Abstract
Avian pathogenic Escherichia coli (APEC) causes colibacillosis that leads to high morbidity and mortality among poultry birds. To date, there is a lack of knowledge about virulence-associated genes (VAGs) and multidrug resistance of APEC isolates from Pakistan. In this study, we determined the VAGs and antibiotic resistance profiles of APEC isolates recovered from colibacillosis affected broilers in Faisalabad region of Pakistan. A total of 84 diseased and dead birds from different local broilers farms were collected and examined for the gross lesions of colibacillosis by conducting postmortem examination. Of these, APEC isolates were recovered from 75 (89.2%) birds. Antibiotic susceptibility tests against 11 antimicrobial agents showed the highest resistance against ampicillin (98.6%) followed by tetracycline (97.3%) and ciprofloxacin (72%). The presence of 11 virulence-associated genes (VAGs) was detected by multiplex polymerase chain reaction (PCR). Of the 75 APEC, 32 (42.6%) harbored > 5 VAGs. Most commonly found genes were increased serum survival (iss; 84%), iron transport (iutA; 74.6%), and colicin V (ColV; 60%). Twenty-two isolates (29.3%) were found to possess a combination of VAGs; iss, tsh, iroN, and iutA, in addition to other VAGs. To the best of our knowledge, this is the first report on the detection of virulence-associated genes and multidrug resistance among APEC isolates in Pakistan. In the future, the strains with the predominant set of VAGs can be used for colibacillosis diagnosis and as a potential vaccine candidate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Avian pathogenic Escherichia coli (APEC) has been reported as an etiologic agent of colibacillosis in poultry, responsible for great economic losses worldwide. The losses are due to mortality, carcass condemnations, costs acquired in the prevention and control of the disease (Ronco et al. 2017). Avian colibacillosis causes several local and systemic infections like septicemia, omphalitis, swollen head syndrome, cellulitis, pericarditis, perihepatitis, yolk sac infection, or an amalgamation of these syndromes (Filho et al. 2015). APEC strains are responsible for 2–3% reduction in egg production and 3–4% for the mortality of birds in a farm (Solà-Ginés et al. 2015).
It has been a frequent practice to use antibiotics to treat birds affected with colibacillosis. Studies have reported that antibiotics like penicillin, chloretetracycline, bacitracin, salinomycin, and colistin have been used in chicken broilers as growth promoters and disease preventive measures (Saleemi et al. 2014; Subedi et al. 2018). The presence of multidrug resistance and resistance genes among APEC isolates is of high significance (Younis et al. 2017; Zhang et al. 2017). The use of antimicrobials in poultry bird’s feed may modify the intestinal flora by creating a selective pressure in favor of resistant bacterial populations (such as resistant E. coli) that can find their way into the food chain and environment. The emergence of multidrug resistance among APEC strains has posed serious challenges in antimicrobial therapy of colibacillosis (Lv et al. 2018). Prolonged medication has resulted in widespread resistance to therapeutic agents. APEC strains are also found to be multidrug resistant as they have acquired resistance genes through horizontal gene transfer (Li et al. 2013).
The ability of APEC to cause disease depends on numerous virulence factors such as adhesins (papC, papG allele I, II, III, fimH, sfaS), invasins (afaD, ibeA, aap), protectins (iss, traT, rfc, ompT), toxins (hlyF, hlyD, sat, astA), and iron acquisition (iroN, iutA, iucD, sitAC, sitAP) mechanisms that protect them from the host immune response and enable their extra-intestinal existence (Olesen 2017). There is no single or a set of specific virulence genes which has been proved to be systemically associated with APEC, thus making it difficult to diagnose APEC and the design of the antimicrobial/vaccine that targets all APEC isolates, simultaneously (Guabiraba and Schouler 2015). The characterization of APEC isolates is critical to understand the pathogenesis of colibacillosis and to reach effective prevention and control strategies for the disease (Lutful Kabir 2010). The diagnostic strategies to identify APEC isolates have relied on detection of several virulence genes of E. coli (Schouler et al. 2012). Knowledge about epidemiology of APEC in broiler chicken has been reported in previous studies throughout the world including Korea (Jeong et al. 2012), UK (Kemmett et al. 2014), Egypt (Mohamed et al. 2014), Brazil (Barbieri et al. 2015), Mexico (Vhm et al. 2017), Algeria (Mohamed et al. 2018), and Nepal (Subedi et al. 2018). The importance and interaction of specific virulence genes that determine pathogenesis of APEC infections are still poorly understood (Díaz et al. 2012). In addition to the presence of VAGs, APEC is commonly characterized by serogrouping. Previous studies have shown the most common APEC belonged to the serogroups O1, O2, and O78 (Lutful Kabir 2010).
