Abstract
Chickpea straw (CS) and sunflower stalks (SS) are agricultural wastes with high fibre content and low digestibility. To improve their nutritional value and ruminal digestibility, the effects of NaOH and urea treatments combined with exogenous fibrolytic enzymes (EFE) were investigated. The untreated CS (CCS) and SS (CSS), 4% NaOH treated CS (NCS) and SS (NSS), and 4% urea-treated CS (UCS) and SS (USS) were supplemented by two enzymatic complexes (DCX and MaxFiber) composed mainly of cellulase and xylanase activities at increasing doses: 0, 1, 2, 5, and 10 μL DCX/g DM and 0, 0.5, 1, 2, and 4 mg MaxFiber/g DM. The results of in vitro ruminal fermentation proved that the DCX was more efficient than the MaxFiber complex for both CCS and CSS. Indeed, it improved the rate and the extent of ruminal fermentation, metabolizable energy, organic matter digestibility, and volatile fatty acids (p-value <0.05) by 5 %, 47%, 12%, 12.8%, and 23.8%, respectively, for CCS using 10 μl/g DM and 20.8%, 27.6%, 12.9%, 11.8%, and 22.8%, respectively, for CSS by using 5 μl/g DM. The association between alkali treatments and EFE was depending to the supplemented enzymatic complex, the treated substrate and the alkali treatment. For the CS, the association between alkali and EFE stimulated the ruminal fermentation and improved the digestive use. However, it decreased the efficiency of EFE for SS. Overall, the use of EFE to CS and SS could provide a valuable source of energy from digestible fibre for ruminants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, there has been an increasing focus on sustainable agricultural practices and the efficient utilization of agricultural waste for livestock feed. Chickpea straw and sunflower stalk are abundant agricultural residues that are underutilized and often treated as waste [1]. However, they possess significant potential as fed resources due to their high fibre content, which may provide valuable energy and nutrients for ruminants [2]. To unlock the nutritional value of these lignocellulosic wastes, enzymatic treatment is proposed as a novel approach. Previously, researches have primarily focused on chemical treatments, while enzymatic treatments remain relatively unexplored [3, 4]. The novelty of the current study focuses on the enzymatic valorization of alkali-treated chickpea straw and sunflower stalks for sustainable ruminant nutrition. The chemical treatments could modify the chemical composition and weaken the structural fibre of the treated biomass [5]. And, the exogenous fibrolytic enzymes (EFE) can break down complex carbohydrates present in the lignocellulosic biomasses, into simpler sugars, which can be more easily fermented by the ruminal microorganisms and provide a better source of energy and nutrition for the animal [6]. So, by using specific enzymes and optimizing dosages supplementation and treatment conditions, the nutritional value, the digestive use, and the feed costs could be optimized [7]. However, their use is subject to some challenges, such as the need for an appropriate balance between the type and dose of enzymatic product, the type of substrate being treated, and the desired animal response [8]. This highlights the need to further research to optimize the use of exogenous fibrolytic enzymes in agricultural waste management and ruminant nutrition. So, this research aimed to test the hypothesis that the alkali treatments of chickpea straw and sunflower stalk could improve the efficiency of EFE to enhance their nutritional value. The objective was to determine the effect of two different exogenous fibrolytic enzyme complexes at increasing dose levels on the in vitro ruminal fermentation and digestive use parameters of untreated and alkali-treated chickpea straw and sunflower stalk.
2 Material and methods
2.1 Collect and alkali treatments of chickpea straw and sunflower stalks
After chickpea and sunflower seeds harvesting, samples from chickpea straw (CS) and sunflower stalks (SS) were randomly collected from fields located in the northwest region of Tunisia. Then, the samples were manually chopped into small stands of almost 5 cm to facilitate alkali treatments. Once well homogenized, the shopped CS and SS were divided into 9 subsamples of 2 kg each. The first 3 subsamples were kept untreated for CCS and CSS. The second 3 subsamples were subject to NaOH treatment. According to Dulphy et al. [9], the CS and SS samples were pulverized by 4% NaOH solution and left uncovered during 48 h for NCS and NSS. The remaining 3 subsamples were subject to urea treatment for UCS and USS. According to Chermiti et al. [10], the CS and SS were pulverized by 4% urea solution and ensiled in hermetic plastic bags for 2 months to prevent oxygen entrance and ammonia losses. Once all treatments were ready, samples of 500 g from each CS and SS preparations (CCS, NCS, UCS, CSS, NSS, and USS) were dried in a forced air oven overnight at 55 °C until constant weight and then grounded through a 1-mm sieve using a Retsch SK 100 standard, Giessen, Germany, for subsequent analysis.
