Abstract
Variation in haematological parameters of Nigerian native chickens was studied using 60 clinically normal frizzle-feathered, naked-neck, and normal-feathered native chickens. These included red blood cell count, haemoglobin, packed cell volume, white blood cell count, mean corpuscular volume, mean corpuscular haemoglobin concentration, serum glucose, urea, cholesterol, albumin, globulin and creatinine. Normal-feathered birds had higher (p < 0.05) mean values compared to frizzled and native neck genotypes except for albumin, red blood and white blood cells, and mean cell haemoglobin concentration. Males generally had higher mean values than their female counterparts across all genotypes. Correlation coefficients among the parameters were significant (p < 0.001) with r values ranging from 0.26 between red blood cell and mean corpuscular haemoglobin to 0.92 between red blood cell and cholesterol. Sufficient genetic variation therefore exists for haematological parameters among Nigerian native chickens that may represent indicator traits for further study. However, the application of molecular tools will provide better understanding and application of these differences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Research on indigenous chickens in Nigeria has increased recently especially on comparative studies of their growth and reproduction (Adebambo 2004; Yakubu et al. 2009; Peters et al. 2010). However, selection in local breeds has been targeted more at adaptation to harsh environments and resistance to diseases rather than enhanced production (Minga et al. 2004). Trail and D’leteren (1992) had earlier reported that tropical breeds were well adapted to tropical conditions and if well-exploited and conserved, are well able to exhibit their potentials since their properties are genetically acquired and nurtured by nature. Information about haematological parameters and blood biochemical indices are very essential in diagnosing various pathological and metabolic disorders in chickens. Haematological changes are routinely used to determine the health status of the body and to determine stresses due to environment, nutritional and pathological factors.
In spite of previously unfavourable reports about these indigenous birds such as poor growth rate, poor body conformation, small egg size (Ibe 1993; Nwagu and Nwosu 1994), and poor reproductive efficiency of both sexes (Egbunike and Nkanga 1999; Gbadamosi and Egbunike 1999), recent efforts have highlighted the need to focus research on their genotypes with the goal of exploiting their genetic potential (Horst 1989; Hoffmann 2005; Peters et al. 2005, 2007 and 2008b).
The Nigerian native chickens (NNC), like most indigenous chickens found around the world, are well adapted to their local environmental and climatic conditions (FAO 2010). They have been reported by Ponsuksili et al. (1996) and Wimmers et al. (2000) to contain a highly conserved genetic system, with high levels of heterozygosity, which may provide biological material for the design of genetic stocks with improved adaptability and productivity. The adaptive potential of the native chickens have been attributed to the possession of major genes of frizzling (Ff -) and naked neck (Na -), both of which have been implicated in heat tolerance (Horst 1983; Yunis and Cahaner 1999). The naked neck gene reduces feather coverage in chickens by 20% and 40% in heterozygous (Nana) and homozygous (NaNa) states, respectively (Deeb and Cahaner 2001). Garces et al. (2001) reported that Ff - chickens showed a reduced density of feather coverage, which provides some heat tolerance to egg-type chickens. The NNC represent valuable resources for local poultry development because their extensive genetic diversity allows for rearing of these chickens under varied environmental conditions, providing a range of products and functions (Yakubu et al. 2009).
The potential of the native chicken has not been fully exploited since there are still growing reports about existing or potential levels of productivity of the local breeds managed under extensive and intensive systems (Mathur et al. 1989; Peters et al. 2002; 2005, 2007, 2008a, and 2008b). Even though several reports on performance of the local chickens in Nigeria have been reported (Ebozoje and Ikeobi 1995; Ikeobi et al. 1996, Adebambo et al. 1999), there are no studies on characterization of NNC based on haematological parameters. Since assessment of variation in haematological parameters in NNC would further help our understanding of diversity, this study was aimed to uncover variation in these parameters of Nigerian native chickens.
Materials and methods
Experimental site and birds
Research was conducted at the Teaching and Research Farm, University of Agriculture, Abeokuta (UNAAB), (latitude 7°10N and longitude 3° 2E) in Southwestern Nigeria. Ambient temperatures range from 28°C in December to 36°C in February with relative humidity of 82% in an area intermediate between tropical rainforest and the derived savannah (Ilori et al. 2010).