However, there is a lack of data regarding virulence genotyping and antimicrobial susceptibility of APEC isolates from Pakistan. Therefore, the objective of this study was to determine the genetic background and to find the pattern of antimicrobial resistance among APEC isolates from broilers that died due to colibacillosis.
Materials and methods
Sample collection and bacterial isolation
Eighty-four diseased and dead broilers in different local poultry farms were collected and examined for the gross lesions of colibacillosis by postmortem at the Veterinary Diagnostic Laboratory located in the Department of Pathology, University of Agriculture, Faisalabad. Samples of the heart (n = 68) and liver (n = 16) showing characteristic fibrinous pericarditis and fibrinous hepatitis were collected separately aseptically for bacterial isolation. Briefly, the sample was minced and a loopful of inoculum from homogenized tissue was streaked on MacConkey agar (Oxoid™) plates and incubated for 18–24 h at 37 °C. Bacterial growth was observed and lactose-fermenting single isolated colony was subjected for biochemical identification. Biochemical tests of the E. coli isolates were done by using a rapid kit by Remel RapID One (Oxoid, UK) as per manufacturer’s instructions. The generated color code was confirmed through ERIC© software.
Antimicrobial susceptibility testing
E. coli isolates were examined for the antimicrobial susceptibility testing using the Kirby-Bauer disc diffusion method. Two to three bacterial colonies from pure culture were picked with sterile loop and inoculated in 5 ml sterile normal saline to produce uniform bacterial lawn on MH-agar (Oxoid, UK) plate. A total of 11 antimicrobials were tested namely ampicillin, colistin, ciprofloxacin, chloramphenicol, cefotaxime, ceftriaxone, gentamicin, tetracycline, imipenem, streptomycin, and sulfamethoxazole-trimethoprim. After incubation at 37 °C for 18–24 h, the zones of inhibition were measured, and the results were interpreted as described by clinical laboratory standards institute guidelines (CLSI 2012). Escherichia coli ATCC-25922 strain was used as negative control.
Virulence genotyping of APEC isolates by multiplex PCR scheme
Briefly, a single colony of bacterial isolate was cultured in brain heart infusion broth overnight at 37 °C and genomic DNA was extracted using commercially available bacterial DNA extraction kit (Vivantis GF-1, USA). The quality of total genomic DNA was estimated by using Nanodrop-1000 spectrophotometer (Thermo-scientific®). The absorbance was measured at A260/A280 and A260/A230 ratios to obtain 50 ng/μl DNA for PCR. The genomic DNA was used to determine the presence of multiple genes known to be associated with APEC virulence. Three multiplex PCR profiles targeting different gene combinations simultaneously were used in this study. Multiplex PCR profile one targeted ColV and papG genes; the profile II was used to target three genes: papG allele I, papG allele II, and papG allele III and a set of six genes: iss, tsh, iutA, iroN, ompT, and hlyF combinations were targeted according to the profile three. The thermal cycler conditions were as described previously (Ewers et al. 2005; Trampel et al. 2007; Wook et al. 2014; Paixao et al. 2016). The description of targeted genes, the primers used in this study, and the sizes (bp) of amplified products are summarized in Table 1.
The amplicons were analyzed by agarose gel electrophoresis with 1.2% agarose gel (Sigma Aldrich), prepared in 1X Tris-acetate-EDTA (TAE) buffer (Thermo-scientific®). After electrophoresis, gel was visualized under UV light in Dolphin ID Gel documentation system (Wealtec, USA).
Results
Based upon postmortem examination, 84 diseased and/or freshly dead birds were collected for this study showing predominant lesions of colibacillosis including pericarditis, perihepatitis, airsacculitis, and splenitis. E. coli isolates were recovered from 75 (89.2%) samples (heart: n = 65; liver: n = 10) based on colony morphology and biochemical characteristics.