2.2 Chemical analyses
The untreated and alkali-treated CS and SS were subject to chemical analysis to determine their dry matter (DM, method ID 930.15), ether extract (EE, method ID 920.30), organic matter (OM, method ID 942.05), crude protein (CP, ID 954.01), and crude fibre (CF, ID 962.09) contents according to the methods of the Association of Official Analytical Chemists [11]. The Neutral detergent fibre (NDF, assayed with a heat stable amylase and expressed inclusive of residual ash), acid detergent fibre (ADF, expressed inclusive of residual ash), and acid detergent lignin (ADL, after extraction with sulphuric acid) were determined using the ANKOM fibre analyzer (ANKOM, A2001, New York, NY, USA) in a fibre filter bag (F57-ANKOM Technology Corporation, Macedon, NY, USA) according to Van Soest et al. [12]. The total phosphorus (P) contents were analysed by the molybdovanadate colorimetric method (method ID 965.17) using a spectrophotometer (Shimadzu UV-1201 UV-Vis). The calcium content (Ca) was measured using an atomic absorption spectrophotometer (Varian AA140, Varian, Australia) (method ID 968.08). All chemical analyses were performed in triplicate for each sample (n = 3), repeated each time the difference between replication was upper 5%, and presented in Table 1.
2.3 Exogenous fibrolytic enzymes and their enzymatic activities
The obtained CS and SS preparations (CCS, NCS, UCS, CSS, NSS, and USS) were supplemented by two different xylanase to cellulase enzymatic complexes. The first was a mixture (1:1, v/v) of two commercial products in liquid form which are Cellulase PLUS and Xylanase PLUS (DCX), produced by the fermentation of non-genetically modified Trichoderma longibrachiatum, and are composed primarily of endo-1,4-β-D-xylanase (E.C. 3.2.1.8) and endoglucanase (EC 3.2.1.4), in addition to other side additional activities such as pectinase, mannanase, amylase, and protease. The DCX was supplemented at increasing dose levels as recommended by Jabri et al. [13]: 0, 1, 2, 5, and 10 μl/g DM. The supplementation was performed by diluting the DCX complex with distilled water (10-fold) and directly sprayed onto the grounded CS and SS preparations with the appropriate dose/g DM. The second enzymatic complex is a commercial protein rich by-product in powdered form, obtained from solid-state fermentation of Aspergillus strains and Neurospora intermedia, contained xylanase, endoglucanase, and exoglucanase activities. The MaxFiber was also supplemented at increasing dose levels according to the manufacturer instructions: 0, 0.5, 1, 2, and 4 mg/g DM. Both enzymatic complexes (DCX and MaxFiber) were assessed in triplicate in each of three runs (n=9) for the xylanase (EC 3.2.1.8, Endo-β-1,4-xylanase), exoglucanase (EC 3.2.1.91, Exo-β-1,4-glucanase), and endoglucanase (EC 3.2.1.4, Endo-β1,4-glucanase) activities according the methods of Wood and Bhat [14] and Bailey et al. [15] (Table 2).
2.4 In vitro ruminal fermentation
The in vitro ruminal fermentation using batch culture technique according to Theodorou et al. [16] was used in this study according the ruminal fermentation workflow (Fig. 1). To collect the fresh ruminal fluid, two cannulated non lactating cows (600–650 kg body weight) were fed a stable diet composed of oat hay ad libitum and 2 kg commercial concentrate formulated for dairy cows (Alfa® 7 standard) with free access to water and mineral/vitamin licks to meet the nutritional requirements as recommended by INRA [17]. The ruminal fluid was collected before morning feeding in prewarmed insulated flasks, from different sites within the rumen via electric pump, and then immediately transferred to the lab and strained through 4 layers of cheesecloth under anaerobic conditions. The fermentation inoculum was prepared by mixing the freshly collected ruminal fluid and the anaerobic buffer medium (pH=6) prepared in advance as described by Menke and Steingass [18] in a ratio of 1:2 (ruminal fluid: buffer medium). Samples of dry 200 ± 10 mg DM ground CCS, NCS, UCS, CSS, NSS, and USS were weighed in the fermentation bottles in triplicate each and then supplemented with the corresponding EFE dose level 20 h before the in vitro incubation as recommended by Beauchemin et al. [19]; then all fermentation bottles were filled with 30 ml of fermentation inoculum, immediately sealed with a butyl rubber stopper and an aluminium crimp cap, and incubated at 39 °C water bath for 96 h. All in vitro ruminal fermentation preparation steps were performed under continuous flushing with CO2 at 39 °C water bath. To ensure results accuracy, negative control bottles containing inoculum fermentation without substrate and positive control bottles containing substrate without enzymatic supplementation (0 μl DCX/g DM and 0 mg MaxFiber/g DM) were used in six replications each. The in vitro ruminal fermentation run was repeated three times with the same procedure (n=9). The incubation was repeated each time the difference of gas production (GP) in positive control bottles was larger than 5% between runs. The GP was measured for each bottle after 2, 4, 6, 8, 12, 24, 48, 72, and 96 h of incubation by inserting a 23-gauge (0.6 mm) needle attached to a pressure transducer connected to a visual display. After each measurement, the transducer was removed, leaving the needle in place to permit venting.
