Abstract
This study aimed to investigate the effects of selenium (Se)-enriched Bacillus subtilis (Se-BS) on growth performance, antioxidant capacity, immune status, and gut health in broilers. A total of 240 one-day-old Arbor Acres broilers were randomly allotted to four groups and fed with basal diet (control group), 0.30 mg/kg Se (SS group), 3 × 109 CFU/g B. subtilis (BS group), and 0.30 mg/kg Se + 3 × 109 CFU/g B. subtilis (Se-BS group) for 42 days. The results showed that Se-BS supplementation increased body weight (BW), average daily gain, the activities of superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), and peroxidase (POD), total antioxidant capacity (T-AOC), and the contents of interleukin (IL)-2, IL-4, and immunoglobulin (Ig) G in plasma, the index and wall thickness of the duodenum, the villus height and crypt depth of the jejunum, and GPx-1 and thioredoxin reductase 1 mRNA levels in liver and intestine and decreased feed conversion ratio (FCR) and plasma malondialdehyde (MDA) content compared with the control group on day 42 (P < 0.05). Compared with groups SS and BS, Se-BS supplementation increased BW, the activities of GPx, CAT, and POD, and the contents of IL-2, IL-4, and IgG in plasma, the index and wall thickness of the duodenum, the crypt depth and secretory IgA content of the jejunum, and GPx-1 mRNA levels in liver and intestine and decreased FCR and plasma MDA content on day 42 (P < 0.05). In conclusion, Se-BS supplementation effectively improved the growth performance antioxidant capacity, immune status, and gut health of broilers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is an essential trace element for humans and animals that has a wide range of biological effects, including improving antioxidant capacity, immune response, and reproductive performance, regulating body metabolism, and anti-tumor and anti-toxic elements [1, 2]. China is one of the regions with the most serious Se deficiency; there are 22 provinces in low Se regions, accounting for 72% of the total area, and the Se-deficient population exceeds 700 million [3, 4]. Se deficiency can reduce the activity of glutathione peroxidase (GPx), damage the biofilm of various tissues and organs, cause apoptosis, degeneration, or necrosis [5, 6], and lead to Keshan disease, Kashin-Beck disease, cancer, and cardiovascular disease in humans [7, 8] and muscle degeneration, pancreas hypoplasia and degeneration, long-term digestive disorders, severe diarrhea, and even death in animals [6]. Therefore, Se supplementation in diets and foods is one of the commonly used measures, and the most commonly used Se preparation today is inorganic Se, sodium selenite (SS); however, inorganic Se has the disadvantages of high toxicity, low absorptivity, low bioavailability, and high environmental pollution [4, 9]. Therefore, it is urgent to develop high quality organic Se sources.
In previous studies, Bacillus subtilis has proved to be beneficial to the host and have the functions of improving growth performance and the intestinal microecological environment, promoting the digestion and metabolism of nutrients, and enhancing the body immunity of animals [10, 11]. Moreover, B. subtilis itself contains a rich biological enzyme transformation system, which can convert inorganic Se into highly active organic Se, Se-enriched B. subtilis (Se-BS) [12, 13]. Se-BS plays the dual role of organic Se and probiotics, which is an ideal Se supplement. Previous studies reported that dietary Se-enriched B. subtilis (Se-BS) supplementation could modify ileal bacterial composition [14, 15], improve Hg-induced intestinal microbial changes, alleviate the abundance of Aeromonas and the inflammation of the fish, and protect common carp against Hg toxicity [16]. Otherwise, the application of Se-BS in animal production is not extensive enough; the majority of scientific researchers need to conduct in-depth investigations on its biological effects, such as its effectiveness and availability. Therefore, this purpose of this study was to evaluate the effects of Se-BS on the growth performance, antioxidant capacity, immunity, and gut health of broiler chickens in order to provide some reference materials for the future application of Se-BS.
Materials and Methods
Preparation of Se-BS
Se-BS was provided by the Laboratory of Animal Nutrition and Poisoning Diseases, Qingdao Agricultural University. The number of effective viable bacteria is ≥ 1 × 1010 /g, and the total Se content is ≥ 1.0 mg/kg, of which organic Se accounts for at least 90%. Se-BS was kept in a sterile container at 4 °C before use.
