive enzyme activities (A) proteases, (B) lipase, and (C) amylase of Japanese quail birds. Treatment groups: BS0—corn-based diet, BS3—1 × 103B. subtilis spores/kg, BS5—1 × 105B. subtilis spores/kg, BS7—1 × 107B. subtilis spores/kg, and BS9—1 × 109B. subtilis spores/kg. Data presented as mean values with their standard errors. Values with different superscript letters are statistically different (P < 0.05)
Nutrients digestibility coefficients
As presented in Table 5, digestion coefficients of DM, OM, CP, and EE were linearly improved by the supplementation levels of B. subtilis spores. The abovementioned parameters were significantly increased in groups BS7 and BS9 and numerically increased in the rest of the treatment groups compared with the control group. However, digestibility coefficients of CF and NFE were insignificantly affected.
Discussion
Growth performance
Results of the present study showed that the use of B. subtilis spores supplementation at different levels in quail diets resulted in linear improvement in body weight gain and feed efficiency. Feeding quails with a B. subtilis-supplemented diet improved BW at all studied ages dependent on the spores’ concentration of the diet; the highest concentrations (BS7 and BS9) obtained better weight. The favorable effect of B. subtilis on DBWG and FCR was expressed throughout the brooding period and the overall one. B. subtilis can improve quail growth performance because of its ability to secret high-active protease, amylase, and lipase to decompose feed nutrients and provide more nutrients available to absorption (Li et al. 2014). Regarding feed efficiency, results of the present study proved that dietary probiotic decreased FCR significantly and had no effect on daily feed intake. Our previous results are consistent with the finding of Gao et al. (2017), Li et al. (2016), and Hossain et al. (2015). Therefore, it might be assumed that, in the present study, the dietary probiotic is involved in enhancing of protease, lipase, and amylase activities (Fig. 3) and increased serum levels of T3 and T4 (Table 4), which play very important role in growth rate, leading to enhancement the nutrients utilization to maintain optimum growth.
Blood biochemical indices
In the current study, dietary supplementation of B. subtilis did not alter serum levels of GLO, UA, and ALP and elevated TP and ALB values as well as decreased urea-N, CR, AST, and ALT values. In line with our results, Kasmani et al. (2012) and Yazhini et al. (2018) reported significant increase in serum proteins due to probiotic treatments. Furthermore, the findings of Kasmani et al. (2012), Hashemzadeh et al. (2013), and Ahmed et al. (2015) agreed with ours concerning the liver and renal function indicators. The increase in serum TP and ALB could be explained by the inhibition exclusion mechanism, where B. subtilis improves dietary protein utilization through its ability to inhibit pathogens growth, which reduces protein breakdown into nitrogen and diminishes dietary protein efficiency, and increases the surface area for nutrient absorption (Abd El-Moneim 2017, Abd El-Moneim et al. 2019, Saleh et al. 2017, Yazhini et al. 2018). In addition, liver protection role of probiotic was interpreted by Rishi et al. (2009) who postulated that probiotic supplementation reduces translocation of harmful bacteria in the liver, which decreases levels of serum transaminases.
Present results revealed that the hypolipidemic effect of feeding B. subtilis spores was greatly observed and enhanced by increasing its inclusion level in quail diet. These results are in line with earlier findings (Abd El-Moneim and Sabic 2019, Aluwong et al. 2013, Pourakbari et al. 2016, Yazhini et al. 2018). These negative impacts of probiotics on blood cholesterols and triglycerides may be due to their ability to incorporate cholesterol into their cellular membrane, produce hydrolyze bile salt enzymes (EC 3.5.1.24) for bile salt deconjugation in the enterohepatic circulation (Klaver and Van der Meer 1993), and the conversion of cholesterol by probiotics in the intestine into coprostanol, which is directly excreted with the feces (Ooi and Liong 2010) or inhibit the rate-limiting enzyme of cholesterogenesis, 3-hydroxyl-3-methylglutaryl-CoA reductase (Kalavathy et al. 2003, Pourakbari et al. 2016). Reduction in serum cholesterol level is mostly associated with reduced cholesterol content in poultry products. Probiotics administration in poultry diets could lead to the production of low-cholesterol eggs and meat. Feeding on probiotics strains enhanced internal egg quality criteria (Abdelqader et al. 2013, Fathi et al. 2018), reduced yolk cholesterol content (Abd El-Moneim and Sabic 2019), decreased TBARS content in broiler muscles, and elevated muscle contents of unsaturated fatty acids (Saleh et al. 2014b, 2011). Probiotics can therefore meet the growing global demand for low-saturated fat and low-cholesterol meat and eggs.