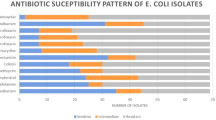
Antimicrobial sensitivity testing results by disc diffusion method showed that 100% APEC isolates possess phenotypic resistance to at least three different antimicrobial drugs; so all the isolates were multidrug resistant (MDR). Out of 75 isolates, resistance was found in 74 (98.6%) isolates to ampicillin, 73 (97.3%) isolates to tetracycline, 54 (72%) isolates to ciprofloxacin, and 52 (69.3%) isolates to chloramphenicol. All the isolates were multidrug resistant but none of the isolate (0%) found resistant against imipenem (Fig. 1).
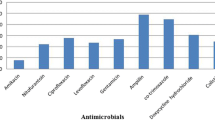
Out of 75 E. coli isolates, 32 (42.6%) isolates were considered as more virulent APEC, according to the genetic criteria of harboring at least five VAGs. Virulence gene combinations of 32 highly virulent isolates from the heart (n = 25) and liver (n = 7) are shown in Fig. 2. Most commonly found genes were increased serum survival (iss; 84%), iron transport (iutA; 74.6%), colicin V (ColV; 60%), temperature sensitive hemagglutinin (tsh; 57.3%), and iron acquisition system (iroN; 57.3%). The percentage of the distribution of VAGs among 75 APEC isolates is listed in Table 2.
Discussion
The primary objective of this study was to provide insight into the distribution of VAGs among avian pathogenic Escherichia coli (APEC) isolates at broiler poultry farms of Pakistan. To the best of our knowledge, there is scarcity of data about the VAGs and multidrug resistance among APEC strains burdening local broiler production in Pakistan. The current investigation showed that highly virulent and multidrug-resistant APEC strains colonized in colibacillosis-affected broilers. This was achieved by screening 84 birds, of which 75 showed virulence genotyped and 32 (42.6%) isolates harboring ≥ 5 VAGs. This is in agreement with previous studies in US (Zhao et al. 2005), Spanish (Solà-Ginés et al. 2015), Brazilian (Rocha et al. 2008), Egyptian (Eid et al. 2016), Malaysian (Roseliza et al. 2017), and Nepalian (Subedi et al. 2018) poultry farms which confirmed the presence of a number of virulence genes among APEC in broiler chickens.
In the present study, we used multiplex PCR for molecular characterization of APEC isolates recovered from diseased broiler chicken. Johnson et al. (2008) described a pentaplex PCR amplifying five VAGs: iss, iutA, iroN, hlyF, and ompT carried by plasmids. Schouler et al. (2012) reported four sets of VAGs in E. coli to be classified as APEC. Moreover, Dissanayake et al. (2014) showed a combination of ompT, iroN, hlyF, and sitAP possessed by 30 out of 55 clinical APEC isolates. In this study, we used three multiplex PCR profiles for the detection of 11 VAGs and found a hexaplex PCR targeting a combination of six VAGs: iss, tsh, iutA, iroN, ompT, and hlyF as more reliable and rapid diagnostic scheme. Out of 22 isolates (29.3%) showing a combination of VAGs, 12 isolates harbor five of six genes targeted by hexaplex PCR scheme. Moreover, this study showed the most prevalent set of VAGs: iss, tsh, iroN, and iutA among 22 (29.3%) isolates.
The results revealed that the frequency of VAGs was different from those previously reported. The increased serum survival gene (iss) has been detected in 51.2% of the APEC analyzed by Roseliza et al. (2017). Similarly, iss gene has been reported in 95.6% and 63.8% of the APEC strains studied by Vhm et al. (2017) and Dou et al. (2015). In this study, 84% of isolates harbored iss gene. Another common VAGs, namely iutA, which involved in E. coli iron transport, has been found in 74.6% APEC isolates. Similarly, Solà-Ginés et al. (2015) detected 95% of the isolates positive for the iutA gene; Mbanga and Nyararai (2015) reported 80% isolates positive for the iutA.
