Abstract
The effect of hemoglobin polymorphism on performance traits in Nigerian indigenous chicken types was investigated in this research. The chickens were obtained through the pure mating of the sire and dam of each population of frizzle feathered, normal feathered, and naked neck chickens to produce F1 offsprings. One hundred fifty-five chicks (37 frizzle, 79 normal, and 39 naked neck) were measured for body weight (g), breast girth (cm), and tibia length (cm). At 20 weeks, 5 mL of blood was collected from the wing vein of each chicken into heparinized tubes and labeled according to its tag number for electrophoresis. The electrophoresis procedures outlined by RIKEN BRC (2006) was used. 0.6 μl of undiluted blood constituent was taken. The prepared buffer (Tris 10.91 g, EDTA 0.60 g, boric acid 3.10 g) at pH 8.5 was used for the gel preparation. The cellulose acetate membrane was used as a supporting medium. The electrophoresis was carried out at a voltage of 150 V for about 50 min at a temperature of 4 °C. The migration of the genotype was from cathode (−) to anode (+). Each bird was scored as either fast (AA), midway (AB), or slow (BB) according to the mobility on the cellulose acetate paper for hemoglobin. Data were analyzed using statistical analysis system (SAS 2002), and significant means were separated using Tukey’s Honestly Significant Difference. Hardy Weinberg’s equation was used to calculate genotypic and allelic frequencies and tested using chi-square (χ2). Hemoglobin had three polymorphic forms viz AA, AB, and BB. The genotypic frequencies of hemoglobin (HbAA, HbAB, and HbBB) were 49, 56, and 50, respectively, while the allelic frequencies were 0.50 for both HbA and HbB. The effect of the polymorphic forms on body weight (g), breast girth (cm), and tibia length (cm) showed that the AA had significantly higher (P < 0.05) body weight (g) than AB and BB (1296.43 g, 1029.59 g, and 884.46 g, respectively). The AA was also higher (P < 0.05) than the AB and BB for breast girth (cm) and tibia length (cm). Heterozygotes adapted and survived better than the homozygotes. The effect of the polymorphic forms of hemoglobin on body weight (g), breast girth (cm), and tibia length (cm) showed that it could be used for body weight selection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The local breeds of chicken also referred to as indigenous chickens are genetically adapted to the harsh conditions prevalent in their native environment such as surviving on limited feed resources and resistance to severe climatic conditions, pathogens, and predators (Mwacharo et al. 2005; Sorenson 2009). They are often utilized for several purposes simultaneously (Sorenson 2009; Adeleke et al. 2011) and possess both superior levels of genetic diversity/variation relative to the commercial breeds (which have been selected for particular performance traits), and they have unique traits of valuable local adaptations (Sorenson 2009). Some local chickens have special characteristics of potential interest to commercial breeders. Therefore, indigenous chickens could be a genetic source for future breeding strategies (Horst 1999).
Hemoglobin is an erythrocyte pigment, conjugated globin-prosthetic group, and also a polymorphic protein (Das and Deb 2008). It carries oxygen and carbon dioxide (Das and Deb 2008; Steppa et al. 2009) and participates in the maintenance of proper blood reaction (Steppa et al. 2009). The occurrence of two or more discontinuous forms of protein in a species/population in such a proportion that the rarest phenotype, which has a frequency of more than 0.1% cannot be maintained merely by recurrent mutation is known as protein polymorphism (Das and Deb 2008). Protein polymorphism is a tool to map (locate) genes responsible for traits such as those causing a disease. For economic traits, it could be used for the selection of superior animals for breeding purposes (Akpa et al. 2011), and they can help match two samples of deoxyribonucleic acid (DNA) to determine if they came from the same source. Das and Deb (2008) reported three hemoglobin polymorphic forms, controlled by two autosomal alleles A1 and A2 with genotypic frequencies as 0.96: 0.04 for White Leghorn, 1.00: 0.00 for local fowl, 1.00: 0.00 for Guinea fowl, and 0.85: 0.15 for Japanese quail. However, Steppa et al. (2009) reported that there were two co-dominant autosomal alleles found in the beta-hemoglobin chain in sheep.
