Abstract
Bovine viral diarrhea virus (BVDV) and bovine herpes virus-1 (BHV-1) remain as the major pathogens with heavy economic consequences in Iran. The prevalence of antibodies against BVDV and BHV-1, the rate of BVDV persistently infected (PI) animals, and associated risk factors were evaluated in a cross-sectional study carried out in Zanjan Province, Northwest Iran, in December 2011. A total number of 562 cattle in 10 herds and five cities were randomly selected, and their serum samples were tested to detect antibodies to these viruses and also BVDV antigen-positive (PI) animals. The data were analyzed with Pearson’s correlation coefficient, chi-square, and logistic regression test. In total, nine and eight of the selected herds were seropositive to BVDV and BHV-1, respectively. The overall seroprevalence of these infections were estimated at 28.6 and 10.7% for BVDV and BHV-1, respectively, and 0.53% of the samples were detected as persistently infected. Statistical analysis revealed that sex, age, and farming system are risk factors for both infections (P < 0.05), while breed was determined as a strong risk factor only for BVDV (P < 0.001). In addition, the present study certainly identifies that infection with BVDV is associated with infection to BHV-1 (OR = 4.52, 95% CI: 2.60–7.80; P ˂ 0.001). The results add our knowledge about the prevalence and associated risk factors of BVDV and BHV-1 in Iran and imply that the prophylactic and surveillance strategies need to be implemented to reduce the risk of spread of these viruses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bovine viral diarrhea virus (BVDV) and bovine herpes virus-1 (BHV-1) are the two substantial pathogens of cattle with worldwide distribution and significant contribution to a series of disease syndromes, especially bovine respiratory disease (BRD) (Ridpath 2010; Hilton 2014; Kirchhoff et al. 2014; Mosier 2014) and reproductive disorders (Brodersen 2014; Newcomer and Givens 2016).
BVDV, a Pestivirus of the Flaviviridae, causes a wide range of clinical manifestations from subclinical infections to gastroenteric, respiratory, reproductive, and hemorrhagic diseases; temporary infertility; and a fatal form known as mucosal disease (Lanyon et al. 2014). The virus is also one of the most significant immunosuppressive agents known to date suppressing both the innate and adaptive immune responses (Chase 2013; Peterhans and Schweizer 2013). BVDV is classified into non-cytopathogenic (ncp) and cytopathogenic (cp) biotypes based on the effect on cell culture. The ncp strains are the causative agents of persistently infected (PI) animals, which enable them to replicate and shed the virus in large amount throughout their life while they remain seronegative (Lanyon et al. 2014). Thus, the PI animals are the permanent reservoirs and play the crucial role in epidemiology of the virus (Houe 1999).
BHV-1, a Varicellovirus within the Herpesviridae, is an important pathogen of cattle and the causative agent of respiratory (Infectious Bovine Rhinotracheitis), genital (infectious pustular vulvovaginitis/balanopostitis), conjunctivitis, and abortion (Maclachlan et al. 2017). The virus is also strongly associated with immunosuppression in infected cattle leading to susceptibility to secondary infections, particularly respiratory pathogens (Jones and Chowdhury 2010; Mosier 2014). As a herpesvirus, BHV-1 is able to establish lifelong latency following primary infection (Ackermann and Wyler 1984; Winkler et al. 2000); the latent virus reactivates periodically and shed which may be transmitted to healthy animals (Turin et al. 1999). Stressful conditions such as movement may trigger reactivation and shedding of the virus complicating control and eradication efforts (Levings and Roth 2013).
Serologic studies have documented a range of prevalence for the BVDV and BHV-1 infections in several regions of Iran (Afshari et al. 2008; Sakhaee et al. 2009; Badiei et al. 2010; Ghaemmaghami et al. 2014; Nikbakht et al. 2015), and due to the considerable economic losses caused by these viruses, inactive vaccines are voluntarily applied in some industrial dairy farms in recent years. To find more information on epidemiology of BVDV and BHV-1, particularly the rate of persistently infected animals for BVDV and association of the potential risk factors with these infections, this study was conducted in Zanjan Province, Northwest Iran.
