Abstract
Bovine herpesvirus type 1 (BoHV 1) is a major bovine pathogen spreading worldwide and causing extensive damage to the livestock industry. BoHV causes respiratory, genital, and neurological disorders. A cross-sectional study was performed for the first time to estimate the seroreactivity to BoHV 1 and related risk factors among Iran’s central desert dairy cattle. A total of 800 blood samples was randomly collected from 76 unvaccinated herds. Samples were tested with an indirect enzyme-linked immunosorbent assay (ELISA) commercial kit to detect BoHV 1 antibodies. The logistic regression model was used to analyze the data. BoHV 1 seroreactivity at animal and herd levels was 50% and 65%, respectively. Herd size was recognized as a risk factor (OR = 2.65, CI = 1.61–4.37) for seroreactivity to BoHV using GLM (p < 0.05). The high prevalence of BoHV 1 antibodies in the study area indicates the need to implement educational programs on the importance of the disease and design methods to control and prevent virus distribution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bovine herpesvirus-1 (BoHV-1) is a critical bovine viral pathogen found worldwide. BoHV infection causes considerable economic losses directly (reproductive problems, respiratory disease, mastitis, and encephalitis) and indirectly (restrictions on national and international trade in livestock and livestock products) to the cattle industry (Benavides et al. 2021; Waldeck et al. 2021). The viruses of the genus Varicellovirus, subfamily Alphaherpesvirinae, and family Herpesviridae have three subtypes: BoHV-1 (associated with infectious bovine rhinotracheitis, IBR, and abortions), BoHV-1.2a (associated to IBR, pustular vulvovaginitis; IPV, infectious balanoposthitis; and IBP, abortions), and BoHV-1.2b (associated to respiratory disease and IPV/IPB). BoHV suppresses the immune system and causes susceptibility to other infectious agents, especially respiratory pathogens (Jones 2019; Iscaro et al. 2021). The main transmission route of the BoHV within the herd is a direct contact between infected cattle and susceptible animals through respiratory, ocular, and genital secretions. The virus is also transmitted indirectly by infected semen, aborted fetal tissues, visitors, and equipment (Fernandes et al. 2019; Hostnik et al.2021; Ince and Sevik 2022; Mandelik et al. 2021). Following initial exposure, BoHV can cause latent infection, and the infected animal may periodically shed the virus. Latent viral infection can happen in the sensory ganglia of the trigeminal nerve, sacral ganglia, tonsils, and blood lymphocytes. Reactivation from latency may be by stressors such as parturition, livestock movement, corticosteroid therapy, and poor management, creating a new cycle of virus replication and transmission to naive livestock. Introducing animals with latent infection to susceptible herds is one of the most important routes of virus transmission between cattle herds (Iscaro et al. 2021; Brock et al. 2020; Chen et al. 2018; Sibhat et al. 2018).
Knowing the prevalence of BoHV infection at the herd and livestock level and identifying related risk factors facilitate the development of virus control and surveillance schemes. (Adeli et al. 2017; Ince and Sevik 2022; Kipyego et al. 2020). Enzyme immunosorbent assay (ELISA) is a specific and sensitive test for the detection of BoHV antibodies and for identifying infected animals in the acute and latent stages (Adeli et al. 2017; Chen et al. 2018; Kipyego et al. 2020). BoHV was first isolated from imported cattle in Iran in 1974 (Hazrati and Amjadi 1975). A wide range (5.1 to 72.2%) of BoHV-1 seropositivity has been observed in different regions of Iran (Hashemi et al. 2022; Noaman and Nabinejad 2020). BoHV seroreactivity has not been evaluated in Iran’s central desert. The study is aimed at estimating the prevalence of BoHV antibodies at the herd and animal level and related risk factors in Iran’s central desert (Yazd and South Khorasan provinces).
Materials and methods
Study area, cattle sampling, and serological evaluation
This project was carried out in Yazd (31° 53′ 55.5″ N, 54° 21′ 16.7″ E) and South Khorasan (32° 54′ 38.9″ N, 59° 10′ 52.9″ E) provinces, located in the central desert of Iran, with hot and dry climatic conditions and annual rainfall of less than 135 mm. There are 62,500 pure (Holstein) and 151,500 crossbreed cattle in the study area (Ministry of Agriculture-Jahad).