2.5 Calculations and statistical analysis
The measured gas pressures for each bottle were converted to gas volume using the following equation:
where GPr is the recorded gas pressure (bar); Vf is the volume of serum bottle (=117.39 ml), Vi is the volume of inoculum added to each bottle, and Patm is the atmospheric pressure (= 1.01325 bar).
Subsequent to the GP measurement, the metabolizable energy (ME), organic matter digestibility (OMD), and volatile fatty acids (VFA) were determined according to Menke and Steingass [18] and Getachew et al. [20] prediction models:
The measured GP kinetics were fitted using the residual least square method of the reduced generalized gradient algorithm of the solver function in Microsoft Excel software according to Groot et al. [21] model:
where A is the estimated potential GP (ml/g DM); B is the required time to produce ½ A (h); C is the curve sharpness. The parameters maximum rate of GP (Rmax) and the time at which Rmax is attained (Tmax) were calculated according to Yang et al. [22] as Eqs. (6) and (7):
All collected data were analysed as a completely randomized design and were conducted using the GLM procedure of SAS Studio (3.6) (2017) according the following statistical model Yijk = μ + Di + Tj + (Di * Tj) + εijk, where Yijk is an individual observation for each dependent variable, μ is the overall mean, Di is the fixed effect of the supplemented EFE dose rate, Tj is the fixed effect of chemical treatment, (Di * Tj) is the interaction between the chemical treatment and the EFE dose rate, and εijk is the residual error.
The mean values of each sample were used as the experimental unit. The polynomial contrasts (linear and quadratic effects) of increasing dose levels supplementation were determined. As the studied dose levels are unequally spaced, the Proc IML from SAS® studio (3.6) (2017) was used to generate coefficients for polynomial contrasts.
The differences between dose levels means were assessed using the multivariate Duncan test [23]. Means were considered significantly different at p-value less or equal to 5% and tendencies were declared at 0.05<p-value<0.1.
3 Results and discussion
3.1 Chemical composition of untreated and alkali-treated chickpea straw and sunflower stalks
The chemical composition of the studied by-products CCS and CSS revealed that both are high fibrous lignified biomasses containing 56.9% and 63.4% NDF, 35.5% and 47.1% ADF, and 7.3% and 11.1% ADL, respectively. So, the cellulose content varied between 28.2 and 36%, the hemicellulose between 21.4 and 16.3%, and the ADL between 7.3 and 11.1% for CCS and CSS, respectively (Table 1). These results were different to those previously reported by de Souza et al. [24], Durmaz and Ates [25], and Maheri-Sis et al. [26]. These differences could be related to the plant species, genetics, and growth conditions. It is noteworthy that different factors could interact with each other’s causing high variability in plant’s chemical composition. Comparing to cereal straw, which is a commonly used by-product for livestock feeding [27], both CS and SS presented an interesting nutritive value richer in CP by 3.7% and 5.7%, respectively, against 3.2% for wheat straw [27], which could significantly contribute to ruminant feeding.
The alkali treatment of CS and SS modified their chemical composition as presented in Table 1. The urea treatment improved the solubilization of hemicellulose and ADL contents by 28.7% and 13.1%, respectively, of UCS as compared to the CCS. As for the SS, it caused lignin and cellulose solubilization by 26.1% and 10.5%, respectively, as compared to the CSS. The NaOH treatment caused a significant solubilization of hemicellulose, cellulose, and ADL especially for the NCS which their contents decreased by 15.8%, 14.8%, and 37.0% respectively (Table 1). Generally, the use of NaOH and urea treatments for agricultural by-product led to an increase of cellulose, hemicellulose, and lignin solubility [28]. However, the efficacy of alkali treatments could vary depending on a number of factors, including the substrate properties, the alkali concentration and its mode of action, the pH of the treatment environment, the temperature, and the duration of the treatment [29]. Indeed, the NaOH is a strong alkali that can dissolve hemicellulose, cellulose, and lignin through depolymerization [30], whereas the urea is an organic compound that can also dissolve lignocellulosic content by breaking the hydrogen bonds between the polysaccharides in anaerobic conditions [31]. The ash content improved significantly after NaOH treatment by 82.9% for NCS and 81.4% for NSS, which could be attributed to the residual NaOH [32]. Moreover, the urea treatment improved significantly the crude protein content for both UCS and USS (Table 1). During the urea treatment, the pH and temperature increased, creating a favourable environment for microorganisms’ growth and protein synthesis from the urea non-protein nitrogen [33, 34].
3.2 Effect of EFE supplementation on in vitro ruminal fermentation of untreated and alkali-treated CS and SS
Both studied EFE complexes (DCX and MaxFiber) supplied xylanase, endoglucanase, and exoglucanase activities under ruminal conditions (pH= 6.6, T°= 39 °C) with a xylanase to cellulase ratio equal to 1.5 and 0.75, respectively, as presented in Table 2.