Thyroid hormones activities
Reducing blood glucose level in the present study may be related to the suppressive effect of probiotics on glucagon, which decrease blood glucose value (Abd El-Moneim 2017, Aluwong et al. 2013). Moreover, dietary inclusion of B. subtilis increased serum levels of T3 and T4. This elevation in thyroid hormones may be explained by the probiotic-enhanced activity of hypothalamus hormone (thyroid-stimulating hormone-releasing hormone; TSH-RH), consequently activating the release of thyroid-stimulating hormone (TSH) from the anterior pituitary (Aluwong et al. 2013). Also, probiotics might stimulate thyrotropin secretion and, hence, T4 secretion by enhancing the activity of corticotrophin-releasing factor (CRF) (Geris et al. 1999, Klieverik et al. 2009). Therefore, it seems more likely that dietary inclusion of B. subtilis may enhance the growth performance in Japanese quail, by taking into account our results in serum T3 and T4 concentration.
Antioxidative status and lipid peroxidation
Results of our study showed linear increase in GSH content and CAT activity and decrease in lipid peroxidation. These findings revealed that probiotic possesses the physiological role of enhancing antioxidant defense system of birds. This promotion impact might be attributed to the ability of probiotics to produce certain factors chelate free radicals, capture reactive oxygen species, and inhibit their cytotoxic activity (Lin and Yen 1999). Moreover, probiotics can promote antioxidant system of the bids via the augmentation of antioxidant enzymatic activities (e.g., superoxide dismutase (SOD) and glutathione peroxidase) as well as total antioxidant status of the host (Wang et al. 2017). The antioxidant enzymatic system of probiotics itself plays major role in promoting host’s antioxidative status. LeBlanc et al. (2011) found that Lactobacillus casei produced SOD which accelerates weight loss recovery, increases gut enzymatic activity, and reduces intestinal inflammation in mice. Zheng et al. (2016) cleared that Enterococcus faecium stimulates the antioxidant capacity of broiler chickens through its reaction with hydroxyl radicals which in turn promotes the oxidation resistance of biological macromolecules. Our results are in close agreement with findings by many investigators (Abd El-Moneim and Sabic 2019, Abd El-Moneim 2017, Abudabos et al. 2016).
Digestive enzyme activities
Gastrointestinal enzymes activities play a fundamental role in nutrient digestion and, eventually, reflected on poultry performance, health and nutrients retention. In the present study, duodenal amylolytic, proteolytic and lipolytic activities were higher in B. subtilis treated groups. These results are in correspondence with the finding of Jin et al. (2000) who reported that the inclusion of Lactobacillus elevated amylase and lipase activities. Moreover, Wang and Gu (2010) observed significant increase in protease and amylase activities in Arbor Acres broilers fed diets supplemented with Bacillus coagulans NJ0517. Contrarily, Zhang et al. (2016) and Zhi-gang et al. (2014) demonstrated that probiotics had no effect on protease activity. Rodjan et al. (2018) and Palamidi et al. (2016) noticed non-significant alternations in amylolytic, lipolytic, or proteolytic activities as influenced by probiotic administration. The present augmentation in digestive enzymes activities may be due to the contribution of exoenzymes secreted by probiotic bacteria along with the endogenous enzymes produced by the host (Bedford and Schulze 1998, Saleh et al. 2014a, Wang and Gu 2010). Higher activity of protease, amylase, and lipase improved the digestion of protein, starch, and lipids, which might consider an explanation for the growth enhancement observed in the present study.