Eight hundred serum samples of 76 unvaccinated Holstein dairy herds were collected from October to December 2019. BVDV seroreactivity (Karimi et al. 2022), history of abortion, cattle introduction to the herd, herd size (< 100 and > 100 heads), age group (1–2, 2–4, and > 4 years old), housing systems (industrial, semi-industrial, and traditional), and health status (poor, moderate, and good) were recorded. The indirect ELISA method (POURQUIRE; France; 95% sensitivity and 97% specificity) was used to evaluate virus antibodies according to Kit guidelines (Erfani et al. 2019).
Statistical analysis
We used GLM for statistical analysis, so that the BoHV antibody was the dependent variable, and animal age was a covariate. The others were used as fixed effects. The odds ratio (OR) of BoHV antibodies was estimated using cross-table data. The relationship between the risk factors and BoHV antibodies was estimated using a logistic regression model with a logit function by SPSS software (version 22) (Fig. 1).
Results
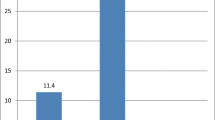
The BoHV antibodies at the individual and herd levels were 50% and 65% (ranged 8–80%), respectively. The prevalence of BoHV antibodies among the age subgroups was the same.
The seroreactivity of animals in large herds (> 100 heads) was 67.7%, while in small herds (< 100 heads), it was 32.3%. The frequency distribution of housing systems, cattle introduction, health status, history of abortion, and BVDV seroreactivity between BoHV seropositive/negative groups was relatively the same (Table 1).
The regression coefficient of age was − 0.063 ± 0.07, but non-significant (p > 0.05). Herd size was predicted as a risk factor (OR = 2.65, CI = 1.61–4.37) using GLM (p < 0.05). Housing systems, cattle introduction, health status, history of abortion, and BVDV seroreactivity were not significant in this study (p > 0.05) (Table 2).
Discussion
This research was the first study that surveyed the candidate risk factors of BOHV antibodies in Iran’s central desert. Pathogen BoHV has a global prevalence and causes extensive economic damage to the livestock industry by causing infections in the respiratory and reproductive systems of cattle (Almeida et al. 2021; Benavides et al. 2021; Hostnik et al. 2021; Jones 2019). BOHV antibodies and the risk factors associated with them, for the first time, were investigated.
The BoHV antibodies indicate a natural infection because the virus vaccination was not performed in the study area. The BoHV seroreactivity at the livestock (50%) and herd (65%) levels in Holstein cows was high in the study area. The prevalence of BoHV antibodies shows differently in other parts of Iran. Antibodies of BoHV in other provinces of Iran at the individual level at 48.69% (Adeli et al. 2017), 27.93% (Badiei et al. 2010), 31.9% (Nikbakht et al. 2015), 58.74% (Bahari et al. 2013), 7.1% (Ezzi et al. 2013), 33.97% (Kargar et al. 2001), 30.39% (Sakhaei et al. 2009), 56% (Sadri. 2012), 72% (Shirvani et al. 2012) 35.6% (Ghaemmaghami et al. 2013), 10.7% (Erfani et al. 2019), 72.7% (Noaman and Nabinejad 2020), and 5.1% (Hashemi et al. 2022) have been reported. In other countries, the prevalence of BoHV antibodies at the animal level is 30.9% (Kipyego et al. 2020), Kenya, 31.1% (Ramirez Vasquez et al. 2016), Colombia, 39.53% (Ince and Sevik 2022), Turkey, 73% (Fernandes et al. 2019) and 48.5% (Almeida et al. 2021), Brazil, 43.2% (Carbonero et al. 2011), Ecuador, 64.5% (Romero-Salas et al. 2013) and 64.4% (Segura- Correa et al. 2016), Mexico, 32.92% (Nezzal et al. 2017), Iraq, 31.17% (Derrar et al. 2019), Algeria, 41% (Sibhat et al. 2018), Ethiopia, and 29.5% (Patil et al. 2021) reported from China. Meta-analysis studies in China (Chen et al. 2018) and Brazil (Pajeu et al. 2021) reported 40% and 54.2% BoHV prevalence, respectively. BoHV seroreactivity at the herd level is similar to different studies conducted in various regions of Iran. Hashemi et al. (2022), Bahari et al. (2013), and Badiei et al. (2010) observed 56.3, 82.93, and 100% BoHV seroprevalence at the herd level in various provinces of the country, respectively. BoHV antibodies have been reported at the herd level from other countries; 30.9% (Kipyego et al. 2020) from Kenya, 73.21% (Ince and Sevik 2022) from Turkey, 84% (Fernandes et al. 2019) from Brazil, 82.1% (Carbonero et al. 2011) from Ecuador, 37.8% (Raaperi et al. 2010) from Estonia, 71.3% (Dias et al. 2013) from Brazil, and 81.8% (Sibhat et al. 2018) from Ethiopia. A meta-analysis study in Brazil (Pajeu et al. 2021) reported 88.53% BoHV seroprevalence.
