Abstract
The present study was conducted to evaluate the adverse effects of an interaction between low levels of dietary aflatoxins (AF) and Eimeria tenella infection on broiler chicks. A set of 1-day-old chicks were raised for 35 days in the following groups: a control group, a group fed AF, a group fed AF and inoculated with E. tenella (AF + E.ten), and a group inoculated with E. tenella alone. AF in the contaminated diet were given at 200 ppb starting from the seventh day after hatching while E. tenella was inoculated at a dose of 5 × 104 sporulated oocysts per chick at the 14th day after hatching. Worsened performance traits and high mortality were all observed in the treated birds, particularly the AF + E.ten group. Lesion scores and oocyst outputs were not different within groups. Chickens fed with AF had significantly increased serum ALT and ALP activities as well as decreased albumin content. They also showed hepatomegaly, hepatocytic vacuolation and necrosis, an atrophied bursa of Fabricius, and a thymus with tissue depletion. E. tenella-infected broilers displayed a significant reduction in packed cell volume, hemoglobin content and lymphocyte percentage, and showed hemorrhagic typhlitis. The deficits in hepatic function and hematologic parameters as well as the gross pathological, and histopathological changes, were more common and more severe in the group that was exposed to both aflatoxicosis and coccidiosis than in the groups exposed to either treatment alone. Thus, the combination of aflatoxicosis and E. tenella infection may influence the course of coccidial infection due to additive effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aflatoxins (AF) are extremely toxic metabolites produced in grains contaminated by the fungi Aspergillus flavus and Aspergillus parasiticus. Among all mycotoxins, AF have generated the greatest public health concern because of the effects that AF-contaminated feeds may have on the growth and health of poultry, and also their possible transmission to humans via meat and egg contamination. Aflatoxicosis is one of the most serious diseases of poultry because of the widespread occurrence of AF in several food grains. AF is particularly present in corn, which comprises between 50% and 60% of most poultry diets (Miazzo et al. 2000). At low levels of feed contamination with AF (<500 ppb), exposed chickens show anorexia, decreased growth rate, poor food utilization, decreased weight gain, decreased egg production, increased susceptibility to environmental and microbial stressors, and increased mortality (Oğuz and Kurtoglu 2000). It is generally accepted that the toxic effects of AF are mainly localized in the liver and are characterized by hepatobiliary damage and increased hepatic enzyme activity (Ortatatli et al. 2005). Moreover, aflatoxicosis is characterized by decreased serum concentrations of total protein, albumin, and total cholesterol (Oğuz et al. 2000); however, the descriptions of the clinical, biochemical, and pathological findings vary considerably because secondary disease arises spontaneously with AF-induced hepatopathy in chickens (Bakshi et al. 2000). In fact, aflatoxicosis decreases resistance to common infectious diseases including coccidiosis because of impairment of humoral and cellular immune responses (Bakshi et al. 2000; Saif 2003).
Coccidiosis is an enteric parasitic disease caused by multiple protozoan parasite species of the genus Eimeria and is one of the most common and expensive diseases in the poultry industry (Shirley et al. 2007). It has a major economic impact on both growers and the poultry industry worldwide (Pinard-van der Laan et al. 2009). It is responsible for 6–10% of all broiler chicken mortalities and causes annual global economic losses due to impaired feed conversion, depressed growth, downgrading at processing, and mortality (Tipu et al. 2002) as well as the cost of medication. Eimeria tenella (E. tenella) is one of the most pathogenic Eimeria organisms that parasitizes growing chickens and causes considerable financial loss to the poultry industry (Williams et al. 1999). In Egypt, the most common Eimeria spp. is E. tenella, which has an infection rate of 20–100% and a mortality rate of 20–60% with concomitant severe reductions in weight gain and feed efficiency (Abd El-Hamid 2007). At present, the literature on aggravation of cecal coccidiosis in broiler chickens fed an AF-contaminated diet is scarce. Consequently, the present study was designed to clarify the complicated clinicopathological findings that result from interactions between low-level dietary AF and E. tenella infection in broiler chickens.
Material and methods
Parasite samples
Field isolates of E. tenella were obtained from a broiler farm with an outbreak of bloody diarrhea with high mortality and were used for the experimental infections. Cultures were identified, purified, and propagated according to Davies et al. (1963). The appropriate infective dose for experimental infection was previously determined to be 5 × 104 sporulated oocysts per chick (Abuakkada and Ellakany 2008).