The indigenous chickens have been reported to have a slow growth rate and lay few eggs (Guèye 1998; Ajayi 2010). Reports on the effect of hemoglobin types on growth and reproductive performance in mammalian species have been documented. (Chineke et al. 2007; Yakubu et al. 2014; Vazic et al. 2017). Most hemoglobin polymorphism reports on Nigeria indigenous chickens have been limited to genotype and allelic frequencies (Salako and Ige 2006; Yakubu and Aya 2012; Ajayi et al. 2013). In the reports of (Das and Deb 2008), hemoglobin polymorphism affects growth rate, hatchability, and susceptibility of chicken to Marek’s disease. In general, this variation in proteins can be used for the study of genetic diversity within a population’s gene pool by applying protein electrophoresis and protein immunology, bearing in mind that the basic principle behind electrophoretic mobility of enzymes as well as other proteins is mobility across gels, which denotes differences in allelic groups responsible for amino acid variations in the protein (Rege and Okeyo 2006).
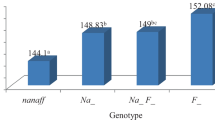
Due to paucity of information on the effect of hemoglobin types on performance characteristics of Nigeria indigenous chicken, this study was carried out to characterize the individual local chicken populations of Nigerian indigenous chickens with the view to establish the genetic basis for the difference in their phenotypic traits such as weight, breast girth, and tibia length (Fig. 1).
Materials and methods
Location of the experiment
The experiment was carried out at the Poultry Unit of the Department of Animal Science Teaching and Research Farm, Ahmadu Bello University, Samaru – Zaria, Kaduna State. The site lies between latitude 11° 091 0611 N and 7° 381 5511 E, at an altitude of 706 m above sea level (Ovimaps 2012).
Experimental birds
Local chickens at growers’ stage were sourced from Kogi State (Anyigba and Okene), Kaduna State (Shika and Sabon Gari markets), and Katsina State (Funtua) and used as the foundation stock. The birds were wing tagged for individual identification according to feather morphology (normal feathered, frizzle feathered, and naked neck). They were reared on deep litter floor, treated against worms and lice, and also vaccinated against Newcastle diseases. The birds were acclimatized to the environment for 3 weeks.
Mating, egg collection, and incubation
Each of the feather morphology group consisted a total of 30 hens and 6 cocks which were naturally mated in the ratio of 1 cock to 5 hens. During the period of egg-laying, laying nests were prepared and kept clean for the laying hens. Eggs were collected early in the morning and also during the day. This was to prevent cracking of the eggs. Eggs were collected separately according to the mating group. Each egg collected was labeled with the pen number and date. Cracked and soiled eggs were removed, and eggs were fumigated in order to prevent microorganisms from invading the eggs during a 6-day period of storage while gathering more eggs for hatching. Eggs were incubated artificially in an automated electric incubator at the optimum temperature of 37 °C and relative humidity of 60–65% and were turned hourly. Candling of the set eggs was done at day 18, at which time, clear eggs without blood or tissue formation and dead embryo were removed. Chicks were hatched at 21 days of incubation.
Management, feed, and feeding
Hatched chicks were collected separately according to the mating group, counted, wing banded, and weighed to the nearest 1 g using Citizen weighing digital scale to determine the weight of the chicks at hatch. The birds were vaccinated against Newcastle (intra-ocular) and Mareks at hatch. Gumboro vaccine was administered at 14 and 28 days while Newcastle (oral) was given at 21 days and Fowl-pox at 56 days of age. Newcastle vaccine was repeated at the 16th week of age. Other routine medication and management operations that were carried out include anti-Coccidia medication, deworming, and delousing. Mortality was recorded. Meanwhile, the birds were weighed on a weekly basis. Body weight was measured using a digital scale, and body measurements were taken with tailor’s tape. Breast girth was taken as the circumference of the breast around the deepest region of the breast while tibia length was measured from the proximal end to the distal end of the shank.
Feed was compounded to meet the nutrient requirements of the birds at all phases of growth. For foundation stock, growers’ diet was compounded during the growing phase. This diet was changed to layer (breeder) mash for the laying period. The hatched chicks were fed on chick mash for the chick stage and growers mash during the grower’s stage. The chick mash contained 2652.45 M.E (Kcal/kg DM) and 20.99% crude protein. Growers mash had 2400.18 M.E (Kcal/kg DM) and 16.08% crude protein while the breeder ration contained 2520.08 M.E (Kcal/kg DM) and 18.05% crude protein. Feed and water were given ad libitum.