Multidrug resistance among E. coli has become an emerging challenge for its control (Azam et al. 2017; Mohsin et al. 2017). In previous published reports, E. coli isolates from poultry showed high rates of resistance against ampicillin, tetracycline, sulfamethoxazole-trimethoprim, streptomycin, and chloramphenicol from the USA (Johnson et al. 2005), Korea (Kim et al. 2007), Bangladesh (Akond 2009), China (Jiang et al. 2011), and Australia (Obeng et al. 2012). In another study, the highest resistance among E. coli isolates from poultry was reported against tetracycline (91.0%) followed by nalidixic acid (83.0%), ampicillin (82.0%), amoxicillin (80.0%), streptomycin (69.0%), ciprofloxacin (67.0%), chloramphenicol (63.5%), co-trimoxazole (60.8%), enrofloxacin (32.0%), kanamycin (31.0%), gentamicin (30.9%), florfenicol (20.9%), and ceftiofur (8.5%) (Nhung et al. 2017). In the present study, all of the APEC were MDR (100%) with the highest resistance against ampicillin (98.6%) followed by tetracycline (97.3%) and ciprofloxacin (72%). Antimicrobial resistance among APEC isolates from poultry is prospective to cause economic losses, consequential from expenditure on ineffective antimicrobials, as well as the burden of untreated poultry disease.
The identification of virulence-associated genes of APEC and antibiotic resistance is imperative to understand its pathogenesis, antimicrobial therapy, and the development of its control strategies. A holistic approach is required for the prevention and control of avian colibacillosis in Pakistan.
References
Akond, M., 2009. Antibiotic resistance of Escherichia Coli isolated from poultry and poultry environment of Bangladesh, American Journal of Environmental Sciences, 5, 47–52
Azam, M., Ehsan, I., Sajjad-ur-Rahman, Saleemi, M.K., Javed, M.R. and Mohsin, M., 2017. Detection of the colistin resistance gene mcr-1 in avian pathogenic Escherichia coli in Pakistan, Journal of Global Antimicrobial Resistance, 11, 152–153
Barbieri, N.L., Matter, B., Regina, S., Pinheiro, S., Ibelli, M., Nolan, L.K., Logue, C.M. and Guimara, B., 2015. Molecular characterization and clonal relationships among Escherichia coli strains isolated from broiler chickens with colisepticemia, 12, 74–83
CLSI, 2012. Performance standards for antimicrobial disk susceptibility tests ; approved atandard Clinical and Laboratory Standars Institute - NCCLS, 32.
Díaz-Sánchez, S., Sánchez, S., Ewers, C. and Höfle, U., 2012. Occurrence of avian pathogenic Escherichia coli and antimicrobial-resistant E. coli in red-legged partridges ( Alectoris rufa ): sanitary concerns of farming, Avian Pathology, 41, 337–344
Dissanayake, D.R.A., Octavia, S. and Lan, R., 2014. Population structure and virulence content of avian pathogenic Escherichia coli isolated from outbreaks in Sri Lanka, Veterinary Microbiology, 168, 403–412
Dou, X., Gong, J., Han, X., Xu, M., Shen, H., Zhang, D. and Zhuang, L., 2015. Characterization of avian pathogenic Escherichia coli isolated in eastern China, Gene, 576, 244–248
Ewers, C., Janssen, T., Kiessling, S., Philipp, H.C. and Wieler, L.H., 2005. Rapid detection of virulence-associated genes in avian pathogenic Escherichia coli by multiplex polymerase chain reaction, Avian Diseases, 49, 269–273
Filho, H.C.K., Brito, K.C.T., Cavalli, L.S. and Brito, B.G., 2015. Avian Pathogenic Escherichia coli ( APEC ) - an update on the control. The battle against microbial pathogens: Basic science, technological advances and educational programs, 598–618
Guabiraba, R. and Schouler, C., 2015. Avian colibacillosis : still many black holes, FEMS, Microbiology Letters, 1–8
Eid, H., M. Algamma, A., A. Nasef, S., Elfeil, W.K. and H. Mansour, G., 2016. Genetic variation among avian pathogenic E. coli strains isolated from broiler chickens, Asian Journal of Animal and Veterinary Advances, 11, 350–356
Jeong, Y.-W., Kim, T.-E., Kim, J.-H. and Kwon, H.-J., 2012. Pathotyping avian pathogenic Escherichia coli strains in Korea, Journal of Veterinary Science, 13, 145
Jiang, H.X., L, D.H., Chen, Z.L., Wang, X.M., Chen, J.R., Liu, Y.H., Liao, X.P., Liu, J.H. and Zeng, Z.L., 2011. High prevalence and widespread distribution of multi-resistant Escherichia coli isolates in pigs and poultry in China, Veterinary Journal, 187, 99–103