Production of aflatoxin
AF were produced by growing standard aflatoxigenic strains on sterile polished rice by the method modified by West et al. (1973). Briefly, the rice was cleaned, washed, and autoclaved at 121°C for 15 min, dispensed into 500-ml Erlenmeyer flasks, and moistened with distilled water (10 ml/flask). Each flask was injected with 10 ml of a fresh saline spore suspension of Aspergillus flavus containing 108 spores per milliliter and then sealed with a tight cotton cork. The flasks were incubated for 2 days at 18°C, then for another 5 days at 26°C. The flasks were shaken vigorously everyday to prevent clumping of the rice, to ensure a homogenous toxin distribution, and to prevent fungal overgrowth. Finally, the flasks were sterilized by autoclaving to kill the fungus and its spores, and the toxins were restored. The rice was dried and ground into powder in an electric blender. No other mycotoxins were produced in this solid substrate fermentation process. The rice powder was incorporated into the diet of broiler chickens to provide a final concentration of 200 ppb total aflatoxins (AF) (Sakhare et al. 2007). AF were detected quantitatively in rations by using affinity column chromatography (Aflatest 10, Naremco, Springfield, IL, USA) and fluorometry (Sequcia Tuner Model 450 with a 360 nm excitation filter and a 450 nm emission filter) according to the method of Nabney and Nesbitt (1965).
Experimental animals
A total of 180 1-day-old Hubbard broiler chicks were reared for 5 weeks on a wire floor with electric starter batteries where feed and water were supplied without restriction. They were fed a commercial broiler homemade starter feed that was free from anticoccidial drugs. All animals received humane care in compliance with the animal care guidelines of the National Institutes of Health, and the local ethics committee approved the experimental design.
Experimental design
On the seventh day after hatching, all chicks were weighed and randomly allocated into one of four main groups (45 chicks each) with each group including three replicates (15 chicks each). The chicks were ranked by restricted randomization procedures that approximately equalized the initial body weights among the different groups. Group I (control group) received the control ration but no AF and served as a control. Group II (AF group) received the AF-containing basal diet. Group III (AF + E.ten group) received the AF-containing diet and was infected with sporulated oocysts of E. tenella. Group IV (E. tenella group) received the control basal diet with no AF but was infected with E. tenella. Before infection, fecal samples from all chicks were examined microscopically to verify that they were free from E. tenella infection.
All birds were individually weighed weekly starting from the seventh day after hatching until the end of the experiment. The AF-containing ration (200 ppb) was administered to chicks in groups II and III starting from the seventh day after hatching until the end of the experiment. On the 14th day after hatching, E. tenella-infected chicks (groups III and IV) were directly inoculated in the crop with 5 × 104 sporulated oocysts per chick using an insulin syringe. Performance traits, including body weight, body weight gain, and feed conversion ratio (FCR) as well as mortality, oocyst count, lesion scoring, hematological and biochemical parameters, and pathological examinations were evaluated.
Measurements and evaluation parameters
Oocyst count and lesion scores
Oocyst count per gram of fecal material (OPG) was evaluated from the seventh to the 14th day post-infection (PI). Five samples from each replicate were collected daily and OPGs were counted using the McMaster counting technique (Long et al. 1976). Seven days PI, the lesion scores of E. tenella were evaluated according to the method of Johnson and Reid (1970), in which three chicks from each replicate were sacrificed by slaughtering, and cecal lesions (Fig. 1) were scored as follows:
-
Score 1 Few scattered petechiae on the cecal wall
-
Score 2 Noticeable blood in the cecal contents with thickened cecal wall
-
Score 3 Blood or cecal cores and severely thickened cecal wall
-
Score 4 Severely distended cecal wall with bloody cores or the bird is dead
Photograph of ceca from broiler chickens inoculated with E. tenella (5 × 104 sporulated oocyst/chick) (groups III and IV). Gross cecal lesions can be seen. a Score 2: noticeable blood in the cecal contents with thickened cecal wall; b Score 3: blood or cecal cores and severely thickened cecal wall; c Score 4: severely distended cecal wall with bloody cores
Hematological and biochemical parameters
On the seventh day PI, blood samples were collected during slaughtering from all groups using disposable tuberculin syringes. Package cell volume (PCV, hematocrit) content (Jain 1993) and hemoglobin content (Hb grams per deciliter) (Drabkin 1948) were measured. The differential leukocytic counts were determined by counting encountered elements on blood smears under the microscope according to Hawk (1965). Blood samples were also collected without anticoagulants for serum separation for the evaluation of liver function. The activities of serum alanine aminotransferase (ALT) and alkaline phosphatase (ALP) were estimated per the method of Kind and King (1954) using commercially available kits supplied by bioMérieux (France). Serum albumin content was determined spectrophotometrically per the method of Varley et al. (1980).
