Abstract
In this study, changes in the chemical composition, pH, cell wall and degradability of ensiled rice straw were investigated when treated with alkali, acid, oxidant agents (1–11 %, w/w) and a combination of an oxidant with either an alkali (ALHP) or an acid (ACHP). The findings of the study revealed that ALHP had a lower efficiency in enhancing fibre degradability compared to alkali alone. Oxidant treatment showed no detectable changes in pH, dry matter (DM) and phenolic compound (PC) solubility, or in silica and fibre content, but led to increased esterified groups formed within the cell wall constituents (hemicellulose and lignin). Increasing acid concentration led to an exponential change in both pH and solubility of DM and hemicelluloses while it quadratically increased PC and silica solubility. Moreover, crystallinity, hydrogen bonding and esterification were enhanced under high acid concentrations (11 %), but decreased under mild acid conditions (5 %). Increased alkalinity led to the linear enhancement of DM and PC solubility. Solubility of silica and hemicellulose did not exhibit any significant changes with alkali concentration above 7 %. A gradual enhancement (29 %) was observed in ruminal DM degradability with increasing oxidant concentration, whereas exponential (91 %) and quadratic (23 %) enhancements were observed with alkaline and acid treatments, respectively. Treatment with acid showed observable reductions in the degradability of both cellulose and hemicellulose, whereas oxidant treatment reduced only that of hemicellulose. Treatment with 7 % alkali (pH ∼ 12) followed by ensiling appeared to be a promising process for improving rice straw quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cereal straw is a potentially large source of energy for ruminants as its gross energy is similar to that of conventional forages (Jung et al. 1999). However, the low availability of straw structural carbohydrates leads to their limited digestibility and intake and, thereby, to their inadequate supply of net energy. Crop residues can be considered as a potential ingredient in the diet of producing animals if their digestibility can be improved (Cameron et al. 1990, 1991; Wanapat et al. 2009). Silicified surface layer, lignin and associated phenolics along with the intrinsic properties of rice straw (RS) cell wall carbohydrates such as crystallinity and substitution of esterified groups on xylan backbone are the major constraints to the digestion of rice straw (Agbagla-Dohnani et al. 2003; Van Soest 2006; Taherzadeh and Karimi 2008). A variety of chemical and biological treatments have been employed to improve rice straw quality (Van Soest 2006). Treatment with sodium hydroxide is generally regarded as the most effective (Sarnklong et al. 2010). The oldest treatment using this alkali, called the Beckman method, involves soaking straw in a solution of sodium hydroxide followed by drainage of the liquid (wet method). This process greatly enhances digestibility but only at the cost of a considerable loss of dry matter (DM) (Van Soest 2006). Efforts to increase nutrient recovery have resulted in the development of spray treatment methods (dry method), with the disadvantage that the animal is forced to consume the added alkali that causes heavy urination and faster rumen washout (Van Soest 2006). Hydrogen peroxide in an alkaline medium (alkaline hydrogen peroxide, AHP) is another chemical widely used for the delignification of lignocelluloses (Sun et al. 2001; Li et al. 2012). It has been shown that at pH 11.5, peroxide decomposition products such as hydroperoxide anion (HOO−) as well as radicals of hydroxyl (HO•) and superoxide anion (O2•) give rise to the disruption of lignin structures (Sun et al. 2001). Moreover, Cameron et al. (1990) have shown that lactating dairy cows fed with AHP-treated wheat straw exhibit a milk production behaviour similar to those fed with lucerne and maize silage. However, to the best of our knowledge, no study has been reported in the literature on pH control using the dry method for animal feed processing. Moreover, few basic studies have been reported on changes in the chemical and cell wall characteristics of rice straw due to different chemical treatments.
The objective of the present study is to adjust pH conditions for the dry treatment of rice straw using hydrogen peroxide. A second objective is to evaluate the effect of different treatments (using alkaline, acidic and oxidative agents) on the chemical characteristics of ensiled rice straw in order to improve its ruminal digestibility.