Data collection
Data were collated on body weight, breast girth, and tibia length at a 4-week interval. At the age of 20 weeks, 2 to 3 ml of blood was collected using a 5 mL syringe from the wing vein of each chicken into heparinized tubes each labeled according to the tag number of the donor bird. The tubes were kept inside a cooler with ice packs till use. The blood samples were then taken to the laboratory and first centrifuged at 2500–3000 revolution per minute (rpm) for 10 min at 4 °C to separate the blood cells from the serum. The blood cell was used for the electrophoretic analysis of hemoglobin as outlined in the standard procedure (RIKEN 2006).
Electrophoresis of blood samples
The electrophoresis procedures outlined by RIKEN BRC (2006) was used. 0.6 μl of undiluted blood constituent was taken. The prepared buffer (Tris 10.91 g, EDTA 0.60 g, boric acid 3.10 g) at pH 8.5 was used for the gel preparation. Cellulose acetate membrane was used as a supporting media. The electrophoresis was carried out at a voltage of 150 V for about 50 min at a temperature of 4 °C. The migration of the genotype was from cathode (−) to anode (+). The direct gene counting method as explained by Christensen (2003) was then used to score the bands based on the separation of the electrophoretic mobility for each chicken as follows:
-
Fast-moving band (homozygous, AA gene)
-
Slow-moving band (homozygous, BB gene). Any mixture, midway between fast and slow (heterozygous, AB gene)
Statistical analysis
Data obtained were subjected to the General Linear Model procedure of Statistical Analysis System (SAS 2002), and significant means were separated using Tukey’s Honestly Significant Difference. Gene and genotypic frequencies were calculated using the Hardy Weinberg’s equation by the expansion of the binomial equation below.
where p stands for the fast alleles and q for the slow types and pq is the midway (heterozygotes). Mendelian inheritance ratio for band (genotype) inheritance was tested using chi-square (χ2) formula below.
The statistical model used for the association of blood polymorphic types with each trait was the following:
where
-
yij = Result of the observation
-
μ = The overall mean
-
bi = Effect of hemoglobin genotype
-
eij = Residual error
The assumptions of this model are as follows:
eijNIID (0, σ2), i. e., it is normally independently and identically distributed
−There is no environmental effect or modification
Results
Hemoglobin genotype, genotypic, and allelic frequencies of the frizzle, normal, and naked neck indigenous chickens of Nigeria
Table 1 shows the hemoglobin genotypic and allelic frequencies of the frizzle, normal, and naked neck indigenous chickens of Nigeria. Three forms of hemoglobin polymorphism were observed as as HbAA, HbAB, and HbBB spreading across the three chicken types. However, there were variations in the frequencies of HbAA, HbAB, and HbBB among the three chicken types. The population of each of the chicken types showed that the normal feathered chicken had the highest population (79) followed by the naked neck chicken (39) and the least was frizzled chicken (37). The overall population had total genotypic frequencies of 49HbAA, 56 HbAB, and 50 HbBB while the allelic frequencies were 0.50 for A and B, respectively.
The variation in the frequency of hemoglobin genotypes among the chicken types revealed that HbAA was the lowest in the females of frizzle, normal, and naked neck chickens (0.00, 0.03, and 0.33, respectively). While the frequency of HbBB was high in the females of frizzle and naked neck chicken (0.53), it was however low in the normal feathered females (0.22). The highest frequency of HbBB was observed in the females of the normal feathered chicken. HbAA had the highest frequency in the combined males while HbBB was the highest in the combined females.
The frequency of A allele was high in males of the frizzle and naked neck chickens (0.78–0.81) while B allele was high in the females of the frizzle and normal feathered chickens (0.74–0.86). The chi-square value of 5.99 at 2 degrees of freedom was the expected limit for which the calculated (observed) chi-square can be compared. The calculated chi-square value for the frizzle population was 0.02, while that of the normal was 10.74, the naked neck was 0.02, and the entire population was 11.93.