Experimental birds
Sixty native chickens comprising 20 each from frizzle-feathered, naked-neck and normal-feathered birds were used for the study. This research was approved by the Institutional Animal Use and Care Committee of the University of Agriculture, Abeokuta, Ogun State, Nigeria.
Egg collection
Eggs were collected from birds maintained at the Poultry Breeding Unit of UNAAB. Females birds were artificially inseminated with 0.1 mL of fresh semen, and fertile eggs were collected once every morning. All eggs collected were first maintained at a temperature of 10–14°C and 75–85% relative humidity for a few days prior to incubation. Eggs were screened and only those without cracks and deformed shapes were sent to the hatchery for incubation.
Management of the birds
Chicks from each genetic group were differentiated and individually identified by wing tagging (sexes combined). The chicks were also vaccinated and medicated routinely. The chicks were transferred to a previously disinfected brooder house where a standard management procedure was strictly adhered to as described in Peters et al. (2005). Brooding was done for 4 weeks during which Neoceryl was administered to reduce stress.
Feeds and feeding
The chicks were fed on a commercial starter ration that provided 20% crude protein and 2,600 kcal/kg ME from 0 to 8 weeks of age. Thereafter, they were fed with commercial growers ration that supplied 17% crude protein and 2850 kcal/kg ME from 8 weeks of age to the end of the experiment. Feed and water were provided ad libitum throughout the experimental period.
Haematological and biochemical parameters
Blood samples from the three genetic groups were randomly collected at 20 weeks of age from 20 males and females. About 3 ml of blood was collected from the right basilica vein of the wing from each selected bird, divided into two parts; one part consisted of 1 ml in EDTA as an anticoagulant, and the rest were allowed to clot to obtain sera (Samour et al. 2010). The anti-coagulated blood was used to determine red blood cell (RBC) count and white blood cell (WBC) count using a manual haemocytometer. Packed cell volume (PCV) was determined using the haematocrit centrifuge method and haemoglobin (Hb) concentration by the cyanmethaemoglobin method. Mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH) and mean corpuscular haemoglobin concentration (MCHC) were calculations from the RBC, Hb and PCV values as described by Jain (1986). Sera were separated from the clotted blood following centrifugation from which serum metabolites (serum glucose, urea, cholesterol, albumin, globulin and creatinine) were determined by spectrophotometry. Values of all these parameters were analysed using the routine laboratory procedures of Dacie and Lewis (1991).
Data analysis
All parameters were analysed with the Statistical Analysis System (SAS 1999) using a two-way analysis of variance. The model below accommodated the effects of genotype, sex and interaction between genotype and sex.
Where:
- Y ijk = :
-
observed value of the measurable traits of j th sex in the i th genotype.
- μ :
-
overall mean
- G i :
-
effect of i th genotype (i = 1,2,3)
- S j :
-
effect of j th sex (j = 1, 2)
- e ijk :
-
random residual error.
Duncan’s multiple range of test was used to separate means that differed significantly. Pearson correlation (r) was used to ascertain relationships between measurable traits.
Results
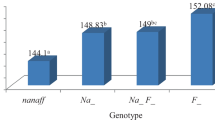
The least square means and standard errors of means for haematological parameters and biochemical indices as influenced by major genes and sex are presented in Tables 1 and 2, respectively. Significant differences (p < 0.05) among chicken genotypes were observed for PCV, globulin, urea, creatinine and cholesterol. Haemoglobin (Hb) was highly significantly (p < 0.01) different among chicken genotypes, with highest values recorded in normal-feathered chickens (11.98 ± 0.12%) followed by the naked neck birds (11.55 ± 0.41%) and finally the frizzled birds (11.42 ± 0.31%). There were no differences in the other haematological parameters and biochemical indices (p > 0.05).