Materials and methods
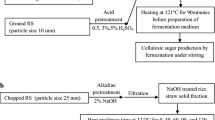
Dry chemical treatment and ensilage
RS was collected from fields in Lenjan in central Iran and chopped to 1.4 cm nominal in length. Chemical treatments included conventional AHP (5 % (w/w) NaOH followed by adding 2 % H2O2 as described in Cameron et al. 1990), pH adjustment to 3 (ACHP) and 11.5 (ALHP) followed by adding 2 % hydrogen peroxide and alkali (NaOH, 98 %), acid (H2SO4, 98 %) and oxidant (H2O2, 30 %) additions at ratios of 1, 3, 5, 7, 9 and 11 % (w/w). The treated straws were compared to a normal ruminant forage of lucerne hay. The amount of alkali or acid required for adjusting pH (to 3 and 11.5) was estimated using titratable capacity. A sample of ground RS was weighed (1.0 g) and suspended in 100 ml of deionized water under continuous mixing with a magnetic stir bar. The suspension was slowly titrated to pH 3 or 11.5 by adding sodium hydroxide or sulphuric acid (0.1 N).
The amount of water used was 1 l/kg RS. The chemical was dissolved in water in all the treatments, sprayed onto the straw, manually mixed and finally compacted tightly in PVC silos (10 cm across and 70 cm high with a one-way valve at the bottom). All silos were kept at room temperature for 5 weeks. After the ensiling period, samples of ensiled RS were dried in a ventilated barn for 5 days and then milled through a 1-mm screen prior to chemical analysis.
Chemical characterization
Pre-ensiling and post-ensiling pH values were determined by adding 10 g of each sample to 200 ml of distilled water. DM values of the straw samples were determined using forced air oven at 105 °C for 16 h (AOAC 2000). Sequential analyses for neutral detergent fibre (NDF; using a heat stable alpha amylase) and acid detergent fibre were measured using an Ankom200/220 fibre analyser (Ankom Technology Corp., Fairport, NY, USA) according to the methods described in Van Soest et al. (1991). Acid detergent lignin (ADL) content in the residues was determined using sulphuric acid (72 %) in a DaisyII incubator (Ankom Technology Corp.) for 3 h at room temperature. To determine the insoluble silica (using method 965.07; AOAC 2000), a sample of RS (250 mg) was suspended in 45 ml of the Na–K buffer solution (pH = 7; Lau and Van Soest 1981) and extracted into an incubator shaker at 39 °C and 20 rpm for 24 h before subjecting to digestion (Elliott and Snyder 1991). Phenolic compounds in RS were also extracted in a manner similar to that used for silica. The liquid sample was centrifuged (10,000×g, 20 min), and the method described by Wang et al. (2004) was used to determine the concentration of soluble phenolic compounds (PC) in the supernatant. Finally, the effects of chemical treatments on DM solubility were evaluated (Liu and Ørskov 2000).
Fourier transform infrared spectroscopy and scanning electron microscopy analyses
The Fourier transform infrared spectroscopy (FTIR) system (Bruker Tensor 27) was used to investigate and quantify the chemical changes in cell wall constituents (i.e. cellulose, hemicellulose and lignin). For this purpose, cell walls of not only lucerne hay but also those of both untreated RS and those treated with AHP, 5 and 11 % sodium hydroxide, sulphuric acid and hydrogen peroxide were collected after extraction using a neutral detergent solution (Van Soest et al. 1991). The samples were then finely ground and pressed uniformly against the diamond surface using a spring-loaded anvil to record mid-IR spectra from 600 to 4,000 cm−1 at a resolution of 2 cm−1. For scanning electron microscopy (SEM) analysis, untreated and treated RS samples were incubated in the rumen for 24 h. The samples were subsequently fixed, dehydrated (Agbagla-Dohnani et al. 2003), affixed and sputter-coated with gold (BAL-TEC SCD 005). The epidermis silicified layers were observed using a SEM (PHILIPS, XL30) at 10 kV.