The high prevalence of BoHV antibodies in animals and herds points to the necessity to address the infection state in Iran’s central desert. The differences in the serum prevalence of BoHV antibodies among regions and countries can be explained by herd size, age, management system, test method, climate, herd density, sample size, study design, statistical model, and biosecurity measures (Adeli et al. 2017; Ince and Sevik 2022; Kipyego et al. 2020).
Our results showed that a herd larger than 100 cattle is a risk factor for serum response to BoHV. Some studies find a positive relationship between larger herd size and BoHV antibodies (Fernandes et al. 2019, Sequra-Correa et al. 2016, Bahari et al. 2013, Ghaemmaghami et al. 2013, Gonzalez-Garcia et al. 2009, Raaperi et al. 2010). The larger herds are more likely to encounter naive livestock with the pathogen. More professional visitors (cattle traders, veterinarians, and animal vaccinators) and more permanent and temporary workers increase the chances of transmitting the virus to larger herds. (Waldeck et al. 2021; van Schaik et al. 2001). Adeli et al. (2017), Derrar et al. (2019), and Erfani et al. (2019) found no significant association between herd size and BoHV seropositivity.
The results of the present study showed that the introduction of livestock to the herd was not significantly associated with BoHV seroprevalence. The finding disagrees with the reports of Gonzalez-Garcia et al. (2009), Boelaert et al. (2005), Waldeck et al. (2021), and van Schaik et al. (1998). Some authors found no significant association between the introduction of the animal to the herd and the status of the BoHV serostatus (Fernandes et al. 2019, Carbonero et al. 2011, Segura-Correa et al. 2016, Derrar et al. 2019, Sibhat et al. 2018, Solis-Calderon et al. 2003, Raaperi et al. 2010). The high prevalence of virus antibodies in the study area and low rate of reactivation of BoHV latent infection may explain the lack of a significant relationship between the introduction of animals into the herd and BoHV seroreactivity (Solis-Calderon et al. 2003; Derrar et al. 2019).
In this study, no significant relationship was found between age and the prevalence of BoHV antibodies. The results contradict reports that found a positive relationship between aging and serum response to BoHV. Reason for the greater chance of being exposed to the virus and being seropositive for life (Kipyego et al. 2020; Waldeck et al. 2021; Adeli et al. 2017; Ramirez Vasquez et al. 2016; Ince and Sevik. 2022; Carbonero et al. 2011; Romero-Salas et al. 2013; Bahari et al. 2013; Ezzi et al. 2013; Derrar et al. 2019; Shirvani et al. 2012; Chen et al. 2018; Hashemi et al. 2022). Ghaemmaghami et al. (2013), Kaddour et al. (2019), and Segura-Correa et al. (2016) did not find a significant relationship between age and serum status of the virus and did not explain.
Our findings did not show a significant relationship between health status and the prevalence of BoHV antibodies. Carbonero et al. (2011) and Kaddour et al. (2019) noted a notable association between health measures and the lower prevalence of BoHV antibodies. The difference in the results can be due to the different evaluation of health status, the planning of the questionnaire, and the partiality of herd holder answers to a questionnaire.