Sex differences were highly significant (p < 0.01) for Hb, with males having mean values of 12.73 ± 0.13% compared to females (10.56 ± 0.17%). In addition, PCV, RBC, glucose, albumin, globulin, urea, creatinine, and cholesterol exhibited sex differences (p < 0.01) but not MCV, MCH and MCHC (p > 0.05). Significantly higher values for these parameters were obtained for the male chickens compared to the female ones.
Least square means for interactions between genotype and sex are presented in Tables 3 and 4. Parameters such as PCV, WBC, MCV, MCH and MCHC were not significantly (p > 0.05) influenced by the interaction effect of genotype and sex. However, Hb, RBC, glucose, albumin, globulin, urea, creatinine, and cholesterol had very highly significant genotype and sex interaction effects (p < 0.001), with the highest red blood cell value of 4.46 ± 0.08 mμ/mm3 recorded for the naked-neck native cocks followed by frizzle-feathered males (4.20 ± 0.08 mμ/mm3), normal-feathered males (4.12 ± 0.03 mμ/mm3), normal-feathered females (3.72 ± 0.10 mμ/mm3) and the least values of 3.38 ± 0.06 mμ/mm3 and 3.36 ± 0.05 mμ/mm3 were recorded for frizzle-feathered females and naked-neck females, respectively.
The correlation coefficients among the studied parameters are presented in Table 5 and showed that correlation between some parameters studied were weak and non-significant (p < 0.05) while some parameters showed negative relationships. Very highly significant (P < 0.001) and strong associations were observed between most parameters with r values ranging from 0.92 between Hb and cholesterol (Chol), Hb and glucose (Glu), PCV and Glu to 0.77 between albumin (Alb) and creatinine (Cret). However, correlation between PCV and white blood cell WBC (r = 0.16), PCV and MCV (r = 0.26) and r = 0.26 between RBC and MCH were low but still significant (P < 0.05).
Discussion
Variation in haematological parameters among chicken genotypes with the exception of MCV agree with Harper and Lowe (1998), but the values for other parameters in this study were higher than previously reported for exotic chickens in Nigeria (Iheukwumere and Herbert 2003; Talebi et al. 2005). But the present results are in agreement with Sturkie (1986), Uko and Ataja (1996), Mushi et al. (1999) and Iheukwumere et al. (2006). Reports from these authors indicated variability in haematological profiles among different breeds. While haematograms of these strains remained almost the same, there are differences in leukograms between the exotic strains and the indigenous chickens as equally reported by Islam et al. (2004). The high value of WBC obtained from this investigation may simply demonstrate the immunological status of the local chicken.
On the other hand, low WBC values in exotic strains may explain the higher level of susceptibility to avian disease-causing agents compared with indigenous chickens that are relatively tolerant to many poultry diseases (Campbell and Coles 1986). These authors reported that since the WBC in avian species serves a phagocytic role as in mammals, they are mainly responsible for defence of the body against infections. Also, Shaniko (2003) reported that leukocyte counts as well as heterophiles and lymphocytes ratio were used as indicators of stress responses and sensitive bio-makers that are crucial to immune functions. Previous reports stated that PCV, Hb and MCH are major indices for evaluating circulating avian erythrocytes, and are very significant in the diagnosis of anaemia and also serve as useful indices of the bone marrow capacity to produce red blood cells as in mammals (Awodi et al. 2005; Chineke et al. 2006). The higher values for PCV, Hb, and MCH values in this investigation in normal feathering birds compared to naked neck and frizzled birds probably reflect inherent genetic differences in agreement with the findings of Agaie and Uko (1998). They reported variation in erythrocyte values due to season and species. In other reports, Oladele et al. (2001), Iheukwumere et al. (2002) and Adejumo (2004), attributed low values of PCV and Hb to poor nutrition especially protein deficiency whereas Ikhimioya et al. (2002) attributed low erythrocyte values to system of management. These reasons cannot be advanced for variations found in our studies where all birds were exposed to common environment for feeding and management. Therefore, the only logical factor implicated is chicken genotype. Chineke et al. (2006) reported that high PCV reading indicated either an increase in number of circulating RBC or reduction in circulating plasma volume. Reports by Brackenbury et al. (1981a, b) and Donkoh (1989) earlier showed that variation in erythrocytes can be attributed to increased body temperature from increase ambient temperature, respiration, respiratory water loss and oxygen consumption of birds, which in turn increase oxygen intake and partial pressure of oxygen in the blood. Increase in partial pressure decreases the production of red blood cells and consequently reduces the mean value of circulating erythrocytes. These mechanisms may partly explain the lower values of PCV, Hb, and MCH that are associated with naked-neck and frizzle-feathered birds in this study. This may be the most likely mechanism by which the expression of naked neck and frizzle genotypes produce thermoregulatory effects. The lower mean values of PCV, Hb and MCH were still within normal ranges as reported by Wayne and Huxtable (1988) and Campbell (1994). These authors reported normal avian PCV as ranging from 35% to 50%, and a PCV less than 35% may be detrimental to the individual animal. Results of other biochemical parameters monitored in this study (serum glucose, serum globulin, serum urea, creatinine, and cholesterol) indicated that the normal-feathered birds had higher mean values than naked and frizzle-feathered genotypes, respectively. These significant differences which may be attributed to strain differences are consistent with Agaie and Uko (1998), Islam et al. (2004) and Chineke et al. (2006), strengthening the argument for inherent genetic differences.