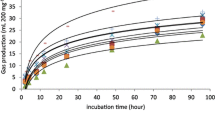
As presented in Figs. 2 and 3, Tables 4 and 6, the in vitro ruminal fermentation results proved that the fermentation profile, the extent and the rate of GP, and the digestive use parameters of CCS and CSS are comparable to cereal straw [27] and higher than some industrial by-products like sesame seed coats [35] and olive cake [36, 37]. Indeed, the CCS and CSS could be an interesting source of nutrients, such as fibre, which is an important diet component for ruminant as it promotes rumen health and function, and it is essential for maintaining optimal animal performances. However, the physical structure of the sunflower stalks can be abrasive to the animal’s mouth and may cause dental issues if not properly processed before inclusion in the diet. So, both chickpea straw and sunflower stalk could be included in ruminant diet; however, it’s important to consider the nutritional value and the physical properties of the biomass, as well as the animal’s requirements. Indeed, properly balancing ruminant’s diet with other feedstuffs, applying adequate processing techniques such as chopping, grinding or ensiling, and adjusting the inclusion levels can help to optimize the digestive use of these agricultural by-products by ruminants.
The NaOH and urea treatments of CS and SS caused variable effects on the in vitro ruminal fermentation due the modifications of their chemical composition. Indeed, for the NCS, the NaOH treatment improved slightly the rate of GP and the digestive use parameters by 3.0% (Rmax), 4.0% (ME), 5.6% (OMD), and 7.1% (VFA) as compared to CCS. As the urea treatment improved the rate and the extent of chickpea straw by 8.2% (A) and 31.8% (Rmax), then the estimated digestive use parameters by 26.0% (ME), 28.5% (OMD), and 16.6% (VFA), as compared to the CCS. The NaOH and urea treatments improved slightly the ruminal fermentation of CS by promoting the hemicellulose and lignin hydrolysis, making the cellulose more accessible to ruminal microorganisms, which can lead to faster and more efficient fermentation of CS as compared to wheat straw [37]. On the other hand, for the SS, both studied alkali treatments decreased the ruminal fermentation profile, and all studied parameters of in vitro ruminal GP and digestive use as compared to the CSS (Fig. 1, Tables 3 and 4). This finding could be attributed to the initial high content of lignin which is more resistant to ruminal fermentation. The NaOH and urea treatments can break down these components, but they can also make them less accessible to rumen microorganisms, decreasing the fermentation efficiency as found by Moradi et al. [38] for pistachio by-products. So, the NaOH and urea treatments efficiency is dependent to the substrate initial lignocellulosic matrix.
The EFE supplementation effects at increasing doses on untreated and alkali-treated CS and SS are depicted in Figs. 1 and 2 and Tables 3, 4, 5, and 6. During this study, the considered optimal dose was the minimum dose required to obtain the greatest significant improvement for the studied fermentation parameters as suggested by Eun et al. [39]. The effect of both studied EFE enzymatic complexes depended significantly to the supplemented dose level and the alkali treatment for most studied parameters as the GP kinetic (Tables 3 and 5) and the in vitro ruminal fermentation and the digestive use parameters (Tables 4 and 6).
Both studied EFE complexes (DCX and MaxFiber) exerted quadratic effects at increasing doses on the GP kinetic, the in vitro ruminal fermentation, and digestive use parameters of CCS and CSS. The same tendencies were recorded by Yang et al. [40] using EFE derived from Trichoderma reesei on ruminal degradability of faba bean silage. This means that as the EFE dosage increased, the fermentation efficiency of the substrate also increased until it reaches optimal improvement, after which further increase in enzyme dosage results in a decrease in fermentation efficiency. However, Souza et al. [41] recorded linear effects of the supplemented EFE on the in vitro ruminal degradation, gas production, and fermentative profile of maize silage and sugarcane silage, whereas Arriola et al. [7] found that the EFE could be ineffective on ruminant’s digestibility. Accordingly, it is important to note that the effect of EFE supplementation depend on the type of the supplemented substrate, the enzyme source, the supplemented fibrolytic activity, and the xylanase to cellulase ratio [13]. For both studied by-products (CCS and CSS), the effect of EFE supplementation was variable depending on the type of used enzymatic complex. Indeed, for the CS, the optimal improvements (p-value <0.05) were recorded by supplementing the DCX complex at the optimal dose D10 at 5 %, 47%, and 31% for A, Rmax, and Tmax, respectively, as compared to the CCS control (D0). Accordingly, the digestive use parameters improved (p-value <0.05) by 12% (ME), 12.8% (OMD), and 23.8% (VFA) as compared to the control D0. As for the CSS, the optimal improvements were recorded by the optimal dose D5= 5 μl/g DM of DCX by 20.8% (A), 27.6% (Rmax), 12.9% (ME), 11.8% (OMD), and 22.8% (VFA). The MaxFiber complex improved only the in vitro ruminal fermentation parameters by 11.6% and 28.7%, respectively, for A and Rmax by the dose M1=1 mg/g DM. Therefore, we may conclude that the DCX complex was more effective as it improved (p-value <0.05) the rate and the extent of GP and then the estimated digestive use parameters of CCS and CSS by their optimal doses D10= 10 μl/g DM and D5= 5 μl/g DM, respectively, proving the presence of enzyme-substrate specificity [42].