Histopathological examination
From chicks examined for lesion scores, cecal parts as well as specimens from liver, bursa, and thymus were collected. The specimens were rapidly fixed in 10% neutrally buffered formalin for at least 24 h. The fixed specimens were processed through the conventional paraffin embedding technique. From the prepared paraffin blocks, 4-μm thick sections were obtained and stained with Hematoxylin and Eosin (H & E).
Statistical analysis
Results were statistically analyzed by one-way analysis of variance followed by Duncan’s multiple range test (SAS 2001). Data are presented as means ± the standard error. The minimum level of significance was set at p < 0.05.
Results
Oocyst count
Table 1 shows that the broiler chickens from the group fed with AF and inoculated with E. tenella (AF + E.ten) had a significantly higher number of oocysts (p < 0.05) at the seventh, 11th, and 14th days PI when compared to birds from the E. tenella group; however, the total oocyst values were similar in all E. tenella-inoculated birds. Non-infected animals had negative oocyst counts.
Mortality rates, performance traits, and lesion scores
The data in Table 2 show that the highest mortality rate occurred in chickens from the AF + E.ten group (20.0%), followed by those infected with E. tenella alone (13.3%) and then the AF-treated chickens (10.0%). There was a significant (p < 0.05) reduction in the final body weight in all groups when compared to controls, with the greatest decrease in the AF + E.ten group (1,133.75 g), followed by the AF group (1,172.22 g), and then the E.ten group (1,354.29 g). Moreover, the FCR at the end of the experimental period was generally worse in the AF-treated and E. tenella-infected groups. Unexpectedly, the values of cecal lesion scores at 7 days PI of chickens infected with E. tenella did not differ significantly (p < 0.05), even with the addition of AF.
Hematological and serum biochemical profiles
Results of hematological and serum biochemical profiles at 7 days PI of the control and investigational broiler chickens infected with E. tenella are given in Table 3. Significant reductions (p < 0.05) in PCV%, Hb content and lymphocyte percentage were observed in E. tenella-infected broiler chickens (groups III and IV) compared to the AF-treated and control specimens. Monocyte, basophil, and eosinophil values were comparable in all groups while heterophils percentage significantly (p < 0.05) increased in E. tenella-infected and/or alflatoxin-treated groups. Administration of AF significantly enhanced (p < 0.05) serum ALT and ALP enzyme activities and lowered serum albumin content when compared to the control group. Control animals had values within normal limits.
Pathological findings
Gross pathology
Broiler chickens fed AF-contaminated rations (groups II and III) each had an enlarged and yellow–colored liver, along with an atrophied bursa of Fabricius and thymus. Conversely, chickens inoculated with E. tenella (groups III and IV) had ceca that were enlarged and thickened with hemorrhagic contents, as well as congested and hemorrhagic mucosa (Fig. 1). No gross pathological changes were observed in the control animals.
Histopathology
Liver
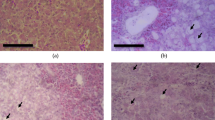
The livers of the control group exhibited normal hepatic architecture of intact portal areas and normal hepatocytes with rounded vesicular nuclei (Fig. 2). The livers of the AF + E.ten group showed severe diffuse hepatocytic vacuolation (Fig. 3) and the presence of multifocal areas of coagulative necrosis represented by pyknotic and karyorrhectic hepatocytes (Fig. 4). These findings were less severe in the AF group.
Photomicrograph of a liver from broiler chickens fed an AF-contaminated diet (200 ppb) and inoculated with E. tenella (5 × 104 sporulated oocyst/chick) (group III, AF + E.ten). Focal areas of coagulative necrosis represented by pyknotic and karyorrhectic hepatocytes (arrow heads) can be seen (H & E, bar = 50 μm)
Cecum
Severe cecal lesions were observed in E. tenella-inoculated broiler chickens (groups III and IV) without dissimilarity in between. These lesions showed severe mucosal and submucosal congestion, edema and hemorrhage, as well as numerous heterophils. Additionally, the mucosal epithelium showed severe diffuse degeneration, necrosis, and desquamation as well as the presence of numerous intracellular developmental schizonts (oval structures containing basophilic banana-shaped merozoites) (Figs. 5, 6). Ceca from the AF group showed degenerative and necrotic changes as well as desquamation of the mucosal epithelium.