Experimental Animals and Treatment
A total of 240 1-day-old Arbor Acres broilers (half male and half female), weighing 43–46 g, were purchased from Qingdao Aote Poultry Breeding Company (Qingdao, China) and randomly divided into 4 groups with six replicate pens of ten chickens each. Group 1 was the control (CON) group, which was fed a basal diet without Se; group 2 was the sodium selenite (SS) group, which was fed the basal diet supplemented with 0.30 mg/kg Se in the form of SS; group 3 was the Bacillus subtilis (BS) group, which was fed the basal diet supplemented with B. subtilis (3 × 109 CFU/g); and group 4 was the Se-BS group, which was fed the basal diet supplemented with 0.30 mg/kg Se and B. subtilis (3 × 109 CFU/g) in the form of Se-BS. The basal diet was formulated to meet the nutrient requirements for broilers [17]. The basal diet formulation and approximate composition are shown in Table 1. Feed ingredients were purchased from a local commercial feed factory (Puxing Biological Technology Co., Ltd., Qingdao, China). During the experiment period of 42 days, all birds were housed in a closed and ventilated building and provided with continuous light. Room temperature was controlled at 32–35 °C for 3 days then gradually reduced by 3 °C/week until reaching 24 °C and maintained for the remainder of the experiment. Water and feed were provided ad libitum.
All animal experiments were approved by the Animal Care and Use Committee of Qingdao Agricultural University in accordance with the Laboratory Animal Guidelines on Ethical Review for Animal Welfare (GB/T35892-2018, National Standards of the People’s Republic of China) and conducted in accordance with the principles and specific guidelines presented in the Guide for the Care and Use of Agricultural Animals in Research and Teaching [18].
Growth Performance
On day 42, all chickens were fasted for 12 h. The remaining feed amount and total body weight of the chickens were weighed in units of replicate pens. The body weight (BW), average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR) were calculated.
Plasma Determination
On days 21 and 42, three chickens were randomly selected from each pen, and the anticoagulated blood was collected through the fin vein and centrifuged for 10 min. Plasma was collected and stored at -20 °C. GPx activity, superoxide dismutase (SOD) activity, peroxidase (POD) activity, catalase (CAT) activity, total antioxidant capacity (T-AOC), and malondialdehyde (MDA) content in the plasma were analyzed according to the instructions of the reagent kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The contents of immunoglobulin (Ig) A, IgM, IgG, interleukin (IL)-2, IL-4, IL-6, interferon γ (IFN-γ), and tumor necrosis factor α (TNF-α) in the plasma were analyzed according to the instructions of the reagent kits (Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China).
Analysis of Gut Health
On day 42, three chickens were randomly selected from each pen, with a total of 18 chickens in each group. After being euthanized with sodium pentobarbitone and depilated, the duodenum was separated and weighed, and the organ index was calculated. Intestinal wall thickness of the duodenum was measured with an electronic digital vernier caliper (Mitutoyo, Japan). At 5 cm of the jejunum segment before the yolk pedicle, the intestinal segment was longitudinally dissected with surgical scissors, and the intestinal mucosa was scraped into a cryopreservation tube and stored at -80 °C for determination of secretory immunoglobulin A (sIgA). The sIgA content was detected according to the instructions of the enzyme linked immunosorbent assay detection kit (Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China). The cecum contents were collected and stored in cryopreservation tubes at 4 °C for determination of gut microbiota. The cecum contents were mixed with the same quantity of normal saline and allowed to stand for 1 min. About 100 μL of the diluted liquid was taken, diluted to ten million times, then 100 μL of the dilution was taken and spread on the nutrient agar, triple sugar iron agar medium, lactic acid bacteria agar medium, and yeast peptone dextrose agar medium. The bacteria colony was observed and counted when these petri dishes were cultured in an anaerobic incubator for 18–24 h. The jejunum segments were collected and stored in formaldehyde solution. The jejunum segments were embedded, dehydrated, sliced, and stained with hematoxylin and eosin, and the intestinal villi length and crypt depth were determined under a light microscope (Optec Instrument Co., Ltd, Chongqing, China) at a magnification of × 100.
Determination of the mRNA Levels of Antioxidant Enzyme Genes
On day 42, the jejunum and liver tissues (not less than 1.0 g per sample) were collected and stored in liquid nitrogen for determination of the mRNA levels of antioxidant enzyme genes. Selenoproteins, including GPx-1 and thioredoxin reductase 1 (TR-1), mRNA levels in jejunum and liver tissues were determined using a quantitative real-time reverse transcription polymerase chain reaction (RT-qPCR) based upon SYBR Green I [2,3,4]. Briefly, total RNA was extracted using the TRIzol reagent (Sangon Biotech Co., Ltd., Shanghai, China), and cDNA was synthesized in accordance with the instructions of the AMV First Strand cDNA Synthesis Kit (Sangon Biotech Co., Ltd., Shanghai, China). The specific primers for gene expression were designed based on Gallus gallus sequences (Table 2). The β-actin housekeeping gene was used as an internal control, and RT-qPCR was performed with an ABI Real-time PCR System (StepOnePlus™; Applied Biosystems, Waltham, MA, USA). The mRNA gene expression levels were calculated using the 2−△△Ct method.