Ruminal DM and fibre degradability
For degradability estimations, F57 bags (25 μm pore size; ANKOM Technology Corp.) were filled with 500 mg of straw. The bags were put into a larger loose mesh cloth sack (50 × 50 cm; 2 mm pore size) and incubated in the rumen of two non-lactating fistulated cows for 24 and 48 h. The 24-h incubation time was used in order to determine changes in the forage degradation during the normal time that feed would be expected to be retained in the rumen of a high producing dairy cow (Eun et al. 2006). The cows were individually housed in 4 × 4-m covered pens where they received a dry cow total mixed ration (45 % NDF and 11.9 % CP). After incubation, the bags were removed from the rumen and hand-washed with tap water until they were clear.
Data analysis
Chemical composition and degradability analyses were accomplished in three and four replicates, respectively. Data were analysed in a completely randomized design using the GLM procedure of SAS (SAS Institute 2001). Polynomial contrasts were used to determine linear and quadratic responses to the reagent concentrations.
Results
Chemical characterization and ruminal degradability
Titratable capacity analysis suggested that 4.1 % alkali and 2.38 % acid were necessary additions to adjust the initial pH value of the untreated RS to 11.5 and 3, respectively, while experimental results showed the corresponding pH values of 11.40 and 2.88 (Table 1). Similar pre-ensiling and post-ensiling pH values were achieved for acid treatments, whereas post-ensiling pH values were substantially reduced for oxidative (2–3 U) and alkaline (1–4 U) treatments (Fig. 1). The solubility levels of DM and PC were greater when treated with AHP compared to that treated with sodium hydroxide (5 %) alone (Table 1). However, AHP gave no advantage over that of alkali treatment with respect to DM degradability. On the contrary, RS treated only with alkali exhibited higher cellulose and hemicellulose degradability than the one treated with AHP. ADL content in the lucerne hay (8.5 %) was almost twice that in raw RS (4.2 %). The lowest ADL value (3.4 %) was observed in RS treated with ACHP. Lucerne hay showed higher values of DM solubility (26.6 %) and degradability (53.2 %), whereas substantially higher values were obtained for fibre (hemicellulose) degradability in both untreated and treated RS samples (P < 0.05).
Effects of reagent concentrations (1–11 %, w/w), including sodium hydroxide (square), sulphuric acid (triangle) and hydrogen peroxide (circle), on chemical characteristics and ruminal degradability of rice straw: a pre-ensiling pH, b post-ensiling pH, c dry matter (DM) solubility, d cellulose, e hemicellulose, f lignin, g silica, h phenolic compounds (PC), i DM degradability after 48 h ruminal incubation, j DM, k hemicellulose (HC) and l cellulose (CL) degradability after 24 ruminal incubation
Minor increases were observed in DM solubility (22 %) as well as in DM and cellulose degradability (29 %) in response to increased hydrogen peroxide concentration. Another effect of the process was the quadratic decrease in hemicellulose degradability (P < 0.01; Fig. 1). The highest concentration of hydrogen peroxide (11 %) exhibited little hydrolytic effect on hemicellulose (12 %) and lignin (14 %) and caused no significant influence on PC, silica and cellulose contents (P > 0.05). In contrast, a greater level of hemicellulose hydrolysis occurred with acid treatments (P < 0.01), reaching its maximum at an acid concentration of 9 %. PC and silica solubilities and DM degradability quadratically changed (P < 0.01) with increasing acid concentration. However, fibre degradability linearly decreased as acid concentration increased (P < 0.01). When RS was treated with increasing quantities of alkali, a linear increment was observed in DM and PC solubility values (P < 0.01). Similarly, ruminal DM and cellulose degradability linearly increased by up to 7 % when sodium hydroxide was used (P < 0.01). Maximum quantities of sodium hydroxide led only to a small rise in DM degradability (P < 0.05). Besides, hemicellulose degradability and silica solubility reached their maximum levels at an alkali concentration of 7 % (P < 0.05), beyond which no further effects were observed (P > 0.05). Cellulose content remained constant in all the different treatments (P > 0.05). The highest concentrations of hydrogen peroxide and sodium hydroxide decreased RS lignin (ADL) content from its original 4.2 to 3.6 and 3.1 %, respectively.