The three genotypes of the hemoglobin obtained were known to be controlled by two co-dominant alleles: the HbA and HbB. Yakubu and Aya (2012), Ajayi et al. (2013), and Ige et al. (2013) observed that the hemoglobin locus is being controlled by two co-dominant alleles and has three genotypes. The overall highest frequency of HbAB obtained in this result agrees with the report of Abdulraheem et al. (2018) who obtained much higher HbAB frequency (88.68% and 72.34%) for cocks and hens of indigenous chicken, respectively, in Borno State, north eastern part of Nigeria. However, the report of Ajayi et al. (2013) revealed that the HbBB genotype had the highest overall frequency. Okamoto et al. (2003) however reported that in general, Asian native fowls were being fixed at the HbB locus with HbA detected at extremely low frequencies in some chickens. While the frequency of HbAA was higher in males than females in this study, a contrary report was given by Ajayi et al. (2013) and Abdulraheem et al. (2018) who observed higher HbAB frequency in males of Nigerian indigenous chickens. However, Yakubu and Aya (2012) reported a higher frequency of AA in females of normal feathered and Fulani ecotype chickens but a lower frequency of AA genotype for males of naked neck chickens. The inconsistency in the frequency of hemoglobin genotypes according to sex shows that sexual dimorphism does not affect the inheritance of hemoglobin.
The differences in the observed frequencies of hemoglobin genotypes in this study and other literature reports could be due to genetic pollution due to indiscriminate mating of the birds while scavenging. Hrinca (2009) had observed that the genetic types of hemoglobin are useful in determining if a breed is indigenous in its area or it has been produced from different crossing systems among other domestic breeds. Moreover, patterns of nucleotide diversity and linkage disequilibrium are indicative of local adaptation of α- and β-globin genes to different altitudinal zones. Hemoglobin genotypes differ in oxygen affinity and aerobic performance (Storz 2010; Cheviron and Brumfield 2011). The high heterozygosity value of 0.56 observed in this study is an index of moderate genetic diversity at the hemoglobin locus of the chickens.
It has been reported that some blood factors are related to the suitability of the breeds under a particular environment. Alphonsus et al. (2012) and Akpa et al. (2013) reported that the frequency of HbA is usually higher than that of HbB in high altitude. Sam et al. (2016) reported high frequency of HbA in goats reared in the north west region of Nigeria characterized by extreme temperature (as high as 38–45 °C) whereas Salako et al. (2007) reported that Red Sokoto goats sampled in the south west of Nigeria (rain forest zone) had a higher frequency of HbB than HbA. However, Sengupta (1976) claimed that HbB was found to be favored in hot arid climate and HbA appeared to be less frequent in hot arid climate than in warm humid climate. Okamoto et al. (2003) also reported that in general, Asian native fowls were being fixed at the HbB locus with HbA detected at extremely low frequencies in some chickens.
In the reports of Pal and Mummed (2014), the authors opined that though differences in blood biochemical parameters exist, the mechanism of polymorphism is not constant. These authors claimed that if hemoglobin type is connected or has any adaptive significance, it should not be different in breeds and species living in the same geographical and climatic region especially hemoglobin whose physiology and role are the same in all animals. This is the situation in this present study with overall equal frequencies (0.5) of HbA and HbB whereas studies on hemoglobin types conducted in the same climatic environment with this work reported higher frequencies of HbA than HbB as reported by Alphonsus et al. (2012), Akpa et al. (2013), and Sam et al. (2016). However, Di vitro et al. (2002) reported that there was no direct evidence of differences among the different hemoglobin types in cattle.
The observed chi-square values for the frizzle population (0.02), normal (10.74), naked neck (0.02), and the entire population (11.93) showed that frizzle and naked neck population were both in Hardy Weinberg’s equilibrium while the normal and entire population were not, considering the expected 5.99 at 2 degrees of freedom. A similar result has also been reported by Yakubu and Aya (2012) who worked on hemoglobin polymorphism in indigenous Fulani, normal, and naked neck chickens of Nigeria and reported that Fulani ecotype and naked neck chickens were in Hardy Weinberg’s equilibrium but the normal feathered chickens were not. The whole population was not in Hardy Weinberg’s equilibrium at the hemoglobin locus, and the chi-square value was 11.93. This may be due to the fact that there was no equal survivability of each genotype, and gene frequency was not the same in each sex. The reason could also be due to the sample size or forces that affect gene and hence genotype frequency in a population (e.g., natural or artificial selection).