Statistical Analysis
IBM SPSS Statistic 22.0 (IBM Corporation, Armonk, USA) was used for statistical analysis. All data were expressed as mean ± standard deviation and analyzed using one-way ANOVA to compare means and the multivariate analysis of the GLM procedure. The least significant difference (LSD) and Dunnett’s T3 tests were used to determine differences between means. The pen was defined as the experimental unit for statistical analysis. Differences were considered as significant at P < 0.05 for all tests.
Results
Growth Performance
The effects of Se-BS on the growth performance of broilers are shown in Table 3. Compared with the control group, SS and B. subtilis supplementation significantly increased BW (P < 0.05), and Se-BS supplementation significantly increased BW and ADG and decreased FCR (P < 0.05). Compared with the SS and BS groups, Se-BS supplementation significantly increased BW (P < 0.05). No differences were observed among the four groups in the ADFI (P > 0.05).
Antioxidant Function
The effects of Se-BS on the antioxidant function of broilers are shown in Table 4 and Fig. 1. Compared with the control group, SS supplementation significantly increased CAT activity on day 21 and the activities of SOD, POD, and CAT and the liver GPx-1 and TR-1 mRNA levels on day 42, and decreased MDA content on day 42 (P < 0.05). Se-BS supplementation significantly increased the activities of SOD, GPx, and CAT on day 21 and the activities of T-SOD, POD, and CAT and the liver GPx-1 and TR-1 mRNA levels on day 42, and decreased MDA content on day 42 (P < 0.05). Compared with the BS group, SS supplementation significantly increased CAT activity on day 21 and the activities of SOD, POD, and CAT and the liver GPx-1 and TR-1 mRNA levels on day 42, and decreased MDA content on day 42 (P < 0.05). Se-BS supplementation significantly increased T-AOC and the activities of SOD, GPx, and CAT on day 21 and the activities of SOD, GPx, POD, and CAT and the liver GPx-1 and TR-1 mRNA levels on day 42, and decreased MDA content on day 42 (P < 0.05). Compared with the SS group, Se-BS supplementation significantly increased GPx activity on day 21 and the activities of GPx, POD, and CAT and the liver GPx-1 mRNA levels on day 42, and decreased MDA content on day 42 (P < 0.05).
Effect of Se-enriched B. subtilis on the relative mRNA levels of GPx-1 and TR-1 in the liver and intestine. Data are presented as means ± SD (standard deviation) (n = 6). Different superscript letters denote significant differences (P < 0.05). CON control group, SS group supplemented with sodium selenite, BS group supplemented with B. subtilis, Se-BS group supplemented with Se—enriched B. subtilis. GPx-1 glutathione peroxidase 1, TR-1 thioredoxin reductase 1
Immune Status
The effects of Se-BS on the immune status of broilers are shown in Table 5. Compared with the control group, Se-BS supplementation significantly increased the contents of IL-2 and IL-4 on day 21 and the content of IL-2, IL-4, and IgG on day 42 (P < 0.05). No differences were observed among the four groups in the contents of TNF-α, IFN-γ, IL-6, IgM, IgG, and IgA on day 21 and TNF-α, IFN-γ, IL-6, IgM, and IgA on day 42 (P > 0.05).
Gut Health
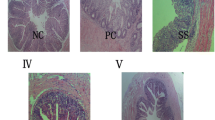
The effects of Se-BS on the gut health of broilers are shown in Tables 6 and 7 and Fig. 1. Compared with the control group, SS supplementation significantly increased the organ index and bowel wall thickness of the duodenum and the villus height and the GPx-1 and TR-1 mRNA levels of the jejunum (P < 0.05). BS and Se-BS supplementation significantly increased the organ index and wall thickness of duodenum and the villus height, crypt depth, and sIgA content of the jejunum and decreased the Enterobacteriaceae content in the cecal contents (P < 0.05). Se-BS supplementation also significantly increased the GPx-1 mRNA levels of the jejunum (P < 0.05). Compared with the SS group, BS supplementation significantly increased the organ index and wall thickness of duodenum and the villus height and the sIgA content of the jejunum (P < 0.05), and Se-BS supplementation significantly increased the organ index and wall thickness of the duodenum and the villus height, crypt depth, sIgA content, and GPx-1 mRNA level of the jejunum (P < 0.05). Compared with the BS group, Se-BS supplementation significantly increased the organ index and wall thickness of the duodenum and the crypt depth, sIgA content, and GPx-1 mRNA level of the jejunum (P < 0.05).