Photomicrograph of a cecum from a broiler chicken fed an AF-contaminated diet (200 ppb) and inoculated with E. tenella (5 × 104 sporulated oocyst/chick) (group III, AF + E.ten). Severe necrosis and desquamation of the mucosal epithelium can be seen, with replacement by schizonts (a) next to the submucosal hemorrhage (b) (H & E, bar = 100 μm)
Photomicrograph of a cecum from a broiler chicken fed an AF-contaminated diet (200 ppb) and inoculated with E. tenella (5 × 104 sporulated oocyst/chick) (group III, AF + E.ten). Numerous intracellular schizonts containing banana-shaped merozoites (arrows) can be seen with severe submucosal hemorrhage and infiltration of numerous heterophils (arrow heads) (H & E, bar = 50 μm)
Lymphoid organs
Bursal follicles exhibited necrotic changes in the AF-treated groups (group II and III), with higher severity in cases of E. tenella infection. There was severe diffuse lymphocytic cell necrosis and depletion that gave the bursal follicle a moth-eaten appearance (Fig. 7). Some bursal follicles showed large cystic structures devoid of lymphocytes and containing faint eosinophilic necrotic debris (Fig. 8). Thymic follicles of AF-treated groups (group II and III) showed apoptotic changes with a resulting “starry-sky” appearance with higher severity in cases of E. tenella infection (Fig. 9) along with inter-follicular congestion.
Photomicrograph of a bursa of Fabricius from a broiler chicken fed an AF-contaminated diet (200 ppb) and inoculated with E. tenella (5 × 104 sporulated oocyst/chick) (group III, AF + E.ten). A large cystic cavitation in a bursal follicle containing faint eosinophilic necrotic debris (a) can be seen (H & E, bar = 100 μm)
Discussion
The profitability of poultry production can be greatly affected by feed contamination with AF and its subsequent detrimental effects on animal performance. In the course of aflatoxicosis in broiler chickens experimentally inoculated with E. tenella, there was a complicated clinical picture of cecal coccidiosis involving impairment of animal performance traits in addition to hematological, biochemical, parasitological, and histopathological alterations. In chickens, AF in the diet can act as a stress factor to increase the susceptibility to, or severity of, cecal coccidiosis (Saif 2003). Increased susceptibility of AF-exposed chickens to infectious diseases indicates an impaired immune response and a breakdown of vaccinal immunity (Bakshi et al. 2000).
The present study shows that the dietary AF not only increases the mortality rates of E. tenella infection in broiler chickens but also causes a significant depression in average body weight and a worsened FCR as also observed by Allameh et al. (2005). The decreased growth and worsened FCR may be due to anorexia, listlessness, and the toxic effects of AF on protein synthesis and lipogenesis (Oğuz and Kurtoglu 2000). AF can also affect the balance between orexigenic and anorexigenic circuits, which normally regulate the homeostatic loop of body weight regulation, but cause cachexia when they malfunction (Rastogi et al. 2001). The mortality changes observed in this study were due to AF-induced decreases in tissue integrity and increased susceptibility to hemorrhage in multiple tissues (Dalvi 1986). AF also interferes with normal blood clotting in chickens, specifically via extrinsic and common clotting pathways (Dalvi 1986). This is in addition to the additive effect of E. tenella infection, which causes death primarily due to hemorrhage.
Our oocyst count and lesion score data were surprising, as there were no significant differences between E. tenella-infected chickens (groups III and IV) even upon exposure to AF. These results clearly indicate that low levels of AF (200 ppb) do not interfere with the developmental stages of E. tenella in broiler chickens. This is different from the effects observed at high levels of AF (1–5 ppm), which cause increased oocyst production and reproductive potential during chicken coccidiosis (Toulah 2007; Shareef 2010). It is also evident from the examined parasitic parameters that lesion scores reflect the oocyst counts (Toulah 2007; Shareef 2010).
The significant reduction in PCV% and Hb content in E. tenella-infected broiler chickens (groups III and IV) were likely derived from E. tenella rather than low levels of AF, which alone did not induce such changes. It was previously reported that broiler chickens exposed to high levels of AF suffered from anemia and malnutrition (Mani et al. 1993). Anemia is a consistent finding with coccidiosis that arises from blood loss, but not from dietary aflatoxicosis, at the level currently studied. The lymphocyte counts were significantly decreased in all treated groups in comparison with the control group, indicating a depression of cell-mediated immunity caused by both AF (Ghosh et al. 1991; Bakshi et al. 2000) and E. tenella (Lillehoj and Trout 1993). The heterophil percentages were increased in treated birds since they are the initial responders to numerous pathogens and irritants (Redmond et al. 2009) and to adapt stress condition (Aengwanich 2007). Moreover, the increases in ALT and ALP activities in AF-treated birds correspond well with the subsequent detrimental changes in the hepatic tissues and biliary system. Marked decreases in the levels of albumin may indicate protein catabolism or decreased plasma amino acid concentrations (Sakhare et al. 2007). Moreover, it might be the consequence of blood loss in the intestine because of hemorrhages caused by Eimeria infection.