Mating or breeding system has a significant effect on the frequency of occurrence of alternative genotypes in a given population. The mating system adopted may be a contributing factor to the deviation from Hardy Weinberg equilibrium since mating among individuals with similar phenotypes was adopted. Besides, the foundation stock was assembled from different geographical locations which may have a migratory effect on gene and genotypic frequencies in the population. Moreover, Iorio et al. (2004) suggested that variables such as protein stability, efficiency of hemoglobin tetramer formation, and other factors can affect the steady state of globin variants and whether quantitative evaluation of both globins and hemoglobins has repeatability. Hardy Weinberg’s equilibrium can only be attained when there is random mating, no selection occurred, no migration, and no mutation and genetic drift (Kinghorn and van-der-Werf 2000).
Effect of hemoglobin genotype on body weight (g), breast girth (cm), and tibia length (cm) of the whole population of indigenous chicken
Table 2 shows the effect of hemoglobin genotype on body weight, breast girth, and tibia length of the combined population of indigenous chicken.
The coefficient of variation (CV%) was low and varied from 7.15 for breast girth at 8 weeks to 18.73% for tibia length at 16 weeks. Birds with HbAA had significantly (P < 0.05) highest value for body weight from day old to 20 weeks (28.18–1296.43 g against 24.73–1029.59 g for HbAB which was also significantly higher than the 22.86–884.46 g obtained for HbBB). This trend of significant differences observed in body weight among hemoglobin genotypes (HbAA, HbAB, and HbBB) was also observed in breast girth and tibia length measured from 4 to 20 weeks of age.
Effect of hemoglobin genotypes on body weight (g), breast girth (cm), and tibia length (cm) within the frizzle indigenous chicken population
Table 3 shows the effect of hemoglobin genotypes on body weight (g), breast girth (cm), and tibia length (cm) within the frizzle indigenous chicken population. The coefficient of variation remains low in this group with only tibia length at 16 weeks of age having moderate value (34.02%). In these frizzled chickens, the trend observed in a combined population of chickens was also observed. HbAA had the highest value for body weight at day old till 20 weeks. However, the values obtained for tibia length at 16 weeks were statistically similar for both HbAA and HbAB (9.05 and 9.06 cm).
Effect of hemoglobin genotypes on body weight (g), breast girth (cm) and tibia length (cm) within the normal indigenous chicken population
Table 4 shows the effect of hemoglobin genotypes on body weight (g), breast girth (cm), and tibia length (cm) within the normal indigenous chicken population. A similar trend as reported earlier was observed in the normal feathered birds. The trend observed here is similar to the earlier values in the combined and frizzle population. The highest coefficient of variation of 15.65% was obtained for body weight at 20 weeks in HbAA. However, there was a similarity in body weight at 16 and 20 weeks for HbAA and HbAB groups.
CV coefficient of variation; SEM standard error of means; LOS level of significance; NS not significant; WT4–20 body weight for weeks 4, 8, 12, 16, and 20, respectively;BG4–20 breast girth for weeks 4, 8, 12, 16, and 20, respectively; TL4–20 tibia length for weeks 4, 8, 12, 16, and 20, respectively
Effect of hemoglobin genotypes on body weight (g), breast girth (cm), and tibia length (cm) within the naked neck indigenous chicken population
Table 5 shows the effect of hemoglobin genotypes on body weight (g), breast girth (cm), and tibia length (cm) within the naked neck indigenous chicken population. The highest coefficient of variation of 17.51% was obtained for body weight at 20 weeks. HbAA group consistently had the highest value for all parameters followed by HbAB and the least was HbBB. However, values obtained for breast girth at 12 weeks were similar in HbAA and HbBB (18.38 and 18.13 cm).
The significant differences (P < 0.05) in weight, breast girth, and tibia length along the different hemoglobin genotypes available (HbAA, HbAB, and HbBB) may be due to differences in polymorphic forms, which might have resulted from their amino acid and carbohydrate compositions. It is believed that genetic polymorphisms at an aminopeptidase locus have direct influences on protein metabolism, including associated influences on energy flux and animal condition. Energy costs associated with protein turnover play a clear role in determining the physiological and evolutionary consequences of genetic polymorphism. Reduced whole-body protein turnover consistently underlies lower energy expenditure and higher growth efficiencies (net energy balance per unit of energy absorbed). In turn, slower protein turnover, lower energy expenditure, and/or higher growth efficiencies have been shown to represent the metabolic basis of positive associations between growth and polymorphic locus (Hawkins and Day 1999).