Material and methods
Study area
Zanjan Province located in the Northwest region of Iran between latitude 35° 33′ and 37 ° 15′ N and longitude 47° 10′ to 49° 26′ E with an area of 22,000 km2 comprising of eight cities. The prevailing climate is temperate with the average annual rainfall of ~ 300 mm. In total, about 120,000 cattle, mainly crossbred, live in the province which are kept in industrial, semi-industrial, and traditional livestock system. For this cross-sectional study, 10 farms in five cities, i.e., Abhar, Ijrood, Khodabandeh, Khorramdareh, and Zanjan, were randomly selected. No vaccination program has been practiced against BVDV or BHV-1 in Zanjan Province and the selected farms.
Blood collection and serum preparation
In order to investigate the herd and animal level prevalence of BVDV and BHV-1 infections in Zanjan Province, this cross-sectional study was performed. Sampling was carried out in December 2011, and the target population was cattle herds and sampling unit was cattle. Sample size was determined based on the published data on BVDV prevalence (Afshari et al. 2008); accordingly, the samples were also tested for BHV-1.
To calculate the number of herds and animals, the prevalence of BVDV which was previously estimated at 37% (Afshari et al. 2008) was considered as the base with 95% confidence level to find at least one seropositive herd (Salman 2003). For selection of the herds, a list of the all including 177 herds was prepared in Excel® and the final list of the herds was achieved using the random selection function, and all the animals were sampled in the selected herds.
The blood was collected from the jugular vein in sterile vacuum tubes. Sera were separated by centrifugation (10 min) at 3000 rpm and were transported on ice to the Animal Virology Department of Razi Vaccine and Serum Research Institute and stored at − 20 °C until tested. Some data including age (newborn animals under 6 months, young animals between 6 months and 2 years and old animals above 2 years), sex (male or female), breed (crossbred or exotic), farming system (traditional, semi-industrial, and industrial), and history of abortion were also recorded.
ELISA test
The sera were tested for antibodies against BVDV and BHV-1 using HERDCHEK* BVDV Ab Test Kit (IDEXX; Switzerland) and IBR-IPV Serum gB Blocking (POURQUIRE; France). The tests were carried out according to the manufacturer’s procedures. The optical density (OD) for both kits was measured at 450 nm; the ratio of sample/positive control (S/P) equal or greater to 0.3 was considered positive for BVDV Ab, and the percentage of inhibition of sample/negative control (S/N) equal or lower to 50% was considered positive for BHV-1 gB Ab. To detect PI cattle, all sera were tested by HERDCHEK* BVDV Ag Test Kit/Serum Plus (IDEXX; Switzerland) and they were classified positive when difference of OD values measured at 450 nm between the samples and the negative control (S-N) was greater than 0.3. All PIs were seronegative to BVDV.
Statistical analysis
For statistical analysis of the data, herd and individual prevalence of BVD and BHV-1 was described. Pearson’s correlation coefficient was used to determine the relationship between herd size and the number of seropositive animals within herds. For the qualitative variables, the relationship between the independent variables and the seropositive cattle was examined using Pearson’s correlation coefficient, chi-square, and logistic regression tests and presented as odds ratios (OR) with 95% confidence interval (CI) and P values which were considered significant at P < 0.05. The associated variables with a univariate P value < 0.2 were offered to multivariable logistic regression. All statistical procedures were conducted with SPSS version 22 software.
Results
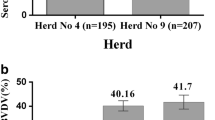
From the 10 herds in five cities selected for sampling, nine in five cities and eight in four cities were seropositive to BVDV and BHV-1, respectively. In a total of 562 samples, the prevalence of antibodies for BVDV and BHV-1 was estimated at 28.6 and 11.4%, respectively. The highest prevalence of antibodies for BVDV and BHV-1 was observed in Khodabandeh (64.3%) and Ijrood (25.5%), respectively, while the lowest rate of both infections was observed in Zanjan, 13.7% for BVDV and 0% for BHV-1. In total, three (0.53%) PI cattle were detected in two cities, two in Khodabandeh and one in Abhar (Table 1 and Graph 1).