Discussion
This purpose of this study was to evaluate the roles of Se-BS supplementation in improving the growth performance, antioxidant capacity, immunity, and gut health of broiler chickens and found that: (1) Se-BS supplementation promoted the growth performance of broilers, which showed an increase in BW and ADG and a decrease in FCR; (2) Se-BS supplementation improved the antioxidant capacity of broilers, which showed an increase in SOD and CAT activities and the GPx-1 mRNA level and a decrease in MDA content; (3) Se-BS supplementation enhanced the immune status of broilers, which showed an increase in the levels of IL-2, IL-4, and IgG; and (4) Se-BS supplementation promoted gut health, which showed an increase in the organ index and wall thickness of the duodenum and the villus height, crypt depth, and sIgA content of the jejunum and a decrease in Enterobacteriaceae content in the cecum.
Growth performance characteristics are a series of the important preferred indicators used for evaluating the economic benefits of additives, including Se, probiotics, and their complexes. Most previous studies had demonstrated that Se, probiotics, and their complexes could improve growth performance and organ development [10, 14, 15]. Similarly, the present study found that BS, SS, and Se-BS increased BW and ADG and decreased FCR, which indicated that the economic benefits were improved. Moreover, Se-BS treatment showed better growth-promoting effects than BS and SS, which suggested that Se-BS treatment was more efficient than B. subtilis or Se alone in regulating the growth performance of broilers.
In general, MDA content, T-AOC, and the activities and gene expression of some antioxidant enzymes could reflect the antioxidant status [19, 20]. The accumulated evidence indicated the antioxidant effects of BS and Se [3, 4, 13]. In the present study, SS supplementation improved the antioxidant capacity of broilers by increasing SOD and CAT activities and reducing MDA content; however, BS supplementation did not cause significant changes in the antioxidant function of chickens. Moreover, we found that Se-BS supplementation showed better antioxidant effects than BS or Se supplementation alone and even enhanced the GPx-1 mRNA levels. These results suggested that Se in the Se-BS complex played a more important role of enhancing the antioxidant function and became more efficient after Se bioculture enrichment. It is noteworthy that the antioxidant properties are one of the most important functions of Se compounds, especially the regulating effects on the antioxidant enzymes [3, 4, 15]. Nonetheless, the research on the antioxidant effects of Se-BS is limited, and the mechanism of antioxidant regulation needs to be further investigated.
As an essential nutritional factor for the immune response, Se is an important life-related element directly involved in immune function and has a promoting effect on humoral immunity and cellular immunity [11, 14]. The levels of immunoglobulins and cytokines can reflect the status of the body's immunity and are also the important indicators for evaluating the quality of additives. IgA, IgG, and IgM, produced by B cells, are important parameters reflecting the humoral immune status. TNF-α, IFN-γ, IL-2, IL-4, and IL-6 are pro-inflammatory cytokines produced by activated monocytes, macrophages, T cells, and natural killer cells, which play important roles in enhancing immune function and resistance [19, 20]. Some previous studies have demonstrated that the addition of Se and B. subtilis sources to the feed can increase serum immunoglobulin and cytokine levels in chickens [21, 22]. In this study, SS and BS did not significantly affect the levels of immunoglobulins and cytokines, while Se-BS significantly increased the levels of IL-2, IL-4, and IgG. Similarly, Bakhshalinejad et al. found that total anti-sheep red blood cells (SRBC), IgG titers, and hypersensitivity were enhanced by using organic sources of Se rather than SS [21]. Dalia et al. reported that Se sources and the application time had a greater impact on immune function: all Se sources increased IgM levels, SS and Se-enriched Stenotrophomonas maltophilia increased IgG levels, and Se-enriched Enterobacter cloacae increased IgA levels in the initial phase. All Se sources increased IgG, IgA, and IgM levels, and no significant differences existed within the Se sources in the finisher phase [22]. Therefore, some factors, such as Se source, application time, and experimental animal, may affect the application results of Se sources on the immune function. We suggest that the mechanism of Se-BS enhancing immune function needs further study.