Significant variation between sexes in this study was consistent with the reports of Islam et al. (2004). Our results showed that males generally had significantly higher mean values in PCV, Hb, RBC, glucose, albumin, globulin, creatinine, and cholesterol than their female counterparts. The higher mean values for haematological and biochemical parameters in males compared to females may also be attributed to physiological status of the birds. Kral and Suchy (2000) attributed high mean values of erythrocytes in male birds as a characteristic of gonadal and spermiogenetic development which occurs during the period of sexual maturation and at the onset of reproductive activity in breeding cocks. In a related report, Sturkie (1986) and Oladele et al. (2001) reported that matured males generally had higher erythrocyte values than females and reported that androgen stimulates erythropoeisis and increases the number of circulating erythrocytes and consequently, PCV and Hb in birds. There was a non-significant effect of the sex of bird recorded for MCV, MCH, and MCHC; and since these parameters derived are likely to be more sensitive to sample size, although female birds had slightly higher values than the males. This is further supported by significant interaction effects between genotype and sex on Hb, RBC, Glu, Alb, Glo, urea, creatinine, and Chol. These results were expected because genotype and sex individually had significant effects on most of these parameters.
The correlation coefficients involving MCV, MCH and MCHC with any other parameter were not significant. However, strong and positive correlations among other parameters such as PCV and Glu, PCV and Alb, PCV and Glo, Hb and Glu, Hb and urea, RBC and Glo, RBC and Glu, RBC and Chol, and RBC and urea clearly demonstrate inter-relationships among these parameters. These relationships further strengthen the roles that PCV, Hb and RBC play in better understanding of normal physiology, pathology and total health monitoring of birds (Maxwell and Burns 1986; Talebi et al. (2005). These authors reported that the levels of PCV and Hb were major indices in evaluating circulating avian erythrocytes and were very significant in the diagnosis of anaemia. However, the non-significant correlation obtained between WBC and other parameters may indicate less dependence on other parameters and diminished role in the immune functions of the native chickens.
In summary, while this study may indicate that sufficient genetic variation exists among NNC as indicator traits for further study in the context of selection and improvement programme, there remains a need to apply molecular tools to characterise these genetic groups for better resolution of these differences and long-term utilisation.
References
Adebambo, O.A., Ikeobi, C.O. N., Ozoje, M. O., Adenowo J. A, and Osinowo, O.A. 1999. Colour variation and performance characteristics of the indigenous chickens of South Western Nigeria, Nigerian Journal of Animal Production, 26, 15–22.
Adebambo, O.A. 2004. Animal genetics and quality of life. Proceedings of the 29th Annual Conference of the Genetics Society of Nigeria, October 11–14, 2004, Abeokuta, Nigeria
Adejumo, D.O. 2004. Haematology, growth and performance of broiler finishers fed rations supplemented with Indian almond (Terminalia catappa) husk and kernel meal. Ibadan Journal of Agricultural Research, 1 (1):1–6.