FTIR and SEM analysis
The broad band at 3,230–3,570 cm−1 is assigned to hydrogen-bonded OH groups in cellulose (Fig. 2; Kobayashi et al. 2009). The absorbance peaks (×10−2) at 3,335 cm−1 were 1.96, 1.26, 1.90, 1.76, 2.67, 1.55, 1.59, 1.14 and 2.21, respectively, for lucerne hay, untreated RS and RS treated with AHP, 5 and 11 % hydrogen peroxide, sodium hydroxide and sulphuric acid. The dry treatment of RS with an acid concentration of 11 % enhanced the absorbance ratio A1367/A2922 (total crystallinity), while minor changes were observed in RS samples treated with AHP, hydrogen peroxide or sodium hydroxide. The absorbance band at 1,728 cm−1 is attributed to the hemicellulose acetyl and uronic ester groups or to linkages in lignin and/or ester hemicellulose ferulic and p-coumaric acid carboxylic groups (Kumar et al. 2009). The values (×10−2) for the corresponding band were 1.59, 0.89, 0.99, 1.15, 1.56, 0.83, 0.78, 0.72 and 1.09 for lucerne hay, untreated RS and those treated with AHP, 5 and 11 % hydrogen peroxide, sodium hydroxide and sulphuric acid, respectively. Using 11 % sodium hydroxide completely eliminated the encrusting silicified layer (Fig. 3). This layer was unaffected by any of the AHP, hydrogen peroxide or sulphuric acid treatments.
Scanning electron micrographs of epidermis surface layer in untreated and treated straw samples after 24 h incubation in the rumen; bar, 20 μm: a untreated, b alkaline hydrogen peroxide, c 5 % sodium hydroxide, d 11 % sodium hydroxide, e 5 % sulphuric acid, f 11 % sulphuric acid, g 5 % hydrogen peroxide and h 11 % hydrogen peroxide
Discussion
AHP treatment is one of the most effective methods used to achieve an improved lignocellulosic enzymatic hydrolysis (Sun et al. 2001). Using this treatment, cows fed with a wheat straw diet have shown production responses similar to those fed with the control diet containing conventional forage (Cameron et al. 1990). The results of the present study showed no improvements in degradability, neither with the controlled nor with the non-controlled AHP treatment, over the simple alkali treatment. In fact, the dry treatment using AHP led to an increase in cellulose rigidity (hydrogen bonds) which is believed to significantly limit the enzymatic saccharification of cellulose (Kumar et al. 2009). Moreover, the enhanced esterified groups within the cell wall components in the hydrogen peroxide treatment might have increased the cross-linkages, thereby reducing hemicellulose digestibility (Li et al. 2012). Further studies are required to determine the optimum conditions for AHP treatment using the dry processing method.
Regarding cellulose resistance to chemical treatments, it was found that the increased crystallinity at the highest acid concentration (11 %) could be the result of the increased cellulose/hemicellulose ratio or the net crystallization of amorphous cellulose. The crystallized regions of cellulose may be claimed to be less hydrolyzed than the noncrystallized regions due to both their low surface area and unavailability of endocellulase (Taherzadeh and Karimi 2008). However, an acid concentration of 5 % had no negative impacts but resulted in enhanced DM degradability. The presence of acid or the low pH values of the acid treated RS and the associated inhibitory effect on microbial activity may explain the reduced fibre degradation with various acid concentrations (Sung et al. 2007). As Mourino et al. (2001) suggested, under substrate-limited conditions, the rate constant of cellulose digestion is a linear function of pH at which cellulose digestion is initiated, an effect that might be due to the reduced bacterial adhesion at low pH levels. On the other hand, fibre degradability was significantly improved in the alkali treatments in which not only hemicellulose and saponified esterified groups are solubilized but also the highest alkali concentration reduces lignin and eliminates the cuticle silica layer. These effects increase the swelling capacity of the cell walls and facilitate the penetration of microbial enzymes that lead to greater digestion of structural carbohydrates (Hendriks and Zeeman 2009). Treatment with sodium hydroxide at concentrations higher than 7 % increased DM solubility but failed to significantly increase ruminal fibre degradability. Spray treatment methods avoid dry matter losses, but may simultaneously suppress the voluntary intake of straw due to high alkalinity (Van Soest 2006). Therefore, the relative alkali requirement may not be anywhere near the amount needed to achieve maximum increase in digestibility. Decreasing pH level in the alkali-treated straw followed by ensiling is an effective way to reduce alkalinity. Another problem encountered in treating straw with high levels of sodium hydroxide is that the increased digestibility levels commonly observed in the in vitro measurements may not be realized in animal digestion (Van Soest 2006). This may be ascribed to both the increased rate of feed passage by the treatment (reduction in forage particle size) and to the high levels of the dietary sodium (Berger et al. 1980). No such effect has, however, been reported for dairy cows fed with diets containing up to 52 % AHP-treated oat hulls (Cameron et al. 1991). Dilution with other feeds may be a practical solution to overcome problems with sodium hydroxide-treated straw. Lucerne hay showed greater DM degradability as compared to RS treated with sodium hydroxide at concentrations lower than 7 %. This might be a consequence of its greater DM solubility (cell content). The greater in vivo fibre degradability in grasses as compared to legumes is due to the greater particle fragility and shorter rumen retention time of legumes (Oba and Allen 1999; Pinos-Rodrıguez et al. 2002). In this study, however, the higher 24-h fibre degradability in RS than that in lucerne hay shows another possibility that the low fibre degradability in lucerne hay is due to its higher lignin content and fibre crystallinity as well as its hydrogen and ester bonds.