In this study, there was no notable association between the history of abortion and BoHV serostatus. It may be due to the impact of various factors on abortion that have not been evaluated in our study. Infection can also occur when cows are not pregnant (Asmare et al. 2018). On the other hand, clinical cases of the disease are rarely observed, which leads to the presence of a large number of seropositive cattle without clinical symptoms (Mandelik et al. 2021). Adeli et al. (2017), Asmare et al. (2018), Erfani et al. (2019), Fernandes et al. (2019), Ince and Sevik (2022), and Ramirez Vasquez et al. (2016) did not report a notable association between the history of abortion and serum reaction to BoHV.
No significant association was observed between BVDV serum status and BoHV seroreactivity. The consistent results have been reported by Carbonero et al. (2011), Segura-Correa et al. (2016), and Hashemi et al. (2022). The result is in contrast to the findings of Nikbakht et al. (2015), Ince and Sevik (2022), Noaman and Nabinejad (2020), and Erfani et al. (2019).
No significant relationship was found between different housing systems and BoHV seroreactivity. The report of Shirvani et al. (2012) was consistent with our results, but Erfani et al. (2019) reported the industrial housing system as a risk factor.
Conclusion
The results showed that herd size is a risk factor in Iran’s central desert. It is necessary to implement disease monitoring and control programs because of the wide prevalence of BoHV antibodies at the animals and herd levels in Iran’s central desert. Cross-sectional studies (such as this and most studies on the prevalence of BoHV) have limited capability for temporarily verifying risk factors for BoHV seroreactivity, suggesting a meta-analysis in the future. European Union animal health and food regulations are a good model for developing local legislation to enable disease surveillance, control, and prevention (Bondoc 2016a, b, c, d). A comprehensive system for collecting and sharing data on the status of BoHV infection is necessary to help adopt disease control strategies.
Data availability
The datasets generated during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Adeli, E., Pourmahdi Borujeni, M., Haji Hajikolaei, M.R. and Seifi Abad Shapouri, M.R., 2017. Bovine Herpesvirus-1 in Khouzestan province in Iran: seroprevalence and risk factors. Iranian journal of ruminants health research, 2(1), pp.47-56. https://doi.org/10.22055/ijrhr.2017.14417.
Almeida, I.C.D., Almeida, Y.V., Donatele, D.M., Clipes, R.C., Barioni, G., Zanini, M.S. and Filippo, P.A.D., 2021. Seroprevalence and associated factors of infectious bovine rhinotracheitis and bovine viral diarrhea in dairy cows in the Caparaó region, Espírito Santo, Brazil. Ciência Rural, 5(12), pp.1-9. https://doi.org/10.1590/0103-8478cr20200220.
Asmare, K., Sibhat, B., Ayelet, G., Gebremedhin, E.Z., Lidete, K.A. and Skjerve, E., 2018. Serological evidence of Bovine herpesvirus-1, Bovine Viral Diarrhea virus and Schmallenberg virus infections in relation to reproductive disorders in dairy cattle in Ethiopia. Acta tropica, 178, pp.236-241. https://doi.org/10.1016/j.actatropica.2017.12.005.
Badiei, K., Ghane, M. and Mostaghni, K., 2010. Seroprevalence of bovine herpes virus type 1 in the industrial dairy cattle herds in suburb of Shiraz-Iran. Australian Journal of Basic and Applied Sciences, 4, pp.4650-4654.
Bahari, A., Gharekhani, J., Zandieh, M., Sadeghi-Nasab, A., Akbarein, H., Karimi-Makhsous, A. and Yavari, M., 2013. Serological study of bovine herpes virus type 1 in dairy herds of Hamedan province, Iran. Veterinary Research Forum, 4(2), pp.111-114.
Benavides, B., Casal, J., Dieguez, J., Yus, E., Moya, S.J. and Allepuz, A., 2021. Quantitative risk assessment of introduction of BVDV and BoHV-1 through indirect contacts based on implemented biosecurity measures in dairy farms of Spain. Preventive Veterinary Medicine, 188, Article Number:105263, pp.1–12. https://doi.org/10.1016/j.prevetmed.2021.105263.