Johnson, T.J., Siek, K.E., Johnson, S.J. and Nolan, L.K., 2005. DNA Sequence and Comparative Genomics of pAPEC-O2-R , an Avian Pathogenic Escherichia coli Transmissible R Plasmid, Antimicrobial agents and chemotherapy, 49, 4681–4688
Johnson, T.J., Wannemuehler, Y., Doetkott, C., Johnson, S.J., Rosenberger, S.C. and Nolan, L.K., 2008. Identification of minimal predictors of avian pathogenic Escherichia coli virulence for use as a rapid diagnostic tool, Journal of Clinical Microbiology, 46, 3987–3996
Kemmett, K., Williams, N.J., Chaloner, G., Humphrey, S., Wigley, P. and Humphrey, T., 2014. The contribution of systemic Escherichia coli infection to the early mortalities of commercial broiler chickens, Avian Pathology, 43, 37–42
Kim, T.E., Jeong, Y.W., Cho, S.H., Kim, S.J. and Kwon, H.J., 2007. Chronological study of antibiotic resistances and their relevant genes in Korean avian pathogenic Escherichia coli isolates, Journal of Clinical Microbiology, 45, 3309–3315
Li, Y., Chen, L., Wu, X. and Huo, S., 2013. Molecular characterization of multidrug-resistant avian pathogenic Escherichia coli isolated from septicemic broilers, Poultry Science, 94, 601–611
Lutful Kabir, S.M., 2010. Avian colibacillosis and salmonellosis: A closer look at epidemiology, pathogenesis, diagnosis, control and public health concerns, International Journal of Environmental Research and Public Health, 7, 89–114
Lv, J., Mohsin, M., Lei, S., Srinivas, S., Talish, R. and Lin, J., 2018. Discovery of a mcr-1- bearing plasmid in commensal colistin-resistant Escherichia coli from healthy broilers in Faisalabad , Pakistan, Virulence, 9, 994–999
Mbanga, J. and Nyararai, Y.O., 2015. Virulence gene profiles of avian pathogenic Escherichia coli isolated from chickens with colibacillosis in Bulawayo, Zimbabwe, The Onderstepoort journal of veterinary research, 82, E1–8
Mohamed, M.A., Shehata, M.A. and Rafeek, E., 2014. Virulence genes content and antimicrobial resistance in escherichia coli from broiler chickens, Veterinary Medicine International, 1–6
Mohamed, L., Ge, Z., Yuehua, L., Yubin, G., Rachid, K., Mustapha, O., Junwei, W. and Karine, O., 2018. Virulence traits of avian pathogenic (APEC) and fecal (AFEC) E. coli isolated from broiler chickens in Algeria, Tropical Animal Health and Production, 50, 547–553
Mohsin, M., Raza, S., Roschanski, N., Guenther, S., Ali, A. and Schierack, P., 2017. Description of the first Escherichia coli resistance Gene mcr-1 from the Indian sub continent, Antimicrobial Agents and Chemotherapy, 61, 1–2
Nhung, T. N., Chansiripornchai, N, Carrique, J. J., 2017. Antimicrobial resistance in bacterial poultry pathogens: A review, Frontiers in Veterinary Sciences, 4, 126
Obeng, A.S., Rickard, H., Ndi, O., Sexton, M. and Barton, M., 2012. Antibiotic resistance, phylogenetic grouping and virulence potential of Escherichia coli isolated from the faeces of intensively farmed and free range poultry, Veterinary Microbiology, 154, 305–315
Olesen, B., 2017. Characterization of four Escherichia coli clonal groups, Acta Pathologica Microbiologica et Immunologica Scandinavica, 125, 1–28
Paixao, A. C., Ferreira A.C., M. Fontes, P. Themudo, T. Albuquerque, M.C. Soares, M. Fevereiro, L. Martins, M.I. Corr and S. De., 2016. Detection of virulence-associated genes in pathogenic and commensal avian Escherichia coli isolates, Poultry Science, 95,1646–1652.
Rocha, A.C.G.P., Rocha, S.L.S., Lima-Rosa, C.A. V, Souza, G.F., Moraes, H.L.S., Salle, F.O., Moraes, L.B. and Salle, C.T.P., 2008. Genes associated with pathogenicity of avian Escherichia coli (APEC) isolated from respiratory cases of poultry, Pesquisa Veterinaria Brasileira, 28, 183–186
Ronco, T., Stegger, M., Olsen, R.H., Sekse, C., Nordstoga, A.B., Pohjanvirta, T., Lilje, B., Lyhs, U., Andersen, P.S. and Pedersen, K., 2017. Spread of avian pathogenic Escherichia coli ST117 O78:H4 in Nordic broiler production, BMC Genomics, 18, 13
Roseliza R., S. Khairani., Z. Zunita., M. Ramlan and E. Khoo, 2017. Phylogenetic grouping and virulence gene profiles of Escherichia Coli isolates from chicken, Malaysian Journal of Veterinary Research. 8, 65–74.