The result of the present study confirms the report of Das and Deb (2008), which stated that hemoglobin polymorphism/polymorphic forms affect growth rate and hatchability. The authors indicated that hatchability was highest in HbAA followed by HbAB and lowest in HbBB. There is a paucity of information on the relationship between hemoglobin polymorphic forms and performance characteristics in chickens. However, Yakubu et al. (2014) reported higher body weight and heart girth of goats with HbAA type. Akinyemi and Salako (2010) reported that wool fiber and horn length were higher in sheep with HbAA hemoglobin type compared to HbBB type while Abdulmojeed et al. (2014) reported a positive relationship of body mass and heart volume in West African dwarf goats in the central Nigeria with HbAA genotype. Vazic et al. (2017) had reported that Ewes with two lams had a higher frequency of HbBB genotype in relation to ewes with single lamb while ewes with single lamb had a higher frequency of HbAA genotype. Also, Iyiola-Tunji et al. (2014) reported that lambs that were born to mothers with HbAB hemoglobin types had a higher survival rate compared to lambs born to ewes with HbAA and HbBB.
The higher body weight, breast girth, and tibia length of birds with HbAA type is an indication of favorable natural selective advantage. The present study showed that the hemoglobin genotypes serve as a better biochemical discriminator of weight, breast girth, and tibia length for selection. This phenomenon suggests that some of the biochemical proteins have powerful biological interactions with physiological growth of the body than others, just as some do not even indicate growth but are recorders of other traits. Das and Deb (2008) reported series of biochemical/biological enzymes and the activities of each of them, namely serum alkaline phosphatase, carbonic anhydrase, plasma albumin variant, pre-albumin variant, and egg albumin variants.
Summary
The present research work examined the possible effect of hemoglobin polymorphisms on performance traits in indigenous chicken genotypes in order to establish the basic genetic activities which contribute to the differences observed in terms of body weight, breast girth, and tibia length among the Nigerian indigenous chicken genotypes (frizzle, normal, and naked neck). There were differences among the hemoglobin polymorphic forms in terms of body weight breast girth and tibia length.
Conclusion
Hemoglobin polymorphism was distinct in three genotypic forms which were HbAA, HbAB, and HbBB. The birds were more of heterozygotes (HbAB) than either of the homozygotes (HbAA or HbBB) with HbAA (49) HbAB (56) and HbBB (50), respectively. This means more of the heterozygotes adapted and survived better than the homozygotes. The total population was not in Hardy Weinberg’s equilibrium. The relationship between body weight (g), breast girth (cm), tibia length (cm), and the polymorphic forms of the biochemical markers showed that the HbAA (1296.43 g) genotype had a significant (P < 0.05) higher body weight than the HbAB (1029.59 g) and HbBB (884.46 g), respectively. This implies that there is an additive genetic superiority of the different polymorphic forms, and therefore, they could be used for selection.
Recommendations
Hemoglobin polymorphic forms may be used for selection for body weight and related parameters in the indigenous chicken. More research is recommended on the effect of the polymorphic forms of blood biochemical parameters on the reproductive traits of the indigenous chickens. There should be further studies relying on molecular tools into the genetic resources of all the indigenous poultry species of the country with the aim of improving their reproduction, production, and general uses to mankind.
References
Abdulmojeed, Y., Haruna, K.A., Ibrahim, S.M.A., Rowland, E.B and Abdulrazak, O.R (2014): Preliminary investigation of hemoglobin polymorphism and association with morphometric traits in West African Dwarf goats in north central Nigeria. Mljekarstvo, 64 (1): 57–63.
Abdulraheem A. O., Alade N. K., Aliyu J., Raji, A. O. and Mohammed I. D. (2018). Analysis of haemoglobin polymorphisms of indigenous chickens in Borno State, Nigeria. Nigerian Journal of Animal Science and Technology,1 (2):53–70
Adeleke, M. A., Peters, S. O., Ozoje, M. O., Ikoebi, C. O. N., Bamgbose, A. M and Adebambo, O. A. (2011). Genetic parameter estimates for body weight and linear body measurements in pure and crossbred progenies of Nigerian indigenous chickens. Livestock Research for Rural Development, 23 (01): 1–4
Ajayi, F. O (2010). Nigerian indigenous chicken: a valuable genetic resource for meat and egg production. Asian Journal of Poultry Science 4:164–172.