Risk factor analysis for BVD and BHV-1
In univariable logistic regression analysis, when traditional system was considered as reference category, a statistically significant relation of cattle kept in industrial and semi-industrial systems was determined for seropositivity to BVDV (OR = 3.775, 95% CI: 2.248–6.34; P ˂ 0.001) and (OR = 2.452, 95% CI: 1.399–4.30; P = 0.002), respectively, while only industrial system was found as a risk factor for BHV-1 (OR = 4.67, 95% CI: 2.50–8.76; P ˂ 0.001). The results also revealed a strong association for concurrent infection with both viruses as BVDV infection is associated with infection to BHV-1 (OR = 4.52, 95% CI: 2.60–7.80; P ˂0.001). In univariable logistic regression analysis, the animals recorded with history of abortion showed no significant relation for BVDV and BHV-1 infections (Table 2).
The association of age, sex, breeds, and farming system with these viruses was calculated using uni- and multivariable logistic regression analysis and exhibited in Tables 2 and 3, respectively. Animals older than 2 years were significantly susceptible to BVDV (P = 0.004), and those between 6 months and 2 years were significantly the most susceptible to BHV-1 (OR = 4.22, 95% CI: 1.76–10.12; P = 0.001). Sex was found to be a risk factor for both infections as females were significantly less susceptible to BVDV (OR = 0.18, 95% CI: 0.08–0.42; P ˂ 0.001) and BHV-1 (OR = 0.2, 95% CI: 0.07–0.54; P = 0.002) than males. The crossbred exhibited a strong association for seropositivity to BVDV (OR = 2.7, 95% CI: 1.57–4.85; P˂0.001) comparing to the exotic breed, but no significant association was observed between the breed and BHV-1 infection in multivariable logistic regression analysis.
Pearson’s correlation coefficient between the herd size and seropositivity to BVD and BHV-1 was evaluated as 0.275 and 0.547, respectively, which were not statistically considered significant correlations (P ˃ 0.05).
Discussion
The result of this study revealed that BVDV and BHV-1 as the major pathogens of cattle are prevalent in Zanjan Province. The result shows high herd-level seroprevalence (90%) for BVDV which is compatible with those reported in the world and Iran as the virus is prevalent in most cattle-raising countries (Sedighinejad 1996; Hajikolaei and Seifi-abad Shapouri 2007; Sakhaee et al. 2009; Ghaemmaghami et al. 2014). The prevalence of seropositive animals is ranged from 13.7% in Zanjan to 64.3% in Khodabandeh with the overall prevalence of 28.6% in the province which seems lower than the average rate of the infection reported in the country. However, the considerable range of variation in prevalence of the infection was described in other regions of Iran as follows: 29.9%, 49.2, 66%, 73.33%, and 54.3% in Ahwaz, Isfahan, Fars, Kerman Provinces, and Arak area, respectively, and 64.4% in four provinces, i.e., Khorasan, Chaharmahal Bakhtiari, Sistan and Baluchestan, and Semnan (Hajikolaei and Seifi-abad Shapouri 2007; Sakhaee et al. 2009; Badiei et al. 2010; Shirvani et al. 2012; Ghaemmaghami et al. 2014; Nikbakht et al. 2015). Also, in a study conducted on 2985 sera samples collected from industrial farms involved in mucosal disease-like syndromes distributed throughout the country, the rate of infection was estimated from 22 to 90.3% in different provinces with the overall seroprevalence of 52.6% (Sedighinejad 1996). The regional difference in the rate of infection, which was also reported in other countries, has been explained to be correlated with density of animals, herd size, rate of animal movement into the herds, and contact with PI animals in the herds (Houe et al. 1995; Van Campen 2010; Almeida et al. 2013). Concerning the role of PIs in epidemiology of BVDV, in total, three (0.53%) PI cattle were detected in our study. This rate is consistent with the low prevalence of antibodies in the region but again is lower than the rate of PIs reported yet in previous studies 3.31 and 4% in Mashhad area and Fars Province, respectively (Garoussi et al. 2011; Farjani Kish et al. 2013). The rate of PIs has been estimated as 1–2% in the world and explained to be strongly associated with entering animals in the herds (Houe 1999; Gates et al. 2014). In our study, the region (Khodabandeh) with high level of animal movement exhibited the highest rate of PIs (1.73%) and seropositive animals against BVDV (64.3%). Regarding the age as a risk factor, animals older than 2 years significantly showed a higher rate of seropositivity than the younger ones which is likely related to the increased chance of exposure to the virus in the older.