Fibrolytic enzymes could modify the cell wall structure of some forages by hydrolyzing polysaccharides bonds and breaking down the cell wall into smaller, more readily soluble molecules [6], which prepares the cell wall to ruminal microorganism attachment and provides endogenous enzymes greater access to the cell wall matrix [42,43,44,45]. These modifications facilitate the extraction of valuable compounds such as sugars and other biomolecules from the plant material, which could help to improve the nutritional value of agricultural wastes for livestock [46]. Consequently, ruminant’s performances could improve. In fact, Jabri et al. [4] support these results by recording improvement of lamb’s average daily gain and nutrient digestibility of wheat straw using the same DCX enzymatic complex. Also, Romero et al. [47] proved that the supplementation of total mixed dairy cattle with EFE increased the DM intake and milk yield.
The effects of combining NaOH or urea treatments with EFE supplementation on ruminal fermentation varied depending on the treated substrate and the specific enzymatic complex. Indeed, for NCS, the DCX supplementation improved (p-value< 0.05) the rate and extent of in vitro ruminal fermentation by 8.1% (A) and 7.3% (Rmax) and estimated digestive use parameters by 5.7% (ME), 5.6% (OMD), and 11.1% (VFA) using lowest DCX dose D2 = 2 μl/g DM. As for the UCS, the highest dose D10 improved linearly the in vitro ruminal fermentation parameters A and Rmax by 5.4% and 15% without affecting the ME, DMO, and VFA (Table 4). As for the MaxFiber effect, it seems to be stimulated by the alkali treatments of CS. Indeed, for the NCS, improvements by 3.6% for A, 25% for Rmax, 4% for ME, 4% for DMO, and 9% for VFA were recorded using the M4 dose. As for the UCS, the highest improvements were recorded by using the lowest MaxFiber dose M0.5, by 12.6%, 21.8%, 6.8%, 6.7%, and 14% for A, Rmax, ME, DMO, and VFA, respectively (Table 6). So, as compared to the chemically untreated by-products, the MaxFiber complex seems to be more effective on alkali-treated chickpea straw since the NaOH and urea treatment modified the structure and chemical composition of the substrate, making it more susceptible to exogenous enzyme hydrolysis. Furthermore, the NaOH treatment decreased the DCX optimal dose form D10 to D2 which could have economic benefits by reducing the cost of the enzymatic treatment.
On the other hand, for the SS, and as compared to the untreated SS, the alkali treatments decreased the efficacy of both studied EFE (DCX and MaxFiber). Indeed, the association between EFE complexes and NaOH or urea treatments had no significant improvements in the GP kinetics throughout the 96 h of incubation and on the estimated fermentation and digestive use parameters of sunflower stalks and may cause detrimental effects when combined with urea treatment. This finding was similar to those reported by Jabri et al. [48] for sunflower head by-products using the same enzymatic complexes. Depending on the biochemical composition and the polysaccharide matrix of the treated substrate, the alkali treatment could modify the cell wall structure [49] by breaking down the lignocellulosic biomass which may generate by-products such as lignin-derived phenolic compounds (e.g. vanillyl alcohol, coniferyl alcohol, and sinapyl alcohol) and decrease the efficiency of enzymatic hydrolysis [50]. Generally, to optimize the efficiency of EFE and the yield of fermentable sugars from plant biomass, it is crucial to optimize the conditions of the alkali treatment process. These findings emphasize the importance of enzyme-substrate specificity to determine the effectiveness of EFE treatments on the ruminal fermentation of agricultural wastes.
4 Conclusion
The obtained results from this study proved that both chickpea straw and sunflower stalk could be included in ruminant diet, but it’s important to consider their nutritional value and their physical properties, as well as the animal’s requirements. The supplementation by two EFE composed mainly of cellulase and xylanase activities improved the rate and the extent of in vitro ruminal fermentation and the digestive use parameters of both studied by-products, proving that lignified agricultural waste could be valorised in ruminant nutrition providing digestible source of fibre. The association between alkali treatments (NaOH and urea) and EFE exerted variable effects depending on the type of used enzymatic complex, the treated substrate and the alkali treatment. For chickpea straw, the association between alkali and EFE stimulated the ruminal fermentation and improved the digestive use. However, the alkali treatment of sunflower stalk decreased the efficiency of both studied EFE. Thus, it is essential to emphasize the specificity of the enzyme-substrate interaction to ensure the effectiveness of exogenous fibrolytic enzymes on the digestive utilization of agricultural by-products. However, further research is needed to explore the presence of potential anti-nutritional factors and validate the in vitro findings through in vivo trials, which will contribute to a comprehensive understanding of the nutritional value, limitations, and practical application of these feed resources in sustainable ruminant nutrition.
Data availability
The datasets and materials used during the current study are available from the corresponding author upon reasonable request.