Nutrients digestibility coefficients
Our results revealed significant increase in the digestion of DM, OM, CP, and EE as well as numerical increase in the digestion of CF and NFE. These results synchronize and harmonize with the increase in duodenal amylolytic, proteolytic, and lipolytic activities observed in the present study. Results of the present study are in agreement with previous reported findings noticed that B. subtilis can significantly increase digestibility coefficients of CP, EE, OM, and DM (Gao et al. 2017, Hossain et al. 2015, Li et al. 2014, Mountzouris et al. 2010).
Conclusion
It can be concluded that different levels of dietary B. subtilis spores supplementation could enhance antioxidative status and digestive enzyme activities. However, the high concentrations (BS7 and BS9) of B. subtilis spores could improve the nutrient digestibility and growth performance of growing Japanese quail birds.
References
Abd El-Moneim, A.E. and Sabic, E.M., 2019. Beneficial effect of feeding olive pulp and Aspergillus awamori on productive performance, egg quality, serum/yolk cholesterol and oxidative status in laying Japanese quails, Journal of Animal and Feed Sciences, 28, 52–61
Abd El-Moneim, E.A., 2017. Influence of in ovo injection with an effective bacterial preparation (Bifidobacterium spp.) on some productive and physiological traits in poultry, (Doctoral dissertation, Ain Shams University)
Abd El-Moneim, E.A., El-Wardany, I., Abu-Taleb, A.M., Wakwak, M.M., Ebeid, T.A. and Saleh, A.A., 2019. Assessment of in ovo administration of Bifidobacterium bifidum and Bifidobacterium longum on performance, ileal histomorphometry, blood hematological, and biochemical parameters of broilers, Probiotics and Antimicrobial Proteins, In Press
Abdelqader, A., Irshaid, R. and Al-Fataftah, A.-R., 2013. Effects of dietary probiotic inclusion on performance, eggshell quality, cecal microflora composition, and tibia traits of laying hens in the late phase of production, Tropical animal health and production, 45, 1017–1024
Abou-Raya, A. and Galal, A.G., 1971. Evaluation of poultry feeds in digestion trials with reference to some factors involved, J Anim Prod United Arab Repub, 207–221
Abudabos, A., Alyemni, A. and Zakaria, H., 2016. Effect of Two Strains of Probiotics on the Antioxidant Capacity, Oxidative Stress, and Immune Responses of Salmonella-Challenged Broilers, Revista Brasileira de Ciência Avícola, 18, 175–180
Ahmed, K., Hasan, M., Asaduzzaman, M., Khatun, A. and Islam, K., 2015. Effects of probiotics and synbiotics on growth performance and haemato-biochemical parameters in broiler chickens, Journal of Science, 5, 926–929
Aluwong, T., Hassan, F., Dzenda, T., Kawu, M. and Ayo, J., 2013. Effect of different levels of supplemental yeast on body weight, thyroid hormone metabolism and lipid profile of broiler chickens, Journal of Veterinary Medical Science, 75, 291–298
AOAC, 2003. Official methods of analysis of AOAC, (AOAC, Gaithersburg)
Bedford, M.A. and Schulze, H., 1998. Exogenous enzymes for pigs and poultry, Nutrition research reviews, 11, 91–114
Boutwell, J.H., 1962. Clinical Chemistry. Laboratory Manual and Methods, Journal of Medical Education, 37, 158
Coles, E., 1986. Veterinary clinical Pathology 4th ed WB Saunders company Philadelphia, London, Toronto, Mexico, Riodejenario, Sydney, Tokyo & Hong Kong, 136–170