Boelaert, F., Speybroeck, N., de Kruif, A., Aerts, M., Burzykowski, T., Molenberghs, G. and Berkvens, D.L., 2005. Risk factors for bovine herpesvirus-1 seropositivity. Preventive veterinary medicine, 69(3-4), pp.285-295. https://doi.org/10.1016/j.prevetmed.2005.02.010.
Bondoc, I., 2016b. European Regulation in the Veterinary Sanitary and Food Safety Area, a Component of the European Policies on the Safety of Food Products and the Protection of Consumer Interests: A 2007 Retrospective. Part Two: Regulations. Universul Juridic, Supliment, pp.16-19.
Bondoc, I., 2016c. European Regulation in the Veterinary Sanitary and Food Safety Area, a Component of the European Policies on the Safety of Food Products and the Protection of Consumer Interests: A 2007 Retrospective. Part Three: Directives. Universul Juridic, Supliment, pp. 20-23.
Bondoc, I., 2016d. European Regulation in the Veterinary Sanitary and Food Safety Area, a Component of the European Policies on the Safety of Food Products and the Protection of Consumer Interests: A 2007 Retrospective. Part Four: Decisions. Universul Juridic, Supliment, pp. 24-27.
Bondoc, I., 2016a. European Regulation in the Veterinary Sanitary and Food Safety Area, a Component of the European Policies on the Safety of Food Products and the Protection of Consumer Interests: A 2007 Retrospective. Part One: the Role of European Institutions in Laying Down and Passing Laws Specific to the Veterinary Sanitary and Food Safety Area. Universul Juridic, Supliment, pp. 12–15.
Brock, J., Lange, M., Guelbenzu-Gonzalo, M., Meunier, N., Vaz, A.M., Tratalos, J.A., Dittrich, P., Gunn, M., More, S.J., Graham, D. and Thulke, H.H., 2020. Epidemiology of age-dependent prevalence of Bovine Herpes Virus Type 1 (BoHV-1) in dairy herds with and without vaccination. Veterinary research, 51(1), pp.1-13. https://doi.org/10.1186/s13567-020-00842-5.
Carbonero, A., Saa, L.R., Jara, D.V., García-Bocanegra, I., Arenas, A., Borge, C. and Perea, A., 2011. Seroprevalence and risk factors associated to Bovine Herpesvirus 1 (BHV-1) infection in non-vaccinated dairy and dual purpose cattle herds in Ecuador. Preventive veterinary medicine, 100(1), pp.84-88. https://doi.org/10.1016/j.prevetmed.2011.03.006.
Chen, X., Wang, X., Qi, Y., Wen, X., Li, C., Liu, X. and Ni, H., 2018. Meta-analysis of prevalence of bovine herpes virus 1 in cattle in Mainland China. Acta tropica, 187, pp.37-43. https://doi.org/10.1016/j.actatropica.2018.07.024.
Derrar, S., Aggad, H., Hammoudi, A., Saim, M.S., Ayad, M.A., Benzineb, F.Z., Abdali, M. and Abdelli, A., 2019. Seroprevalence and risk factors associated with bovine herpesvirus-1 infection in the region of Tiaret, Algeria. Veterinaria, 68(3), pp.127-132.
Dias, J.A., Alfieri, A.A., Ferreira-Neto, J.S., Goncalves, V.S.P. and Muller, E.E., 2013. Seroprevalence and risk factors of bovine herpesvirus 1 infection in cattle herds in the state of Paraná, Brazil. Transboundary and Emerging Diseases, 60(1), pp.39-47. https://doi.org/10.1111/j.1865-1682.2012.01316.x.
Erfani, A.M., Bakhshesh, M., Fallah, M.H. and Hashemi, M., 2019. Seroprevalence and risk factors associated with bovine viral diarrhea virus and bovine herpes virus-1 in Zanjan Province, Iran. Tropical animal health and production, 51(2), pp.313-319. https://doi.org/10.1007/s11250-018-1687-3.
Ezzi, A., Hatami, A., Bakhshesh, M.,Shoukri, M.R. and Gharaghozloyan, M., 2013. Serological study of bovine herpesvirus type 1 and parainfluenza type 3 in cow farms of Qazvin province based on different ages and seasons. Archives of Razi Institute, 68(1), pp.53-57. https://doi.org/10.7508/ari.2013.01.009.