References
Sivakumar D, Srikanth P, Ramteke PW, Nouri J (2022) Agricultural waste management generated by agro-based industries using biotechnology tools. Global J Environ Sci Manage 8(2):281–296. https://doi.org/10.22034/gjesm.2022.02.10
Aghajanzadeh-Golshani A, Maheri-Sis N, Baradaran-Hasanzadeh A, Asadi-Dizaji A, Mirzaei-Aghsaghali A, Dolgari-Sharaf J (2012) Determining nutrients degradation kinetics of chickpea (Cicer arietinum) straw using nylon bag technique in sheep. Open Vet J 2(1):54–57
Khademi AR, Hashemzadeh F, Khorvash M, Mahdavi AH, Pazoki A, Ghaffari MH (2022) Use of exogenous fibrolytic enzymes and probiotic in finely ground starters to improve calf performance. Sci Rep 12(1):11942. https://doi.org/10.1038/s41598-022-16070-0
Jabri J, Abid K, Ben Said S, Yaich H, Malek A, Rekhis J, Kamoun M (2022) Effect of fibrolytic enzyme supplementation of urea-treated wheat straw on nutrient intake, digestion, growth performance, and blood parameters of growing lambs. Small Rumin Res 217. https://doi.org/10.1016/j.smallrumres.2022.106840
Bachmann M, Martens SD, LeBrech Y, Kervern G, Bayreuther R, Steinhöfel O, Zeyner A (2022) Physicochemical characterisation of barley straw treated with sodium hydroxide or urea and its digestibility and in vitro fermentability in ruminants. Sci Rep 12(1):20530. https://doi.org/10.1038/s41598-022-24738-w
Carrillo-Díaz MI, Miranda-Romero LA, Chávez-Aguilar G, Zepeda-Batista JL, González-Reyes M, García-Casillas AC, Tirado-González DN, Tirado-Estrada G (2022) Improvement of ruminal neutral detergent fibre degradability by obtaining and using exogenous fibrolytic enzymes from white-rot fungi. Animals 12(7):843. https://doi.org/10.3390/ani12070843
Arriola KG, Oliveira AS, Ma ZX, Lean IJ, Giurcanu MC, Adesogan AT (2017) A meta-analysis on the effect of dietary application of exogenous fibrolytic enzymes on the performance of dairy cows. J Dairy Sci 100(6):4513–4527. https://doi.org/10.3168/jds.2016-12103
Tirado-González DN, Tirado-Estrada G, Miranda-Romero LA, Ramírez-Valverde R, Medina-Cuéllar SE, Salem AZM (2021) Effects of addition of exogenous fibrolytic enzymes on digestibility and milk and meat production—a systematic review. Ann Anim Sci 21(4):1159–1192. https://doi.org/10.2478/aoas-2021-0001
Dulphy JP, Breton J, Bienaime A, Louyot JM (1982) Etude de la valeur alimentaire des pailles de céréales traitées ou non à la soude : I-Influence du traitement à la soude. Annales de Zootechnie 31:195–214
Chermiti A, Nefzaoui A, Cordesse R, Amri T, Laajili M (1989) Paramètres d’uréolyse et digestibilité de la paille traitée à l’urée. Ann Zootech, INRA/EDP Sci 38:63–72
Association of Official Analytical Chemists (1995) Official Methods of Analysis of AOAC International, 16th edn. AOAC International, Arlington, VA
VanSoest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74:3583–3597. https://doi.org/10.3168/jds.S0022-0302(91)78551-2
Jabri J, Abid K, Yaich H, Malek A, Rekhis J, Kamoun M (2022) Evaluation of the efficacy of varying xylanase to cellulase ratio on ruminal fermentation of untreated and alkali treated oat straw. Research Square Preprint (version 1) [accessed 2023 January 30]. https://doi.org/10.21203/rs.3.rs-2199970/v1
Wood TM, Bhat KM (1988) Biomass part A: cellulose and hemicellulose. Meth Enzymol 160:87–112. https://doi.org/10.1016/0076-6879(88)60109-1
Bailey MJ, Biely P, Poutanen K (1992) Inter laboratory testing of methods for assay of xylanase activity. J Biotech 23:257–270. https://doi.org/10.1016/0168-1656(92)90074-J
Theodorou MK, Williams BA, Dhanoa MS, McAllan AB, France JA (1994) Simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim Feed Sci Technol 48:185–197. https://doi.org/10.1016/0377-8401(94)90171-6
INRA (2007) Alimentation des bovins, ovins et caprins. Besoins des animaux - valeurs des aliments. Tables Inra 2007. Quae éditions
Menke KH, Steingass H (1988) Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim Res Dev 28:7–55
Beauchemin KA, Rode L, Vincent JS (1998) Enzyme additives for ruminant feeds. United States Patent, 5,720,971.