Agaie, B.M. and Uko, O. 1998. Effect of season, sex and species on the packed cell volume of guinea and domestic fowls in Sokoto State of Nigeria. Nigeria Veterinary Journal, 19, 95–99.
Awodi, S., Ayo, J.O., Atodo, A.D. and Dezende, T. 2005. Some haematological parameters and the erythrocyte osmotic fragility in the Laughing Dove (Streptopella senegalensis) and the village Weaver bird (Ploceus cucullatus). In: Dairo, F.A, Fajemilehin, S.O.K and Onibi, G.E. (Eds), Proceedings of 10th Annual Conference of Animal Science Association of Nigeria, held on 12–15 September at University of Ado Ekiti, Nigeria, 384–387
Brackenbury, J.H., Avery, P. and Gleeson, M. 1981a. Respiration in exercising fowl I: Oxygen consumption, respiratory rate and respired gases, Journal of Experimental Biology, 93, 317–325.
Brackenbury, J.H., Gleeson, M. and Avery, P. 1981b. Respiration in exercising fowl II: Respiratory water loss and heat balance, Journal of Experimental Biology, 93, 327–332.
Campbell, T.W. 1994. Avian Haematology. In: Ritchie, B.W., Harrison, G.J. and L.R Harrison (eds), Avian Medicine: Principle and Application, Saunders, Philadelphia, 426.
Campbell, T.W. and Coles, E.H. 1986. Avian clinical pathology. In: Coles, E.H. (Ed), Veterinary Clinical Pathology, 4th Edition (Saunders Company, Philadelphia, USA), 279–300.
Chineke, C.A., Ologun, A.G. and Ikeobi, C.O.N. 2006. Haematological parameters in rabbit breeds and crosses in humid tropics, Pakistani Journal of Biological Science, 9(11): 2102–2106
Dacie, J.V and Lewis, S.M. 1991. Practical Haematology, 8th Edition (Longman Group Limited, London) 22-68
Deeb, N. and Cahaner, A., 2001. Genotype-by-environment interaction with broiler genotypes differing in growth rate 1. The effects of high ambient temperatures and naked neck genotype on lines differing in genetic background, Poultry Science, 80, 695–702.
Donkoh, A. 1989. Ambient temperature: a factor affecting performance and physiological response of broilers chickens, International Journal of Biometeorology, 33(2): 259–265.
Ebozoje, M.O. and Ikeobi, C.O.N. 1995. Productive performance and occurrence of major genes in the Nigerian local chicken, Nigerian Journal of Genetics, 10, 67–77.
Egbunike, G.N. and Nkanga, E.E. 1999. Effects of breed and season on the semen characteristics of the cocks in the humid tropics, Tropical Journal of Animal Science, 1, 127–134.
FAO, 2010. The state of food and agriculture: livestock in the balance, 176.
Gbadamosi, A. J and Egbunike, G. N. 1999. Studies on cock semen: effects of frequent ejaculation and breed on physical characteristics, Tropical Journal of Animal Science, 1:157–104.