Fernandes, L.G., Denwood, M.J., Santos, C.D.S.A.B., Alves, C.J., Pituco, E.M., Romaldini, A.H.D.C.N., De Stefano, E., Nielsen, S.S. and de Azevedo, S.S., 2019. Bayesian estimation of herd-level prevalence and risk factors associated with BoHV-1 infection in cattle herds in the State of Paraiba, Brazil. Preventive veterinary medicine, 169, Article Number: 104705, pp1-12. https://doi.org/10.1016/j.prevetmed.2019.104705.
Ghaemmaghami, S., Ahmadi, M., Deniko, A., Mokhberosafa, L. and Bakhshesh, M., 2013. Serological study of BVDV and BHV-1 infections in industrial dairy herds of Arak, Iran. Iranian Journal of Veterinary Science and Technology, 5(2), pp.53-61. https://doi.org/10.22067/veterinary.v5i2.22723.
Gonzalez-Garcia, M.A., Maldonado, J.L., Gomez-Pacheco, J.M., Arenas-Casas, A., Carbonero-Martínez, A., Borges-Rodríguez, C., Bocanegra, I.G. and Perea-Remujo, J.A., 2009. Seroprevalence and risk factors associated with bovine herpesvirus type 1 (BHV1) infection in non-vaccinated cattle herds in Andalusia (South of Spain). Spanish Journal of Agricultural Research, 7(3), pp.550-554.
Hashemi, M., Bakhshesh, M., Khezri, M., Gharagouzlouian, M.M. and Tavakoli, G., 2022. A two-year serological study of bovine viral diarrhea virus, bovine alphaherpesvirus 1 and bovine parainfluenza virus type 3 in Qazvin dairy cattle farms, Northwestern of Iran. Veterinarski arhiv, 92(1), pp.1-10. https://doi.org/10.24099/vet.arhiv.1123.
Hazrati, A. and Amjadi, A.R., 1975. The isolation and identification of infectious bovine rhinotracheitis virus in Iran. Archives of Razi Institute, 27(1), pp.21-35.
Hostnik, P., Cerner, D., Mrkun, J., Staric, J. and Toplak, I., 2021. Review of infections with bovine herpesvirus 1 in Slovenia. Frontiers in veterinary science, 8, Article Number:676549, pp.1-9. https://doi.org/10.3389/fvets.2021.676549.
Ince, O.B. and Sevik, M., 2022. Risk assessment and seroprevalence of bovine herpesvirus type 1 infection in dairy herds in the inner Aegean Region of Turkey. Comparative immunology, microbiology and infectious diseases, 80, Article Number: 101741, pp.1-6. https://doi.org/10.1016/j.cimid.2021.101741.
Iscaro, C., Cambiotti, V., Petrini, S. and Feliziani, F., 2021. Control programs for infectious bovine rhinotracheitis (IBR) in European countries: an overview. Animal Health Research Reviews, 22(2), pp.136-146. https://doi.org/10.1017/S1466252321000116.
Jones, C., 2019. Bovine herpesvirus 1 counteracts immune responses and immune-surveillance to enhance pathogenesis and virus transmission. Frontiers in Immunology, 10, Article Number: 1008,pp.1-8. https://doi.org/10.3389/fimmu.2019.01008.
Kaddour, A., Bouyoucef, A., Fernandez, G., Prieto, A., Geda, F. and Moula, N., 2019. Bovine herpesvirus 1 in the northeast of Algiers, Algeria: Seroprevalence and associated risk factors in dairy herd. Journal of advanced veterinary and animal research, 6(1), pp.60-65. https://doi.org/10.5455/javar.2019.f312.
Kargar, M.R., Bokaei, S., Akhavizadegan, M.A., Charkhkar, S. and Meshkot, M., 2001. Seroepidemological Survey for Antibodies against Infectious Bovine Rhinotracheitis and Bovine Herpes 4 Viruses among Cattle in Different Provinces of Iran. Archives of Razi Institute, 52(1), pp.93-100.