Saleemi, M.K., M.F.U. Hussan, M.Z. Khan, A. Khan, R.Z. Abbas and A. Ahmad, 2014. Hematobiochemical effects of colistin administered intramuscularly in growing broiler birds, Pakistan Veterinary Journal, 34, 78–81.
Schouler, C., Schaeffer, B., Brée, A., Mora, A., Dahbi, G., Biet, F., Oswald, E., Mainil, J., Blanco, J. and Moulin-Schouleur, M., 2012. Diagnostic strategy for identifying avian pathogenic Escherichia coli based on four patterns of virulence genes, Journal of Clinical Microbiology, 50, 1673–1678
Solà-Ginés, M., Cameron-Veas, K., Badiola, I., Dolz, R., Majó, N., Dahbi, G., Viso, S., Mora, A., Blanco, J., Piedra-Carrasco, N., González-López, J.J. and Migura-Garcia, L., 2015. Diversity of multi-drug resistant avian pathogenic Escherichia coli (APEC) Causing outbreaks of colibacillosis in broilers during 2012 in Spain, PLoS ONE, 10, 1–14
Subedi, M., Luitel, H., Devkota, B., Bhattarai, R.K., Phuyal, S. and Panthi, P., 2018. Antibiotic resistance pattern and virulence genes content in avian pathogenic Escherichia coli (APEC) from broiler chickens in Chitwan , Nepal, BMC Veterinary Research, 4–9
Trampel, D.W., Wannemuehler, Y., Nolan, L.K., Trampel, D.W., Wannemuehler, A.C.Y. and B, L.K.N., 2007. Characterization of Escherichia coli isolates from peritonitis lesions in commercial laying hens, Avian Diseases, 51, 840–844
Vhm, L., Iq, S., Pdpm, D., Lev, R., Shs, R., Mal, L., Af, D.T. and Rmr, S., 2017. Genes of virulence and phylogenetic group in isolates of avian pathogenic Escherichia coli, IMedPub Journals, 9, 1–5
Wook, K., Young, H., Kuk, H., Kim, W. and Seok, I., 2014. Virulence factors of uropathogenic Escherichia coli of urinary tract infections and asymptomatic bacteriuria in children, Journal of Microbiology, Immunology and Infection, 47, 455–461
Younis, G., Awad, A. and Mohamed, N., 2017. Phenotypic and genotypic characterization of antimicrobial susceptibility of avian pathogenic Escherichia coli isolated from broiler chickens, Veterinary World, 10, 1167–1172
Zhang H, Rehman MU, Li K, Luo H, Lan Y, Nabi F, Shahzad M, Huang S, Liu X, Mehmood K, Iqbal MK and Li J, 2017. Antimicrobial resistance of Escherichia coli isolated from tibetan piglets suffering from white score diarrhea, Pakistan Veterinary Journal, 37, 43–46
Zhao, S., Maurer, J.J., Hubert, S., Villena, J.F. De, Mcdermott, P.F., Meng, J., Ayers, S., English, L. and White, D.G., 2005. Antimicrobial susceptibility and molecular characterization of avian pathogenic Escherichia coli isolates, Veterinary Microbiology, 107, 215–224
Funding
This work was supported by the fund of the International Foundation for Science, Sweden (IFS B/5793-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Ethical approval was obtained from the Institute of Biosafety Committee (IBC), University of Agriculture, Faisalabad, Pakistan. The animal samples were processed according to animal research guidelines approved by research ethics committee of Department of Pathology, University of Agriculture, Faisalabad, Pakistan. All authors have seen and approved the content and have contributed considerably to the work.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Azam, M., Mohsin, M., Sajjad-ur-Rahman et al. Virulence-associated genes and antimicrobial resistance among avian pathogenic Escherichia coli from colibacillosis affected broilers in Pakistan. Trop Anim Health Prod 51, 1259–1265 (2019). https://doi.org/10.1007/s11250-019-01823-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-019-01823-3