BHV-1 was found in eight herds representing a seroprevalence of 80% at herd-level in Zanjan Province. Although this rate is in the range found in other regions of Iran, the animal-level prevalence of the virus is considered low (11.4%) comparing with the reports from other regions: 72%, 58.74%, 30.39%, 31.48%, 35.6%, and 27.93% in Isfahan; Hamedan; Kerman; and suburbs of Arak, Shiraz, and Ahwaz, respectively, and 31.9% in four provinces of Khorasan, Chaharmahal Bakhtiari, Sistan and Baluchestan, and Semnan (Hajikolaei and Seifi-abad Shapouri 2007; Sakhaee et al. 2009; Badiei et al. 2010; Shirvani et al. 2012; Bahari et al. 2013; Ghaemmaghami et al. 2014; Nikbakht et al. 2015). The rate of infection was not, however, comparable in the different cities of Zanjan Province ranging from 0.0% in Zanjan to 25.5% in Ijrood. The wide range of variation in seroprevalence of BHV-1 which has been correlated with some risk factors may significantly exceed higher numbers in favorable conditions (Raaperi et al. 2010; Raaperi et al. 2014). Age has been frequently reported as a risk factor for BHV-1 expressing higher seropositivity in animals ˂ 24 months which has been described to be correlated with waning of maternal antibodies and exposure to the natural infection (Raaperi et al. 2014). Consistent with this, we also report that animals between 6 months and 2 years exhibit a significantly higher chance for seroconversion against BHV-1.
Analysis of other risk factors including sex, farming system, and breed revealed that they were significantly associated with the BHV-1 and BVDV infections. Males exhibited greater risk for these infections than females; this is an exception for BVDV infection (Mockeliuniene et al. 2004; Hajikolaei and Seifi-abad Shapouri 2007); however, comparable reports exist for BHV-1 indicating a higher prevalence of the virus in bulls than cows (Boelaert et al. 2005; Guarino et al. 2008). To assess the actual effect of sex on susceptibility to these viruses, more research is needed to be especially conducted. Analyses of farming system as a risk factor revealed that industrial system was associated with both BVDV and BHV-1 seropositivity while semi-industrial was associated only with BVDV seropositivity. The significantly low prevalence of both infections in traditional system can be related to the less chance of contact between animals and reduced risk of exposure to the pathogens in this system. Also, it has been found that crossbred cattle were more susceptible to BVDV than exotic cattle while such a difference was not found for BHV-1 infection.
In accordance with the previous studies (Paton et al. 1998; Kampa et al. 2004; Ghaemmaghami et al. 2014; Nikbakht et al. 2015; Portela Rêgo et al. 2016), these results also confirmed a highly significant association of concurrent infection with both viruses. This relation has been explained to be likely resulted from similar risk factors (Paton et al. 1998) or immunosuppressive effect of both viruses (Ghaemmaghami et al. 2014). The immunosuppression and synergistic effects of these viruses can also increase pathogenesis of other viral and bacterial pathogens involved in BRD (Jones and Chowdhury 2010; Ridpath 2010). It has also been reported that concurrent infections with BVDV and BHV-1 strongly raise the risk of reproductive disorders (Biuk-Rudan et al. 1999). Most studies reported positive association between the herd size and prevalence of antibodies against BVDV and BHV-1 (Mockeliuniene et al. 2004; Guarino et al. 2008; Talafha et al. 2009; Badiei et al. 2010; Segura-Correa et al. 2010; Almeida et al. 2013; Bahari et al. 2013). On the contrary, our results showed no statistically significant correlation between the herd size and these infections. This effect can be resulted from the influence of other risk factors within the herds which were not recorded during the study. The lack of association between history of abortion and seropositivity to BVDV and BHV-1 may be biased by insufficient and inaccessible information recorded during sampling, especially in traditional farming system.