Karimi, O., Bitaraf Sani, M., Bakhshesh, M., Harofteh, J.Z. and Poormirzayee, H., 2022. Seroprevalence of bovine viral diarrhea virus antibodies and risk factors in dairy cattle from the central desert of Iran. Tropical Animal Health and Production, 54(3), pp.1-7. https://doi.org/10.1007/s11250-022-03180-0.
Kipyego, E.S., Gitau, G., Vanleeuwen, J., Kimeli, P., Abuom, T.O., Gakuya, D., Muraya, J. and Makau, D., 2020. Sero-prevalence and risk factors of infectious bovine rhinotracheitis virus (type 1) in Meru County, Kenya. Preventive Veterinary Medicine, 175, Article Number:104863, pp.1-6. https://doi.org/10.1016/j.prevetmed.2019.104863.
Mandelik, R., Bires, J., Ozsvari, L., Hodnik, J.J. and Vilcek, S., 2021. Infectious bovine rhinotracheitis control program in Slovakia. Frontiers in veterinary science, 8, Article Number:675521,pp.1-7. https://doi.org/10.3389/fvets.2021.675521.
Nezzal, S.F., Hassan, I.Q. and Ali, R.M., 2017. Serosurveillance and molecular detection of bovine herpesvirus-1 (bhv-1) in cattle and buffaloes in Baghdad. Advances in Animal and Veterinary Sciences, 5, pp.283-288. https://doi.org/10.17582/journal.aavs/2017/5.7.283.288.
Nikbakht, G., Tabatabaei, S., Lotfollahzadeh, S., Nayeri Fasaei, B., Bahonar, A. and Khormali, M., 2015. Seroprevalence of bovine viral diarrhoea virus, bovine herpesvirus 1 and bovine leukaemia virus in Iranian cattle and associations among studied agents. Journal of Applied Animal Research, 43(1), pp.22-25. https://doi.org/10.1080/09712119.2014.883995.
Noaman, V. and Nabinejad, A.R., 2020. Seroprevalence and risk factors assessment of the three main infectious agents associated with abortion in dairy cattle in Isfahan province, Iran. Tropical animal health and production, 52(4), pp.2001-2009. https://doi.org/10.1007/s11250-020-02207-8.
Pajeu, B., Aragao, B.B. and Junior, J.W.P., 2021. Economic impacts of Bovine alphaherpesvirus 1 infection in Brazil: Meta-analysis based on epidemiological indicators. Semina: Ciências Agrárias, 42(6), pp.3355-3378.
Patil, S.S., Ravindran, R., Sowjanyakumari, R., Suresh, K.P., Hiremath, J., Hemadri, D., Shivamallu, C. and Rahman, H., 2021. Seroprevalence of Infectious Bovine Rhinotracheitis (IBR) In North Eastern (NE) States Of India. Journal of Experimental Biology and Agricultural Sciences, 9(3) ,pp 305 – 310. https://doi.org/10.18006/2021.9(3).305.310.
Raaperi, K., Nurmoja, I., Orro, T. and Viltrop, A., 2010. Seroepidemiology of bovine herpesvirus 1 (BHV1) infection among Estonian dairy herds and risk factors for the spread within herds. Preventive veterinary medicine, 96(1-2), pp.74-81. https://doi.org/10.1016/j.prevetmed.2010.06.001.
Ramirez Vasquez, N.F., Villar Argaiz, D., Fernandez Silva, J.A., Londono Pino, J., Chaparro Gutierrez, J.J. and Olivera Angel, M.E., 2016. Seroprevalence and risk factors of several bovine viral diseases in dairy farms of San Pedro de los Milagros, Antioquia, Colombia. CES Medicina Veterinaria y Zootecnia, 11(1), pp.15-25.
Romero-Salas, D., Ahuja-Aguirre, C., Montiel-Palacios, F., Garcia-Vazquez, Z., Cruz-Romero, A. and Aguilar-Dominguez, M., 2013. Seroprevalence and risk factors associated with infectious bovine rhinotracheitis in unvaccinated cattle in southern Veracruz, Mexico. African journal of microbiology research, 7(17), pp.1716-1722. https://doi.org/10.5897/AJMR12.1334.