The gut is now considered to be the center of nutrient production and the first line of defense for health, as it contains 78% microorganisms and 60%–70% immune cells. Microbial composition, histomorphology, and sIgA content are the most important indicators for evaluating gut health [22, 23]. Previous studies have evaluated the positive effects of Se and probiotics on the gut health of animals [12, 18], and Se-enriched probiotics exhibited better gut health than Se and probiotics alone [23, 24]. Similarly, the present study indicated that SS, BS, and Se-BS improved gut health, while BS and Se-BS showed better gut health than SS, and Se-BS showed a stronger promoting effect on gut morphology and immunity than SS and BS. These results suggested that BS and Se-BS played a better role in promoting gut health, which may be related to the anaerobic properties of bacteria and its multiple secretory products [14, 25], and the probiotic effects of B. subtilis on different animals are highly strain-specific due to the different characteristics of the gastrointestinal environment [26]. In addition, B. subtilis is able to incorporate Se from the growth medium to their cells, which may enhance their growth and activity. On the other hand, Se as an antioxidant may modulate the diversity of gut microbiota via oxidative stress suppression and provide a better medium for the growth of beneficial bacteria.
Conclusion
In summary, dietary Se-BS supplementation improved the growth performance, antioxidant ability, immune status, gut health, and gene expression of selenoproteins in broilers and can be considered as a more effective alternative source of Se in broiler chickens.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Bakhshalinejad R, Hassanabadi A, Swick RA (2019) Dietary sources and levels of selenium supplements affect growth performance, carcass yield, meat quality and tissue selenium deposition in broilers. Anim Nutr 5:256–263
Chen F, Hou L, Zhu L, Yang C, Zhu F, Qiu H, Qin S (2020) Effects of selenide chitosan sulfate on glutathione system in hepatocytes and specific pathogen-free chickens. Poult Sci 99:3979–3986
Hou L, Qiu H, Zhu L, Gao S, Chen F (2022) Selenide chitosan sulfate improved the hepatocyte activity, growth performance, and anti-oxidation capacity by activating the thioredoxin reductase of chickens in vitro and in vivo. Biol Trace Elem Res 200:3798–3807
Hou L, Qiu H, Sun P, Zhu L, Chen F, Qin S (2020) Selenium-enriched Saccharomyces cerevisiae improves the meat quality of broiler chickens via activation of the glutathione and thioredoxin systems. Poult Sci 99:6045–6054
Ye R, Huang J, Wang Z, Chen Y, Dong Y (2021) Trace element selenium effectively alleviates intestinal diseases. Int J Mol Sci 22:11708. https://doi.org/10.3390/ijms222111708
Huang J, Xie L, Song A, Zhang C (2022) Selenium status and its antioxidant role in metabolic diseases. Oxid Med Cell Longev 2022:7009863. https://doi.org/10.1155/2022/7009863
Barchielli G, Capperucci A, Tanini D (2022) The role of selenium in pathologies: an updated review. Antioxidants (Basel) 11:251. https://doi.org/10.3390/antiox11020251
Tinggi U (2008) Selenium: its role as antioxidant in human health. Environ Health Prev Med 13:102–108
Khan MT, Mahmud A, Zahoor I, Javed K (2017) Organic and inorganic selenium in Aseel chicken diets: Effect on hatching traits. Poult Sci 96:1466–1472
Oladokun S, Koehler A, MacIsaac J, Ibeagha-Awemu EM, Adewole DI (2021) Bacillus subtilis delivery route: effect on growth performance, intestinal morphology, cecal short-chain fatty acid concentration, and cecal microbiota in broiler chickens. Poult Sci 100:100809. https://doi.org/10.1016/j.psj.2020.10.063
Guo M, Li M, Zhang C, Zhang X, Wu Y (2020) Dietary administration of the bacillus subtilis enhances immune responses and disease resistance in chickens. Front Microbiol 11:1768. https://doi.org/10.3389/fmicb.2020.01768
Qiu K, Li CL, Wang J, Qi GH, Gao J, Zhang HJ, Wu SG (2021) Effects of dietary supplementation with bacillus subtilis, as an alternative to antibiotics, on growth performance, serum immunity, and intestinal health in broiler chickens. Front Nutr 8:786878. https://doi.org/10.3389/fnut.2021.786878