The statistical data provided on the prevalence and the main risk factors for BVDV and BHV-1 as the two crucial agents of cattle are valuable for establishment of any control strategies. These results, in comparison with other regional studies, emphasize that any control program needs to be initially assessed with the rate of infections in a specific region. Particularly, implementation of biosecurity strategies and elimination of PI animals are the fundamental parts of any control program. Additionally, an extensive surveillance program which also includes other susceptible species, i.e., wild life, to these viruses needs to be applied throughout the country.
References
Ackermann, M., Wyler, R., 1984. The DNA of an IPV strain of bovid herpesvirus 1 in sacral ganglia during latency after intravaginal infection. Veterinary microbiology, 9, 53–63.
Afshari, G., Bahonar, A., M;, L., Mosakhni, F., 2008. Study on Bovine Herpes Virus -1 antibodies in milk samples of dairy cattle in Karaj city by. Iranian Veterinary Journal, 4, 89–94.
Almeida, L.L., Miranda, I.C., Hein, H.E., Neto, W.S., Costa, E.F., Marks, F.S., Rodenbusch, C.R., Canal, C.W., Corbellini, L.G., 2013. Herd-level risk factors for bovine viral diarrhea virus infection in dairy herds from Southern Brazil. Research in veterinary science 95, 901–907.
Badiei, K., Ghane, M., Mostaghni, K., 2010. Seroprevalence of Bovine Herpes Virus Type 1 in the Industrial Dairy Cattle Herds in Suburb of Shiraz-iran. Australian Journal of Basic & Applied Sciences, 4, 4650.
Bahari, A., Gharekhani, J., Zandieh, M., Sadeghi-Nasab, A., Akbarein, H., Karimi-Makhsous, A., Yavari, M., 2013. Serological study of bovine herpes virus type 1 in dairy herds of Hamedan province, Iran. Veterinary research forum: an international quarterly journal, 4, 111–114.
Biuk-Rudan, N., Cvetnic, S., Madic, J., Rudan, D., 1999. Prevalence of antibodies to IBR and BVD viruses in dairy cows with reproductive disorders. Theriogenology 51, 875–881.
Boelaert, F., Speybroeck, N., de Kruif, A., Aerts, M., Burzykowski, T., Molenberghs, G., Berkvens, D.L., 2005. Risk factors for bovine herpesvirus-1 seropositivity. Preventive veterinary medicine, 69, 285–295.
Brodersen, B.W., 2014. Bovine viral diarrhea virus infections: manifestations of infection and recent advances in understanding pathogenesis and control. Veterinary pathology 51, 453–464.
Chase, C.C., 2013. The impact of BVDV infection on adaptive immunity. Biologicals: journal of the International Association of Biological Standardization, 41, 52–60.
Farjani Kish, G., Khodakaram - Tafti, A., Mohammadi, A., 2013. Serological survey of bovine viral diarrhoea virus by antigen capture ELISA in dairy herds in Fars provine, Iran. Bulgarian Journal of Veterinary Medicine, 16, 217–222.
Talebkhan Garoussi, Haghparast, A.R., Rafati, M.S., 2011. The prevalence of bovine viral diarrhea virus in persistently infected cows in industrial dairy herds in suburb of Mashhad- Iran. Iranian Journal of Veterinary Medicine, 5, 198–203.
Gates, M.C., Humphry, R.W., Gunn, G.J., Woolhouse, M.E.J., 2014. Not all cows are epidemiologically equal: quantifying the risks of bovine viral diarrhoea virus (BVDV) transmission through cattle movements. Veterinary Research, 45, 110.