Conclusions
The results obtained from this study indicate that titratable capacity can be a suitable predictor for adjusting pH under dry processing conditions. Considering the reagent consumption and degradability, treatment with sodium hydroxide at a concentration of 7 % (pH ∼ 12) followed by ensilage may be regarded as the most effective dry method to improve the ruminal degradability of rice straw.
References
Agbagla-Dohnani, A., Noziere, P., Gaillard-Martinie, B., Puard, M., and Doreau, M., 2003. Effect of silica content on rice straw ruminal degradation, Journal of Agricultural Science, Cambridge 140, 183–192.
AOAC, 2000. Official methods of analysis, 17th ed. Association of Official Analytical Chemists, Washington, DC.
Berger, L.L., Klopfenstein, T.J. and Britton, R.A., 1980. Effect of sodium hydroxide treatment on rate of passage and rate of ruminal fiber digestion, Journal of Animal Science, 50, 745–749.
Cameron, M.G., Fahey Jr, G.C., Clark, J.H., Merchen, N.R. and Berger, L.L., 1990. Effects of feeding alkaline hydrogen peroxide-treated wheat straw-based diets on digestion and production by dairy cows, Journal of Dairy Science, 73, 3544–3554.
Cameron, M.G., Cameron, M.R., Fahey Jr, G.C., Clark, J.H., Berger, L.L. and Merchen, N.R., 1991. Effects of treating oat hulls with alkaline hydrogen peroxide on intake and digestion by midlactation dairy cows, Journal of Dairy Science, 74, 177–189.
Elliott, C.L. and Snyder, G.H., 1991. Autoclave-induced digestion for the colorimetric determination of silicon in rice straw, Journal of Agricultural Food Chemistry, 39, 1118–1119.
Eun, J.-S., Beauchemin, K.A., Hong, S.-H. and Bauer, M.W., 2006. Exogenous enzymes added to untreated or ammoniated rice straw: Effects on in vitro fermentation characteristics and degradability, Animal Feed Science and Technology, 131, 86–101.
Hendriks, A.T.W.M. and Zeeman, G., 2009. Pretreatments to enhance the digestibility of lignocellulosic biomass, Bioresource Technology 100, 10–18
Jung, H.G., Varel, V.H., Weimer, P.J. and Ralph, J., 1999. Accuracy of Klason lignin and acid detergent lignin methods as assessed by bomb calorimetry, Journal of Agricultural and Food Chemistry, 47, 2005–2008.
Kobayashi, G.P., Okada, N.N., Hirakawa, A., Sato, T., Kobayashi, J., Hatano, Sh., Itaya, Y. and Mori, Sh., 2009. Characteristics of solid residues obtained from hot-compressed-water treatment of woody biomass, Industrial and Engineering Chemistry Research, 48, 373–379.
Kumar, R.G., Mago, V., Balan, Ch. and Wyman, E., 2009. Physical and chemical characterizations of corn stover and poplar solids resulting from leading pretreatment technologies, Bioresource Technology, 100, 3948–3962.