Ebeid, T., Fathi, M., Al-Homidan, I., Ibrahim, Z. and Al-Sagan, A., 2019. Effect of dietary probiotics and stocking density on carcass traits, meat quality, microbial populations and ileal histomorphology in broilers under hot-climate conditions, Animal Production Science, In Press
Fathi, M., Al-Homidan, I., Al-Dokhail, A., Ebeid, T., Abou-Emera, O. and Alsagan, A., 2018. Effects of dietary probiotic (Bacillus subtilis) supplementation on productive performance, immune response and egg quality characteristics in laying hens under high ambient temperature, Italian Journal of Animal Science, 17, 804–814
Fathi, M., Ebeid, T., Al-Homidan, I., Soliman, N. and Abou-Emera, O., 2017. Influence of probiotic supplementation on immune response in broilers raised under hot climate, British poultry science, 58, 512–516
Gao, Z., Wu, H., Shi, L., Zhang, X., Sheng, R., Yin, F. and Gooneratne, R., 2017. Study of Bacillus subtilis on growth performance, nutrition metabolism and intestinal microflora of 1 to 42 d broiler chickens, Animal Nutrition, 3, 109–113
Geris, K., Laheye, A., Berghman, L., Kühn, E. and Darras, V., 1999. Adrenal inhibition of corticotropin-releasing hormone-induced thyrotropin release: A comparative study in pre-and posthatch chicks, Journal of Experimental Zoology, 284, 776–782
Hashemzadeh, F., Rahimi, S., Torshizi, M. and Masoudi, A.A., 2013. Effects of probiotics and antibiotic supplementation on serum biochemistry and intestinal microflora in broiler chicks, Int J Agri Crop Sci, 5, 2394
Hooge, D., 2003. Bacillus spores may enhance broiler performance. feedstuffs, 2003, (Miller Pub. Co., Minneapolis, Minnesota, USA), 28-32
Hossain, M., Begum, M. and Kim, I., 2015. Effect of Bacillus subtilis, Clostridium butyricum and Lactobacillus acidophilus endospores on growth performance, nutrient digestibility, meat quality, relative organ weight, microbial shedding and excreta noxious gas emission in broilers, Veterinarni Medicina, 60, 77–86
Huang, J.-M., La Ragione, R.M., Nunez, A. and Cutting, S.M., 2008. Immunostimulatory activity of Bacillus spores, FEMS Immunology & Medical Microbiology, 53, 195–203
Huang, M., Choi, Y., Houde, R., Lee, J.-W., Lee, B. and Zhao, X., 2004. Effects of Lactobacilli and an acidophilic fungus on the production performance and immune responses in broiler chickens, Poultry Science, 83, 788–795
Jacobsen, D., Gertovey, S. and Nielson, H., 1960. Digestibility trials with poultry. 322 Bertning fra forsg slabooratoriel udgbet of statens, (Kobengaven)
Jin, L., Ho, Y., Abdullah, N. and Jalaludin, S., 2000. Digestive and bacterial enzyme activities in broilers fed diets supplemented with Lactobacillus cultures, Poultry Science, 79, 886–891
Kalavathy, R., Abdullah, N., Jalaludin, S. and Ho, Y., 2003. Effects of Lactobacillus cultures on growth performance, abdominal fat deposition, serum lipids and weight of organs of broiler chickens, British poultry science, 44, 139–144
Kasmani, B., Karimi Torshizi, M., Allameh, A. and Shariatmadari, F., 2012. A novel aflatoxin-binding Bacillus probiotic: Performance, serum biochemistry, and immunological parameters in Japanese quail, Poultry Science, 91, 1846–1853
Klaver, F. and Van der Meer, R., 1993. The assumed assimilation of cholesterol by Lactobacilli and Bifidobacterium bifidum is due to their bile salt-deconjugating activity, Applied and Environmental Microbiology, 59, 1120–1124
Klieverik, L.P. et al., 2009. Thyroid hormone modulates glucose production via a sympathetic pathway from the hypothalamic paraventricular nucleus to the liver, Proceedings of the National Academy of Sciences, 106, 5966–5971