Ghaemmaghami, S., Ahmadi, M., Deniko, A., Mokhberosafa, L., Bakhshesh, M., 2014. Serological study of BVDV and BHV-1 infections in industrial dairy herds of Arak, Iran. Iranian Journal Of Veterinary Science And Technology, 5, 53–61.
Guarino, H., Nunez, A., Repiso, M.V., Gil, A., Dargatz, D.A., 2008. Prevalence of serum antibodies to bovine herpesvirus-1 and bovine viral diarrhea virus in beef cattle in Uruguay. Preventive veterinary medicine, 85, 34–40.
Hajikolaei, M.R., Seifi-abad Shapouri, M.R., 2007. seroepidemiological stydy of bovine herpes virus 1 (BHV-1) infection in cattle in ahvaz. iranian veterinary journal 2, 23-31.
Hilton, W.M., 2014. BRD in 2014: where have we been, where are we now, and where do we want to go? Animal health research reviews, 15, 120–122.
Houe, H., 1999. Epidemiological features and economical importance of bovine virus diarrhoea virus (BVDV) infections. Veterinary microbiology 64, 89–107.
Houe, H., Baker, J.C., Maes, R.K., Wuryastuti, H., Wasito, R., Ruegg, P.L., Lloyd, J.W., 1995. Prevalence of cattle persistently infected with bovine viral diarrhea virus in 20 dairy herds in two counties in central Michigan and comparison of prevalence of antibody-positive cattle among herds with different infection and vaccination status. Journal of veterinary diagnostic investigation: official publication of the American Association of Veterinary Laboratory Diagnosticians, Inc, 7, 321–326.
Jones, C., Chowdhury, S., 2010. Bovine herpesvirus type 1 (BHV-1) is an important cofactor in the bovine respiratory disease complex. The Veterinary clinics of North America. Food animal practice, 26, 303–321.
Kampa, J., Ståhl, K., Moreno-López, J., Chanlun, A., Aiumlamai, S., Alenius, S., 2004. BVDV and BHV-1 Infections in Dairy Herds in Northern and Northeastern Thailand. Acta Veterinaria Scandinavica, 45, 181–192.
Kirchhoff, J., Uhlenbruck, S., Goris, K., Keil, G.M., Herrler, G., 2014. Three viruses of the bovine respiratory disease complex apply different strategies to initiate infection. Veterinary Research, 45, 20–20.
Lanyon, S.R., Hill, F.I., Reichel, M.P., Brownlie, J., 2014. Bovine viral diarrhoea: pathogenesis and diagnosis. Veterinary journal (London, England: 1997) , 199, 201–209.
Levings, R.L., Roth, J.A., 2013. Immunity to bovine herpesvirus 1: II. Adaptive immunity and vaccinology. Animal Health Research Reviews, 14, 103–123.
Maclachlan, N.J., Dubovi, E.J., Barthold, S.W., Swayne, D.E., Winton, J.R., 2017. Fenner’s veterinary virology. Academic Press United states of America.
Mockeliuniene, V., Salomskas, A., Mockeliunas, R., Petkevicius, S., 2004. Prevalence and epidemiological features of bovine viral diarrhoea virus infection in Lithuania. Veterinary microbiology, 99, 51–57.
Mosier, D., 2014. Review of BRD pathogenesis: the old and the new. Animal health research reviews, 15, 166–168.
Newcomer, B.W., Givens, D., 2016. Diagnosis and Control of Viral Diseases of Reproductive Importance: Infectious Bovine Rhinotracheitis and Bovine Viral Diarrhea. The Veterinary clinics of North America. Food animal practice, 32, 425–441.
Nikbakht, G., Tabatabaei, S., Lotfollahzadeh, S., Nayeri Fasaei, B., Bahonar, A., Khormali, M., 2015. Seroprevalence of bovine viral diarrhoea virus, bovine herpesvirus 1 and bovine leukaemia virus in Iranian cattle and associations among studied agents. Journal of Applied Animal Research, 43, 22–25.