Ajayi, F. O., Agaviezor, B. O. and Wihioka S. N. (2013). Haemoglobin genotypes in the Nigerian indigenous chicken in the Niger Delta region of Nigeria. International Journal of Advanced Biological Research, 3(1): 13–16
Akinyemi, M.O and Salako, A.E (2010) Haemoglobin polymorphism and morphometrical correlates in the West African Dwarf sheep of Nigeria. International Journal of Morphology, 28: 205–208
Akpa, G. N., Ibrahim, O. A., Yakubu, H. and Kabir, M. (2011). Sexual dimorphism, haemoglobin polymorphism and body mensuration characteristics of red sokoto goats. In: Ado, S. G., Akpa, G. N., Dahiru, A. U., Mu’azu, S., Kabir, M., Adeyinka, I. A., Adamu, A. K. and Usman I. S. (Eds.), Genetics in Food Security, Health and Environment, pp 2–3. Proceedings of 35th Annual Conference of Genetics Society of Nigeria (GSN) held at Ahmadu Bello University, Zaria Kaduna State.
Akpa, G. N., Alphonsus, C and Usman, N (2013). Effect of age, sex and haemoglobin type on adaptive and blood biochemical characteristics in red Sokoto Goats. Journal of Research in Biology, 3(3): 870–875
Alphonsus, C., Akpa, G. N., Usman, N., Barje, P. P. and Byanet, O. (2012). Haemoglobin polymorphism and its distribution in smallholder goat herds of Abuja Nigeria. Global Journal of Molecular Sciences, 7(1): 11–14
Cheviron, Z.A., Brumfield, R.T. (2011): Genomic insights into adaptation to high-altitude environments. Heredity https://doi.org/10.1038/hdy.2011.85
Chineke, C.A., Ologun, A.G and Ikeobi, C.O.N 2007. Haemoglobin types and production traits in rabbit breeds and crosses. Journal of Biological Sciences. 7:210–214
Christensen, K. (2003). Population genetics. Retrieved on www.ihh.kvl.dk/htm/kc/popgen/genetics/genetik.htm. P 1–20. Accessed 11 May 2014
Das, A. K. and Deb, R. (2008). Biochemical polymorphism and its relation with some traits of importance in poultry. Veterinary World, Indian Veterinary Research Institute. 1(7): 220–222.
Di Vitro, A., Schwantes, A.R and Schwwantes, M.I.B (2002). Functional properties of the three hemoglobin phenotypes of Nelore cattle. Genetics and Molecular Biology, 25 (2): 135–138
Guèye, H.F. (1998). Village egg and fowl meat production development, 15, 5:2003. http://www.cipav.org.co/ in Africa. World’s Poult. Sci. J., 54: 73–86. Accessed 1 Jan 2013
Hawkins, A. J. S. and Day, A. J. (1999). Metabolic interrelations underlying the physiological and evolutionary advantages of genetic diversity. American Zoology, 39:401–411.
Horst, P. (1999). Evaluation of local poultry resources for creating genetic stock with improved adaptability, productivity and disease resistance in tropical environments. Summary reports of European Commission supported STD-3 projects (1992–1995). CTA. Wageningen. Pp 196–203
Hrinca, G. H (2009): Redefining the historical relations in ovicaprinae using biochemical-genetic and immunoserological tests. Annals of RSCB 14, 79–90.
Ige, A.O., Salako, A.E., Ojedapo, L.O and Adedeji, T.A (2013). Biochemical characterization of indigenous Fulani and Yoruba ecotypes chicken of Nigeria. African Journal of Biotechnology, 12(50): 7002–7008
Iorio , M., Vincenti, D., Annunziata , M., Rullo , R., Bonamassa , R., Luccia, A. D and Pieragostini , E (2004). Biochemical and molecular investigations on qualitative and quantitative Hb polymorphism in the river buffalo (Bubalus bubalis L.) population reared in Southern Italy. Genetics and Molecular Biology, 27 (2): 167–173
Iyiola-Tunji, A.O., Akpa, G.N., Nwagu, B.I and Adeyinka, I.A (2014). Survivability of lambs in relation to their dams haemoglobin variants. Biotechnology in Animal Husbandry, 30 (2): 215–223
Kinghorn, B. and van-der-Werf, J. (2000). Identifying and incorporating genetic markers and major genes in animal breeding programs. Retrieved on www.personal.une.edu.au/~jvanderw/Coursecover.PDF, 17/5/2012. Accessed 5 Jan 2018
Mwacharo, J. M., Otieno, C. J., and Okeyo A. M. (2005). Suitability of blood protein polymorphisms in assessing genetic diversity and relationships in population genetic studies. In: Makkar, H. P. S. and Viljoen, G. J. (eds). Applications of gene-based technologies for improving animal production and health in developing countries, Pp 585–591.