La Ragione, R.M. and Woodward, M.J., 2003. Competitive exclusion by Bacillus subtilis spores of Salmonella enterica serotype Enteritidis and Clostridium perfringens in young chickens, Veterinary microbiology, 94, 245–256
LeBlanc, J.G. et al., 2011. Use of superoxide dismutase and catalase producing lactic acid bacteria in TNBS induced Crohns disease in mice, Journal of Biotechnology, 151, 287–293
Leser, T., Knarreborg, A. and Worm, J., 2008. Germination and outgrowth of Bacillus subtilis and Bacillus licheniformis spores in the gastrointestinal tract of pigs, Journal of applied microbiology, 104, 1025–1033
Li, W., Bai, J., Li, Y., Qin, Y. and Yu, D., 2014. Effects of Bacillus subtilis on meat quality, nutrient digestibility and scrum biochemical index of broilers, Chin. J Vet Sci, 34, 1682–1685
Li, Y., Xu, Q., Huang, Z., Lv, L., Liu, X., Yin, C., Yan, H. and Yuan, J., 2016. Effect of Bacillus subtilis CGMCC 1.1086 on the growth performance and intestinal microbiota of broilers, Journal of applied microbiology, 120, 195–204
Lin, M.-Y. and Yen, C.-L., 1999. Antioxidative ability of lactic acid bacteria, Journal of agricultural and food chemistry, 47, 1460–1466
Lowry, O.H., Rosebrough, N.J., Farr, A.L. and Randall, R.J., 1951. Protein measurement with the Folin phenol reagent, Journal of biological chemistry, 193, 265–275
Mountzouris, K., Tsitrsikos, P., Palamidi, I., Arvaniti, A., Mohnl, M., Schatzmayr, G. and Fegeros, K., 2010. Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition, Poultry Science, 89, 58–67
NRC, 1994. Nutrition Requirements of Poultry, (National Academy Press, Washington)
Ooi, L.-G. and Liong, M.-T., 2010. Cholesterol-lowering effects of probiotics and prebiotics: a review of in vivo and in vitro findings, International journal of molecular sciences, 11, 2499–2522
Palamidi, I., Fegeros, K., Mohnl, M., Abdelrahman, W., Schatzmayr, G., Theodoropoulos, G. and Mountzouris, K., 2016. Probiotic form effects on growth performance, digestive function, and immune related biomarkers in broilers, Poultry Science, 95, 1598–1608
Pourakbari, M., Seidavi, A., Asadpour, L. and Martínez, A., 2016. Probiotic level effects on growth performance, carcass traits, blood parameters, cecal microbiota, and immune response of broilers, Anais da Academia Brasileira de Ciências, 88, 1011–1021
Rishi, P., Mavi, S.K., Bharrhan, S., Shukla, G. and Tewari, R., 2009. Protective efficacy of probiotic alone or in conjunction with a prebiotic in Salmonella-induced liver damage, FEMS microbiology ecology, 69, 222–230
Rodjan, P., Soisuwan, K., Thongprajukaew, K., Theapparat, Y., Khongthong, S., Jeenkeawpieam, J. and Salaeharae, T., 2018. Effect of organic acids or probiotics alone or in combination on growth performance, nutrient digestibility, enzyme activities, intestinal morphology and gut microflora in broiler chickens, Journal of animal physiology and animal nutrition, 102, e931-e940
Saleh, A., Hayashi, K., Ijiri, D. and Ohtsuka, A., 2014a. Beneficial effects of Aspergillus awamori in broiler nutrition, World’s Poultry Science Journal, 70, 857–864
Saleh, A.A., 2014. Effect of dietary mixture of Aspergillus probiotic and selenium nano-particles on growth, nutrient digestibilities, selected blood parameters and muscle fatty acid profile in broiler chickens. Animal Science Paper and Reports, 32, 65–79