Paton, D.J., Christiansen, K., Alenius, S., Cranwell, M.P., Pritchard, G.C., Drew, T.W., 1998. Prevalence of antibodies to bovine virus diarrhoea virus and other viruses in bulk tank milk in England and Wales. Veterinary Record 142, 385–391.
Peterhans, E., Schweizer, M., 2013. BVDV: a pestivirus inducing tolerance of the innate immune response. Biologicals: journal of the International Association of Biological Standardization, 41, 39–51.
Portela Rêgo, M.J., Batista Filho, B., Fernando, A., Fernandes de Oliveira, P.R., de Melo Borges, J., Barbosa de França, C.A., Pestana Ribeiro, C., Maristela Pituco, E., Pinheiro Junior, J.W., 2016. Epidemiological analysis of infection by the bovine viral diarrhea virus on family farms in Brazil. Semina: Ciências Agrárias, 37, 4119–4130.
Raaperi, K., Nurmoja, I., Orro, T., Viltrop, A., 2010. Seroepidemiology of bovine herpesvirus 1 (BHV1) infection among Estonian dairy herds and risk factors for the spread within herds. Preventive veterinary medicine, 96, 74–81.
Raaperi, K., Orro, T., Viltrop, A., 2014. Epidemiology and control of bovine herpesvirus 1 infection in Europe. Veterinary journal (London, England: 1997) 201, 249-256.
Ridpath, J., 2010. The contribution of infections with bovine viral diarrhea viruses to bovine respiratory disease. The Veterinary clinics of North America. Food animal practice, 26, 335–348.
Sakhaee, E., Khalili, M., Kazeminia, S., 2009. Serological study of bovine viral respiratory diseases in dairy herds in Kerman province, Iran. Iranian Journal of Veterinary Research, 10, 49–53.
Salman, M., 2003. Animal Disease Surveillance and Survey Systems, Methods and Applications. Blackwell Publishing Iowa.
Sedighinejad, S., 1996. bovine viral diarrhoea mucosal disease in Iran. pajouhesh & Sazandeghi, 30, 128-131 [in persian].
Segura-Correa, J.C., Solorio-Rivera, J.L., Sanchez-Gil, L.G., 2010. Seroconversion to bovine viral diarrhoea virus and infectious bovine rhinotracheitis virus in dairy herds of Michoacan, Mexico. Tropical animal health and production, 42, 233–238.
Shirvani, E., Lotfi, M., Kamalzadeh, M., Noaman, V., Bahriari, M., Morovati, H., Hatami, A., 2012. Seroepidemiological study of bovine respiratory viruses (BRSV, BoHV-1, PI-3V, BVDV, and BAV-3) in dairy cattle in central region of Iran (Esfahan province). Tropical animal health and production, 44, 191–195.
Talafha, A.Q., Hirche, S.M., Ababneh, M.M., Al-Majali, A.M., Ababneh, M.M., 2009. Prevalence and risk factors associated with bovine viral diarrhea virus infection in dairy herds in Jordan. Tropical animal health and production, 41, 499–506.
Turin, L., Russo, S., Poli, G., 1999. BHV-1: new molecular approaches to control a common and widespread infection. Molecular medicine (Cambridge, Mass.) ,5, 261–284.
Van Campen, H., 2010. Epidemiology and control of BVD in the U.S. Veterinary microbiology, 142, 94–98.
Winkler, M.T., Doster, A., Jones, C., 2000. Persistence and reactivation of bovine herpesvirus 1 in the tonsils of latently infected calves. Journal of virology, 74, 5337–5346.
Acknowledgements
The authors are gratefully thankful to the veterinary general office of Zanjan Province and Razi Vaccine and Serum Research Institute for financial support and technical assistance during sampling and laboratory procedures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Rights and permissions
About this article
Cite this article
Erfani, A.M., Bakhshesh, M., Fallah, M.H. et al. Seroprevalence and risk factors associated with bovine viral diarrhea virus and bovine herpes virus-1 in Zanjan Province, Iran. Trop Anim Health Prod 51, 313–319 (2019). https://doi.org/10.1007/s11250-018-1687-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-018-1687-3