Okamoto S., Inafuku K., Ting Z., Maeda Y., Hou D., Tamg Y., Yun Z., Xu W., Shi L and Hashiguchi T. (2003): Blood protein polymorphisms in native chicken breeds in Yunnan province in China. Animal Science Journal, 74( 6), 471–476.
Ovimaps, (2012). Ovilocation maps; ovi earth imaginary. Dated July, 2012.
Pal S. K and Mummed Y. Y (2014). Investigation of haemoglobin polymorphism in Ogaden cattle. Veterinary World, 7(4): 229–233.
Rege, J. E. O and Okeyo, A. M. (2006). Improving our knowledge of tropical indigenous animal genetic resources. In: Ojiango, J. M.; Malmfors, B. and Okeyo, A.M. (Eds)., Animal Genetic Training Resource, version 2. International Livestock Research Institute, Kenya, Nairobi and Swedish University of Agricultural Sciences, Uppsala. Retrieved on www.agtr.ilri.cgiar.org/Documents/Modules/Improvingourknowledge.pdf. Accessed 1 Jan 2013
RIKEN (2006). Genetic quality monitoring by biochemical marker isoenzymes. Experimental Animal Division of RIKEN BioResource Center, Illinois. Pg 1–12. Retrieved on www.brc.riken.jp/lab/animal/en/quality.shtml. Accessed 27 Feb 2019
Salako A.E and Ige A.O (2006). Haemoglobin polymorphisms in Nigerian indigenous chickens. Journal of Animal and Veterinary Advances.5(11):897–900
Salako, A. E., Ijadunola, T. O. and Agbesola, Y. O. (2007). Haemoglobin polymorphism in Nigerian indigenous small ruminant populations: preliminary investigation. African Journal Biotechnology, 6(22): 2636–2638
Sam, I.M., Akpa, G.N., Alphonsus, C., Nwagu, B.I and Adeyinka, I.A (2016). Haemoglobin and potassium polymorphism in agro-pastoral goats herd from Sudan savannah zone of Nigeria. Animal Research International, 13(3):2475–2482
SAS, (2002). Statistical Analysis System, Users Guide, Statistical Analysis Institute Inc. Cary, North Carolina
Sengupta, B.P (1976). Hemoglobin polymorphism and its positive significance in Zebu cattle. India Journal of Animal Science, 46(2): 72–76
Sorenson, P. (2009). Poultry genetic resources in the context of high pathogenic avian influenza (HPAI). Family Poultry, 18 (1 and 2): 26.
Steppa, J., Wójtowski, J., Sylwia, B. and Maria K. (2009). Effect of transferrin and haemoglobin polymorphism on hygienic quality of milk in sheep. Züchtungskunde, 81(2): 125–132,
Storz, J.F. (2010): Genes for high altitude. Science 329, 40–41.
Vazic, B.S., Rogic, B.S., Drinic, M.S and Przulj, N.M (2017). Relationship between the genetic hemoglobin polymorphism, morphometry and fertility of Pramenka sheep breed from cental Bosnia. Genetika 49 (1): 151–160
Yakubu, A. and Aya, V. E. (2012). Analysis of genetic variation in normal feathered, naked neck and Fulani-ecotype Nigerian indigenous chickens based on haemoglobin polymorphism. Biotechnology in Animal Husbandry, 28 (2): 377–384.
Yakubu, A., Aimiku,H.K., Musa-Azara, I.S., Barde, R.E and Raji, A.O 2014. Preliminary investigation of haemoglobin polymorphism and association with morphometric traits in West African Dwarf goats in north central Nigeria. Mljekarstvo, 64(1): 57–63
Acknowledgments
The authors are grateful to the Department of Animal Science, Ahmadu Bello University, Zaria, Nigeria, for providing the facilities for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
The study was carried out following standard guidelines for handling animals.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Orunmuyi, M., Muhammad, H., Musa, A.A. et al. Effect of hemoglobin polymorphism on performance traits in indigenous chicken genotypes in Nigeria. Trop Anim Health Prod 52, 2395–2403 (2020). https://doi.org/10.1007/s11250-020-02257-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-020-02257-y