Saleh, A.A., Amber, K., El-Magd, M.A., Atta, M.S., Mohammed, A.A., Ragab, M.M. and Abd El-Kader, H., 2014b. Integrative effects of feeding Aspergillus awamori and fructooligosaccharide on growth performance and digestibility in broilers: promotion muscle protein metabolism, BioMed research international, 2014, 1–8
Saleh, A.A., Eid, Y.Z., Ebeid, T.A., Kamizono, T., Ohtsuka, A. and Hayashi, K., 2011. Effects of feeding Aspergillus awamori and Aspergillus niger on growth performance and meat quality in broiler chickens, The Journal of Poultry Science, 48, 201–206
Saleh, A.A. et al., 2017. Synergistic effect of feeding Aspergillus awamori and lactic acid bacteria on performance, egg traits, egg yolk cholesterol and fatty acid profile in laying hens, Italian Journal of Animal Science, 16, 132–139
Wang, X., Yi, Z. and Ji, C., 2006. Effects of fructo-oligosaccharide and Bacillus subtilis on intestinal microflora, fecal emission of ammonia and sulfureted hydrogen and nutrient availability in broilers, Acta Veterinaria et Zootechnica Sinica, 37, 337–341
Wang, Y. and Gu, Q., 2010. Effect of probiotic on growth performance and digestive enzyme activity of Arbor Acres broilers, Research in Veterinary Science, 89, 163–167
Wang, Y., Wu, Y., Wang, Y., Xu, H., Mei, X., Yu, D., Wang, Y. and Li, W., 2017. Antioxidant properties of probiotic bacteria, Nutrients, 9, 521
Yazhini, P., Visha, P., Selvaraj, P., Vasanthakumar, P. and Chandran, V., 2018. Dietary encapsulated probiotic effect on broiler serum biochemical parameters, Veterinary world, 11, 1344
Yurong, Y., Ruiping, S., ShiMin, Z. and Yibao, J., 2005. Effect of probiotics on intestinal mucosal immunity and ultrastructure of cecal tonsils of chickens, Archives of animal nutrition, 59, 237–246
Zhang, L., Zhang, L., Zeng, X., Zhou, L., Cao, G. and Yang, C., 2016. Effects of dietary supplementation of probiotic, Clostridium butyricum, on growth performance, immune response, intestinal barrier function, and digestive enzyme activity in broiler chickens challenged with Escherichia coli K88, Journal of animal science and biotechnology, 7, 3
Zheng, A. et al., 2016. Probiotic (Enterococcus faecium) induced responses of the hepatic proteome improves metabolic efficiency of broiler chickens (Gallus gallus), BMC genomics, 17, 89
Zhi-gang, T., Naeem, M., Chao, W., Tian, W. and Yan-min, Z., 2014. Effect of dietary probiotics supplementation with different nutrient density on growth performance, nutrient retention and digestive enzyme activities in broilers, The Journal of Animal & Plant Sciences, 24, 1309–1315
Acknowledgments
The authors acknowledge the Biological Application Department, Nuclear Research Center, Egyptian Atomic Energy Authority, Poultry and Fish Production Dept., Faculty of Agriculture, Menoufia University, Egypt, and Department of Poultry Production, Faculty of Agriculture, Kafrelsheikh University, Kafr El-Sheikh, Egypt, for their cooperation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All the procedures used in this trial were approved by Local Experimental Animals Care Committee, and approved by the Institutional Ethics Committee.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdel-Moneim, AM.E., Selim, D.A., Basuony, H.A. et al. Effect of dietary supplementation of Bacillus subtilis spores on growth performance, oxidative status, and digestive enzyme activities in Japanese quail birds. Trop Anim Health Prod 52, 671–680 (2020). https://doi.org/10.1007/s11250-019-02055-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-019-02055-1