Getachew G, Blummel M, Makkar HPS, Becker K (1998) In vitro gas measuring techniques for assessment of nutritional quality of feeds: a review. Anim Feed Sci Technol 72:261–281. https://doi.org/10.1016/S0377-8401(97)00189-2
Groot JCJ, Cone JW, Williams BA, Debersaques FMA, Lantinga EA (1996) Multiphasic analysis of gas production kinetics for invitro fermentation of ruminant feeds. Anim Feed Sci Technol 64:77–89. https://doi.org/10.1016/S0377-8401(96)01012-7
Yang HJ, Tamminga S, Williams BA, Dijkstra J, Boer H (2005) In vitro gas and volatile fatty acids production profiles of barley and maize and their soluble and washout fractions after feed processing. Anim Feed Sci Technol 120:125–140. https://doi.org/10.1016/j.anifeedsci.2005.01.007
Duncan DB (1955) Multiple F and multiple “F” test. Biometrics 11:1–42. https://doi.org/10.2307/3001478
de Souza JB, Michelin M, Amâncio AmÃcncio FLR, Vital Brazil OA, Polizeli MTM, Ruzene DS, Silva DP, Mendonça MC, López JA (2020) Sunflower stalk as a carbon source inductive for fungal xylanase production. Ind Crops Prod 153. https://doi.org/10.1016/j.indcrop.2020.112368
Durmaz E, Ates S (2021) Comparison of properties of cellulose nanomaterials obtained from sunflower stalks. Cellul Chem Technol 55(7-8):755–770
Maheri-Sis N, Aghajanzadeh-Golshani A, Cheraghi H, Ebrahimnezhad Y, Ghoaso JG, Asaadi-Dizaji A (2011) Dry matter degradation kinetics and metabolizable energy of chickpea (Cicer arietinum) straw in ruminants. Res J Biol Sci 6(12):635–638
Jabri J, Abid K, Yaich H, Malek A, Rekhis J, Kamoun M (2019) Effect of combining exogenous fibrolytics enzymes supplementation with alkali and acid pre-treatments on wheat straw hydrolysis and ruminal fermentation. Indian J Anim Sci 89:780–785. https://doi.org/10.56093/ijans.v89i7.92051
Li J, Liu X, Zheng Q, Chen L, Huang L, Ni Y, Ouyang X (2019) Urea/NaOH system for enhancing the removal of hemicellulose from cellulosic fibres. Cellulose 26:6393–6400. https://doi.org/10.1007/s10570-019-02587-7
Schroeder BG, Istanbullu HB, Schmidt M, Logroño W, Harms H, Nikolausz M (2023) Effect of alkaline and mechanical pretreatment of wheat straw on enrichment cultures from Pachnoda marginata larva gut. Fermentation 9:60. https://doi.org/10.3390/fermentation9010060
Wang W, Wang X, Zhang Y, Yu Q, Tan X, Zhuang X, Yuan Z (2020) Effect of sodium hydroxide pretreatment on physicochemical changes and enzymatic hydrolysis of herbaceous and woody lignocelluloses. Ind Crops Prod 145:112145. https://doi.org/10.1016/j.indcrop.2020.112145
Lou H, Lin M, Zeng M, Cai C, Pang Y, Yang D, Qiu X (2018) Effect of urea on the enzymatic hydrolysis of lignocellulosic substrate and its mechanism. Bioenerg Res 11:456–465. https://doi.org/10.1007/s12155-018-9910-7
Martens S, Wildner V, Schulze J, Richardt W, Greef JM, Zeyner A, Steinhöfel O (2022) Chemical treatment of straw for ruminant feeding with NaOH or urea – investigative steps via practical application under current European Union conditions. J Sci Food Agric 31(4):260–281. https://doi.org/10.23986/afsci.115262
dos Santos APM, Santos EM, Silva de Oliveira J, Pinto de Carvalho GG, Leal G, de Araújo G, Moura Zanine A, Martins Araújo Pinho R, Ferreira DJ, da Silva Macedo AJ, Pereira Alves J (2021) Effect of urea on gas and effluent losses, microbial populations, aerobic stability and chemical composition of corn (Zea mays L.) silage. Rev Fac Cienc 53(1):309–319. https://doi.org/10.48162/rev.39.030
Yitbarek M, Tamir B (2014) Silage additives: review. Open J Appl Sci 4:258–274. https://doi.org/10.4236/ojapps.2014.45026
Abid K, Jabri J, Yaich H, Malek A, Rekhis J, Kamoun M (2022) Improving the nutritional value and rumen fermentation characteristics of sesame seed coats through bioconversion approach using exogenous fibrolytic enzymes produced by Trichoderma longibrachiatum. Biomass Conv Bioref, 1–9. https://doi.org/10.1007/s13399-022-03402-3
Marcos CN, García-Rebollar P, deBlas C, Carro MD (2019) Variability in the chemical composition and in vitro ruminal fermentation of olive cake by-products. Animals (Basel) 9(3):109. https://doi.org/10.3390/ani9030109
Han L, Feng J, Zhang S, Ma Z, Wang Y, Zhang X (2012) Alkali pretreated of wheat straw and its enzymatic hydrolysis. Braz J Microbiol 43(1):53–61. https://doi.org/10.1590/S1517-83822012000100006