Yang J, Huang K, Wang J, Wu D, Liu Z, Yu P, Wei Z, Chen F (2021) Combined use of Bacillus subtilis yb-114,246 and Bacillus licheniformis yb-214,245 improves body growth performance of Chinese Huainan partridge shank chickens by enhancing intestinal digestive profiles. Probiotics Antimicrob Proteins 13:327–342
Yang J, Wang J, Huang K, Liu Q, GuofangLiu XuX, Zhang H, Zhu M (2021) Selenium-enriched Bacillus subtilis yb-114246 improved growth and immunity of broiler chickens through modified ileal bacterial composition. Sci Rep 11:21690. https://doi.org/10.1038/s41598-021-00699-4
Yang J, Zhang M, Zhou Y (2019) Effects of selenium-enriched Bacillus sp. compounds on growth performance, antioxidant status, and lipid parameters breast meat quality of Chinese Huainan partridge chicks in winter cold stress. Lipids Health Dis 18:63. https://doi.org/10.1186/s12944-019-1015-6
Hu Y, Dun Y, Li S, Zhao S, Peng N, Liang Y (2014) Effects of Bacillus subtilis KN-42 on growth performance, diarrhea and faecal bacterial flora of weaned piglets. Asian-Australas J Anim Sci 27:1131–1140
NRC (1994) Nutrient requirements of poultry, 9th rev. National Research Council, National Academy Press, Washington, DC
National Academies of Sciences, Engineering, and Medicine (2020) Guide for the care and use of laboratory animals. The National Academies Press, Washington, DC. https://doi.org/10.17226/5140
Bakhshalinejad R, Akbari MoghaddamKakhki R, Zoidis E (2018) Effects of different dietary sources and levels of selenium supplements on growth performance, antioxidant status and immune parameters in Ross 308 broiler chickens. Br Poult Sci 59:81–91
Lauridsen C (2019) From oxidative stress to inflammation: redox balance and immune system. Poult Sci 98:4240–4246
Wang Y, Wang H, Zhan X (2016) Effects of different dl-selenomethionine and sodium selenite levels on growth performance, immune functions and serum thyroid hormones concentrations in broilers. J Anim Physiol Anim Nutr (Berl) 100:431–439
Dalia AM, Loh TC, Sazili AQ, Jahromi MF, Samsudin AA (2017) The effect of dietary bacterial organic selenium on growth performance, antioxidant capacity, and Selenoproteins gene expression in broiler chickens. BMC Vet Res 13:254. https://doi.org/10.1186/s12917-018-1578-x
Ren Z, Zhao Z, Wang Y, Huang K (2011) Preparation of selenium/zinc-enriched probiotics and their effect on blood selenium and zinc concentrations, antioxidant capacities, and intestinal microflora in canine. Biol Trace Elem Res 141:170–183
Lv CH, Wang T, Regmi N, Chen X, Huang K, Liao SF (2015) Effects of dietary supplementation of selenium-enriched probiotics on production performance and intestinal microbiota of weanling piglets raised under high ambient temperature. J Anim Physiol Anim Nutr (Berl) 99:1161–1171
Keerqin C, Rhayat L, Zhang ZH, Gharib-Naseri K, Kheravii SK, Devillard E, Crowley TM, Wu SB (2021) Probiotic Bacillus subtilis 29,784 improved weight gain and enhanced gut health status of broilers under necrotic enteritis condition. Poult Sci 100:100981. https://doi.org/10.1016/j.psj.2021.01.004
Larsen N, Thorsen L, Kpikpi EN, Stuer-Lauridsen B, Cantor MD, Nielsen B, Brockmann E, Derkx PM, Jespersen L (2014) Characterization of Bacillus spp. strains for use as probiotic additives in pig feed. Appl Microbiol Biotechnol 98:1105–1118
Acknowledgements
This work was financially supported by the Shandong Natural Science Foundation (ZR2021MC150), Shandong Science and Technology Small and Medium Enterprises Innovation Ability Improvement Project (2021tsgc1303), Qingdao Science and Technology Benefit the People Demonstration and Guidance Project (22-3-7-xdny-11-nsh), and Shandong Modern Agricultural Technology and Industry System, China (SDAIT-11-07).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Materials preparation, data collection, and analysis were performed by Huiling Qiu, Shansong Gao, Lele Hou, and Fu Chen. The first draft of the manuscript was written by Huiling Qiu, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qiu, H., Gao, S., Hou, L. et al. Selenium-enriched Bacillus subtilis Improves Growth Performance, Antioxidant Capacity, Immune Status, and Gut Health of Broiler Chickens. Biol Trace Elem Res 201, 5756–5763 (2023). https://doi.org/10.1007/s12011-023-03610-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03610-6