Garces, A., Casey, N.H. and Horst, P. 2001. Productive performance of naked neck, frizzle and dwarf laying hens under various natural climates and two nutritional treatments, South African Journal of Animal Science, 31:174–180
Harper, E.J. and Lowe, B. 1998. Haematology values in a colony of Budgerigars (Melopsittacus undulates) and changes associated with aging, Journal of Nutrition, 128 (12): 2639–2640
Hoffmann, I. 2005. Research and investment in poultry genetic resource: challenges and options for sustainable use, World Poultry Science Journal, 61(10):57–70
Horst, P., 1983. The concept of ‘productive adaptability’ of domestic animals in tropical and subtropical regions, Journal of the South African Veterinary Association, 54, 159–164
Horst, P. 1989. Native fowl as reservoir for genomes and major genes with direct and indirect effects on adaptability and their potential for tropically oriented breeding plans, Arch. Guflugelk, 5313, 93–101
Ibe, S.N. 1993. Growth performance of normal, frizzle and naked neck chickens in a tropical environment, Nigerian Journal of Animal Production, 20 (1&2): 25–29
Iheukwumere, F.C., Herbert, U. and Ewulu, C. 2002. Effect of quantitative feed restrictions on performance, haematology and serum biochemistry of broiler chickens, Journal of Sustainable Tropical Agriculture Research, 3, 56–60
Iheukwumere, F.C. and Herbert, U. 2003. Physiological responses of broiler chickens to quantitative water restriction; haematology and serum biochemistry, International Journal of Poultry Science, 2(2):117–119
Iheukwumere, F.C., Abu, A.H. Ameh, M. 2006. Effect of human menopausal gonadotropin on haematological and serum biochemical parameters of the Nigerian Indigenous chickens, International Journal of Poultry Science 5(7):632–634
Ikeobi, C.O.N., Ozoje, M.O., Adebambo, O.A., Adenowo, J.A. and Osinowo, O.A. 1996. Genetic differences in the performance of the local chicken in south western Nigeria, Nigerian Journal of Genetics, 11, 33–39
Ikhimioya, I.A., Arijeniwa, I.T., Oteku, A. and Ahmed, A. 2002. Preliminary investigation on the haematology of indigenous chicken, Proceedings of the 5th Annual Conference of Animal Science Association of Nigeria, September 19–22, Port-Harcourt, Nigeria, 10–12
Ilori, B.M, Peters, S.O., Ikeobi, C.O.N, Bamgbose, A.M, Isidahomen, C.E and Ozoje, M.O. 2010. Comparative assessment of growth in pure and crossbred turkeys in a humid tropical environment, International Journal of Poultry Science, 9, 368–375
Islam, M.S., Lucky, N.S., Islam, M.R., Ahad, A., Das, B.R., Rahman, M. and Siddiui, M. 2004. Haematological parameters of Fayoumi, Assil and local chickens reared in Sylhet region in Bangladesh, International Journal of Poultry Science, 13(2): 144–147
Jain, N.C. 1986. Schalman’s Veterinary Haematology, 4th edition (Lea and Febiger Philadelphia) 224
Kral, I. and Suchy, P. 2000. Haematological studies in adolescent breeding cocks, ACTA Vet. Brno. 69, 189–194
Mathur, P.K., El-Hammady, H. and Sharara, H. 1989. Specific use of high yielding strain carrying major genes for improving performance of local fowls in the tropics (Case study: upper Egypt), Proceedings DLG Symposium on poultry production in developing countries, Hemeln, Germany, June 19–22, 1989
Maxwell, M.H. and Burns, R.B. 1986. Experimental stimulation of eosinophil production in domestic fowl. Research of Veterinary Science, 41, 114–123
Minga, U., Msoffe, P.L. and Gwakisa, P.S. 2004. Biodiversity (variation) in disease resistance in pathogens within rural chicken, World Poultry Congress, June 8–21, Istanbul, CD proceedings.
Mushi, E.Z., Binta, M.G., Choabo, O.O and Ndebele, R.T. 1999. Haematological studies on apparently healthy Tswana indigenous chickens (Gallus domesticus) around Gaborone, Botswana, INFPD Newsletter 9, 83–88
Nwagu, B.I. and Nwosu, C.C. 1994. Growth performance of crosses between Arbor broilers and local chicken of Nigeria, Indian Journal Animal Science, 64(6): 651–653
Oladele, S.B., Ogundipe, S., Ayo, J.O. and Esievo, K.A.N. 2001. Effect of season and sex on packed cell volume, haemoglobin and total protein of indigenous pigeons in Zaria, Northern Nigeria, Veterinarski Arhiv 71(5): 277–286