Lau, N.M., and Van Soest, P.J., 1981. Titratable groups and soluble phenolics compounds as indicators of digestibility of chemically treated roughages, Animal Feed Science and Technology, 6, 123–131.
Li, M., Foster, C., Kelkar, S., Pu, Y., Holmes D., Ragauskas A., Saffron, C.M. and Hodge, D.B., 2012. Structural characterization of alkaline hydrogen peroxide pretreated grasses exhibiting diverse lignin phenotypes, Biotechnology for Biofuels, 5, 38.
Liu, J.X. and Ørskov, E.R., 2000. Cellulase treatment of untreated and steam pre-treated rice straw—effect on in vitro fermentation characteristics, Animal Feed Science and Technology, 88, 189–200.
Mourino, F., Akkarawongsa, R. and Weimer, P.J., 2001. Initial pH as a determinant of cellulose digestion rate by mixed ruminal microorganisms in vitro, Journal of Dairy Science, 84, 848–859.
Oba, M., and Allen, M.S., 1999. Evaluation of the importance of the digestibility of neutral detergent fiber from forage: effects on dry matter intake and milk yield of dairy cows, Journal of Dairy Science, 82, 589–596.
Pinos-Rodrıguez, J.M., Gonzalez, S.S., Mendoza, G.D., Barcena, R., Cobos, M.A., Hernandez, A. and Ortega, M.E., 2002. Effect of exogenous fibrolytic enzyme on ruminal fermentation and digestibility of alfalfa and rye-grass hay fed to lambs, Journal of Animal Science, 80, 3016–3020
Sarnklong, C., Cone, J.W., Pellikaan, W. and Hendriks, W.H., 2010. Utilization of rice straw and different treatments to improve its feed value for ruminants: A review, Asian-Australian Journal of Animal Science, 23, 680 – 692.
SAS User’s Guide: Statistics, Version 8.1 Edition. 2001. SAS Inst., Inc., Cary, NC.
Sun, R.C., Tomkinson, J., Mao, F.C. and Sun, X.F., 2001. Physicochemical characterization of lignins from rice straw by hydrogen peroxide treatment, Journal of Applied Polymer Science, 79, 719–732.
Sung, H.G., Kobayashi, Y., Chang, J., Ha, A., Hwang, I.H. and Ha, J.K., 2007. Low ruminal pH reduces dietary fiber digestion via reduced microbial attachment, Asian-Australian Journal of Animal Science, 20, 200–207.
Taherzadeh, M.J. and Karimi, K., 2008. Pretreatment of lignocellulosic wastes to improve in ethanol and biogas production. A review, International Journal of Molecular Sciences, 8, 1–30.
Van Soest, P.J., 2006. Rice straw, the role of silica and treatments to improve quality, Animal Feed Science and Technology, 130, 137–171.
Van Soest, P.J., Robertson, J.B. and Lewis, B.A., 1991. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition, Journal of Dairy Science, 74, 3583–3592.
Wanapat, M., Polyrach, S., Boonnop, K., Mapato, C. and Cherdthong, A., 2009. Effect of treating rice straw with urea and calcium hydroxide upon intake, digestibility, rumen fermentation and milk yield of dairy cows. Livestock Science, 125, 238–243.
Wang, Y., Spratling, B.M., ZoBell, D.R., Wiedmeier R.D. and McAllister, T.A., 2004. Effect of alkali pretreatment of wheat straw on the efficacy of exogenous fibrolytic enzymes. Journal of Animal Science, 82, 198–208.
Acknowledgments
The authors would like to acknowledge Iran National Science Foundation and Isfahan Jihad-e-Agriculture for their financial support of this study. Our thanks also go to Dr. Ezzatollah Roustazadeh, from ELC of IUT, and Glenn Nader from California University for having edited the final version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghasemi, E., Khorvash, M., Ghorbani, G.R. et al. Dry chemical processing and ensiling of rice straw to improve its quality for use as ruminant feed. Trop Anim Health Prod 45, 1215–1221 (2013). https://doi.org/10.1007/s11250-012-0349-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-012-0349-0