Moradi M, Afzalzadeh A, Behgar M, Norouzian MA (2015) Effects of electron beam, NaOH and urea on chemical composition, phenolic compounds, in situ ruminal degradability and in vitro gas production kinetics of pistachio byproducts. Vet Res Forum. 6:111–117
Eun JS, Beauchemin KA, Schulze H (2007) Use of exogenous fibrolytic enzymes to enhance in vitro fermentation of alfalfa hay and corn silage. J Dairy Sci 90:1440–1451. https://doi.org/10.3168/jds.S0022-0302(07)71629-6
Yang JC, Guevara-Oquendo VH, Refat B, Yu P (2022) Effects of exogenous fibrolytic enzyme derived from Trichoderma reesei on rumen degradation characteristics and degradability of low-tannin whole plant faba bean silage in dairy cows. Dairy 3:303–313. https://doi.org/10.3390/dairy3020023
Souza JM, Souza JCSM, Sousa DO, Del Valle TA, Ghizzi LG, Alcântara AHD, Mesquita LG, Sousa RLM, Bueno ICS, Balieiro JCC (2021) The effects of compound treatment of Aspergillus oryzae and fibrolytic enzyme on in vitro degradation, gas production and fermentative profile of maize silage and sugarcane silage. J Agric Sci. 159(1-2):147–158. https://doi.org/10.1017/S002185962100037X
Tirado-González DN, Miranda-Romero LA, Ruíz-Flores A, Medina-Cuéllar SE, Ramírez-Valverde R, Tirado-Estrada G (2018) Meta-analysis: effects of exogenous fibrolytic enzymes in ruminant diets. J App Anim Res. 46(1):771–783. https://doi.org/10.1080/09712119.2017.1399135
Colombatto D, Morgavi D, Furtado A, Beauchemin K (2003) Screening of exogenous enzymes for ruminant diets: Relationship between biochemical characteristics and in vitro ruminal degradation. J Anim Sci 81(10): 2628–2638. https://doi.org/10.2527/2003.81102628x
Vyver WFJ, Cruywagen CWC (2013) Exogenous fibrolytic enzymes to unlock nutrients: histological investigation of its effects on fibre degradation in ruminants. South Afr J Anim Sci 43:S54–S59. https://doi.org/10.4314/sajas.v43i5.10
Mao HL, Wu CH, Wang JK, Liu JX (2013) Synergistic effect of cellulose and xylanase on in vitro rumen fermentation and microbial population with rice straw as substrate. Anim Nutr Feed Tech 13:477–487
Morgavi DP, Beauchemin KA, Nsereko VL, Rode LM, Iwaasa AD, Yang W, Mcallister TA, Wang Y (2000) Synergy between the ruminal fibrolytic enzymes and enzymes from T. longibrachiatum. J Dairy Sci 83:1310–1321
Romero JJ, Macias EG, Ma ZX, Martins RM, Staples CR, Beauchemin KA, Adesogan AT (2016) Improving the performance of dairy cattle with a xylanase-rich exogenous enzyme preparation. J Dairy Sci 99:3486–3496. https://doi.org/10.3168/jds.2015-10082
Jabri J, Ammar H, Abid K, Beckers Y, Yaich H, Malek A, Rekhis J, Morsy AS, Soltan YA, Soufan W, Almadani MI, Chahine M, Marti ME, Okla MK, Kamoun M (2022) Effect of exogenous fibrolytic enzymes supplementation or functional feed additives on in vitro ruminal fermentation of chemically pre-treated sunflower Heads. Agriculture 12:696. https://doi.org/10.3390/agriculture12050696
Achyuthan KE, Achyuthan AM, Adams PD, Dirk SM, Harper JC, Simmons BA, Singh AK (2010) Supramolecular self-assembled chaos: polyphenolic Lignin’s barrier to cost-effective lignocellulosic biofuels. Molecules 15:8641–8688. https://doi.org/10.3390/molecules15118641
Qin L, Li WC, Liu L, Zhu JQ, Li X, Li BZ, Yuan YJ (2016) Inhibition of lignin-derived phenolic compounds to cellulase. Biotechnol Biofuels Bioprod 9:70. https://doi.org/10.1186/s13068-016-0485-2
Funding
This research was supported by the Laboratory of Animal Nutrition: Management of the Health and Quality of Animal Production [LR14AGR03] (Ministry of Higher Education and Scientific Research, Tunisia).
Author information
Authors and Affiliations
Contributions
Conceptualization: JJ and MK. Format analyses and investigation: JJ, KA, and HY. Writing draft: JJ. Resource: AM, JR, and MK. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
The article does not contain any studies with human participants. It also does not perform experiments directly on animals. So, this experience does not need ethics statement.
Consent to participate
All the authors of this article are consented to participate.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jabri, J., Abid, K., Yaich, H. et al. Enzymatic valorization of alkali-treated chickpea straw and sunflower stalks as high fibrous agricultural wastes for sustainable ruminant nutrition. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04659-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04659-y