Peters, S.O., Ikeobi, C.O.N., Ozoje, M.O. and Adebambo, O.A. 2002. Genetic variation in the reproductive performance of the Nigerian indigenous chicken, Tropical Animal Production Investigations, 5, 37–46
Peters, S.O., Ikeobi, C.O.N., Ozoje, M.O. and Adebambo, O.A. 2005. Modelling growth in seven chicken genotypes, Nigerian Journal of Animal Production, 32, 28–38
Peters, S.O, Ikeobi, C.O.N., Ozoje, M.O., Famakinwa, O.A., Oshodi, Y.S. and Adebambo, O.A. 2007. Egg quality of the Nigerian local chicken as influenced by some major genes, Nigerian Journal of Animal Production, 34, 25–31
Peters, S.O., Shoyebo, O.O., Ilori, B.M., Ozoje, M.O., Ikeobi, C.O.N and Adebambo, O.A. 2008a. Semen quality traits of seven strains of chickens raised in the humid tropics, International Journal of Poultry Science 7(10): 949–953
Peters, S.O., Ilori, B.M., Ozoje, M.O., Ikeobi, C.O.N and Adebambo, O.A. 2008b. Gene segregation effects on fertility and hatchability of pure and crossbred chicken genotypes in the humid tropics, International Journal of Poultry Science 7(10): 954–958
Peters, S.O., Idowu, O.M.O., Agaviezor, B.O, Egbede, R.O and Fafiolu, A.O. 2010. Genotype and Sex effect on gastrointestinal nutrient content, microflora and carcass traits in Nigerian native chickens. International Journal of Poultry Science 9(8):731–737
Ponsuksili, S., Wimmers, K. and Horst, P. 1996. Valuation of different combinations of oligosaccharides probes and restriction enzymes to generate DNA fingerprints reflecting genetic variability in different strain of chicken. Archiv fur Gerflugelkunde, 60, 227–235
Samour, J., Naldo, J., Rahman, H and Sakkir, M. 2010. Hematologic and plasma biochemical reference values in Indian peafowl (Pavo cristatus), Journal of Avian Medicine and Surgery, 24, 99–106.
SAS 1999. SAS User’s guide. Statistical Analysis Institute Inc, Cary, North Carolina.
Shaniko, S. 2003. Physiological responses of laying hens to the alternative housing system, International Journal of Poultry Science, 2, 357–360.
Sturkie, P.D. 1986, Avian Pathology. 4th Edition (Springer-Verlag, New York, USA.
Uko, O.J. and Ataja, A.M. 1996. Effects of anticoagulant and storage (40 C) on packed cell volume (PCV) of Nigerian domestic fowl (Gallus domesticus) and guinea fowl (Numida meleagris), British Poultry Science, 37, 997–1002.
Talebi, A., Asri-Rezaei, S., Rozeh-Chai, R. and Sahraei, R. 2005. Comparative studies on haematological values of broiler strain (Ross, Cobb, Arbor- Acres and Arian), International Journal of Poultry Science, 4(8): 573–579.
Trail. J.C.M., and D’leteren, G.D.M. 1992. Characterization of trypanotolerant cattle; case study. Proceedings of the Research Planning Workshop, African Animal Genetic Resources (International Livestock Center for Africa) Addis Ababa, Ethiopia 19th–21st February, 1992, 39–41.
Wayne, F.R. and Huxtable, C.R.R. 1988. Clinicopathologic Principles for Veterinary Medicine, (Cambridge University Press, New York, Melburne) 240.
Wimmers, K., Ponsuksili, S., Hardge, T., Valle-Zarate, A., Mathur, P.K. and Horst, P. 2000. Genetic distinctiveness of African, Asian and South American local chickens, Animal Genetics, 31, 159–165
Yakubu, A., Kuje, D and Okpeku, M. 2009. Principal components as measures of size and shape in Nigerian indigenous chickens, Thai Journal of Agricultural Science, 42, 167–176.
Yunis, R. and Cahaner, A., 1999. The effects of the naked neck (Na) and frizzle (F) genes on growth and meat yield of broilers and their interaction with ambient temperatures and potential growth rate, Poultry Science, 78, 1347–1352.
Acknowledgement
Financial support from grant RG180 by the Research and Development Centre of the University of Agriculture, Abeokuta, Nigeria is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peters, S.O., Gunn, H.H., Imumorin, I.G. et al. Haematological studies on frizzled and naked neck genotypes of Nigerian native chickens. Trop Anim Health Prod 43, 631–638 (2011). https://doi.org/10.1007/s11250-010-9743-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-010-9743-7