Sadri, R., 2012. A new way of occurrence and serodiagnosis for Infectious Bovine Rhinotrchitis in Iranian cattle herds. Iranian Journal of Veterinary Medicine, 6(2), pp.99-103. https://doi.org/10.5897/AJMR12.1334.
Sakhaei, E.A., Khalili, M. and Kazeminia, S., 2009. Serological study of bovine viral respiratory diseases in dairy herds in Kerman province, Iran. Iranian Journal of Veterinary Research. 10, pp.49-53.
Segura-Correa, J.C., Zapata-Campos, C.C., Jasso-Obregon, J.O., Martinez-Burnes, J. and Lopez-Zavala, R., 2016. Seroprevalence and risk factors associated with bovine herpesvirus 1 and bovine viral diarrhea virus in North-Eastern Mexico. Open veterinary journal, 6(2), pp.143-149. https://doi.org/10.4314/ovj.v6i2.12.
Shirvani, E., Lotfi, M., Kamalzadeh, M., Noaman, V., Bahriari, M., Morovati, H. and Hatami, A., 2012. Seroepidemiological study of bovine respiratory viruses (BRSV, BoHV-1, PI-3V, BVDV, and BAV-3) in dairy cattle in central region of Iran (Esfahan province). Tropical animal health and production, 44(1), pp.191-195. https://doi.org/10.1007/s11250-011-9908-z.
Sibhat, B., Ayelet, G., Skjerve, E., Gebremedhin, E.Z. and Asmare, K., 2018. Bovine herpesvirus-1 in three major milk sheds of Ethiopia: Serostatus and association with reproductive disorders in dairy cattle. Preventive veterinary medicine, 150, pp.126-132. https://doi.org/10.1016/j.prevetmed.2017.12.019.
Solis-Calderon, J.J., Segura-Correa, V.M., Segura-Correa, J.C. and Alvarado-Islas, A., 2003. Seroprevalence of and risk factors for infectious bovine rhinotracheitis in beef cattle herds of Yucatan, Mexico. Preventive Veterinary Medicine, 57(4), pp.199-208. https://doi.org/10.1016/s0167-5877(02)00230-1.
van Schaik, G., Dijkhuizen, A.A., Huirne, R.B., Schukken, Y.H., Nielen, M. and Hage, H.J., 1998. Risk factors for existence of Bovine Herpes Virus 1 antibodies on nonvaccinating Dutch dairy farms. Preventive Veterinary Medicine, 34(2-3), pp.125-136. https://doi.org/10.1016/s0167-5877(97)00085-8.
van Schaik, G., Schukken, Y.H., Nielen, M., Dijkhuizen, A.A. and Benedictus, G., 2001. Epidemiology: Risk factors for introduction of BHV1 into BHV1-free Dutch dairy farms: A case-control study. Veterinary Quarterly, 23(2), pp.71-76. https://doi.org/10.1080/01652176.2001.9695085
Waldeck, H.F., van Duijn, L., Mars, M.H., Santman-Berends, I.M., Biesheuvel, M.M. and van Schaik, G., 2021. Risk Factors for Introduction of Bovine Herpesvirus 1 (BoHV-1) Into Cattle Herds: A Systematic European Literature Review. Frontiers in Veterinary Science, 8, Article Number: 688935, pp.1-10. https://doi.org/10.3389/fvets.2021.688935.
Acknowledgements
We want to thank the General Veterinary Administration of Yazd Province and the Razi Vaccine and Serum Research Institute for their financial and laboratory support.
Funding
This research project was funded by the Veterinary Administration of Yazd Province, Iranian Veterinary Organization.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Omid Karimi, Morteza Bitaraf Sani, Mehran Bakhshesh, Javad Zareh Harofteh, and Hamid Poormirzayee. The first draft of the manuscript was written by Omid Karimi, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Razi Vaccine and Serum Research Institute (Date: Nov 25. 2018. Ethics committee code: 4–64–18–87071).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karimi, O., Bitaraf Sani, M., Bakhshesh, M. et al. Prevalence of bovine herpesvirus 1 antibodies and risk factors in dairy cattle of Iran’s central desert. Trop Anim Health Prod 55, 23 (2023). https://doi.org/10.1007/s11250-022-03426-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-022-03426-x