One of the most important signs of AF toxicity in broilers is the change in internal organs. The liver often increases in size while the bursa of Fabricius and the thymus decrease in size (Sur and Celik 2003; Saif 2003). Ortatatli et al. (2005) found no macroscopic changes in these target organs at 50–100 ppb of AF. This observation suggests that macroscopic investigations of organs may be a reliable parameter at more than 200 ppb of AF but should not supersede a histopathological investigation. Moreover, gross investigations in this study showed that both with and without dietary AF, the changes in the appearance of E. tenella-infected ceca clearly represented hemorrhagic typhlitis (Saif 2003). Hepatomegaly, fatty changes, hepatocytic vacuolation, and necrosis were all observed, similar to the previous findings described for aflatoxicosis (Karaman et al. 2005; Ortatatli et al. 2005). These findings were all more pronounced in the AF + E.ten group. Such detrimental changes could be ascribed either to a general inhibition of lipid transport (Tung et al. 1972) or to interference with lipogenesis (Donaldson et al. 1972) as a response to aflatoxicosis. The hemorrhagic and degenerative changes in the ceca of E. tenella-inoculated broiler chickens at 7 days PI are often a consequence of E. tenella localization in this site. In this study, such changes were consistent with those associated with infection induced by Eimeria spp. (Stoev et al. 2002; Saif 2003; Zulpo et al. 2007). Moreover, the degenerative changes and decreased lymphoid tissues in the bursa of Fabricius and the thymus in the AF-treated groups (groups II and III) may be the cause of the high severity of cases of E. tenella infection. These changes are indicative of the immunosuppressive consequences of AF administration and the consequent sensitivity to various infections and parasitic diseases (Stoev et al. 2002).
In conclusion, our results indicate that alterations in performance traits and mortality rate, impairments of hepatic function and hematological parameters, and gross and histopathological changes were greater when broiler chickens were exposed to dietary AF at 200 ppb and concurrently infected with E. tenella. This synergy arises because these effects are derived from both diseases.
References
Abd El-Hamid, H.S., 2007. A project studies on chicken coccidiosis in Egypt presented to the Academy of Scientific Research and Technology, p. 173.
Abuakkada, S.S. and Ellakany, H.F., 2008. Sensitivity of two field isolates of Eimeria tenella from broiler chickens to salinomycin and diclazuril in a battery trial, Proceedings of the 13th Scientific Congress of the Faculty of Veterinary Medicine (Assuit University, Egypt), 347–364.
Aengwanich, W., 2007. Comparative ability to tolerate heat between Thai indigenous chicken, Thai indigenous chicken crossbred and broilers by using heterophil/lymphocyte ratio, Pakistan Journal of Biological Science, 10, 1840–1844.
Allameh, A., Safamehr, A., Mirhadi, S.A., Shivazad, M., Razzaghi-Abyaneh, M. and Afshar-Naderi, A., 2005. Evaluation of biochemical and production parameters of broiler chicks fed ammonia treated aflatoxin contaminated maize grains, Animal Feed Science and Technology, 122, 289–301.
Bakshi, C.S., Asikdar, T., Johri, S., Malik, M. and Singh, R., 2000. Effect of grade dietary levels of aflatoxin on humoral immune response in commercial broilers, Indian Journal of Comparative Microbiology, Immunology and Infectious Diseases, 21,163–164.
Dalvi, R.R., 1986. An overview of aflatoxicosis of poultry: Its characteristics, prevention and reduction, Veterinary Research Communications, 10, 429–443.
Davies, S.F.M., Joyner, L.P. and Kendall, S.B., 1963. Coccidiosis, (Oliver and Boyd Company, London).
Donaldson, W.E., Tung, H.T. and Hamilton, P.B., 1972. Depression of fatty acid synthesis in chick liver (Gallus domesticus) by aflatoxin, Comparative Biochemistry and Physiology - B Biochemistry and Molecular Biology, 41, 843–847.
Drabkin, D.L. 1948. The standardization of hemoglobin measurement, The American Journal of the Medical Sciences, 215, 110–111.
Ghosh, R.C., Chauban, H.V.S. and Jha, G.J., 1991. Suppression of cell-mediated immunity by purified aflatoxin B1 in broiler chicks, Veterinary Immunology and Immunopathology, 28, 165–172.
Hawk, P.B., 1965. Hawk’s Physiological Chemistry. 14th Ed, (McGraw-Hill, New York).
Jain, N.C., 1993. Essentials of Veterinary Hematology, (Lea and Febiger, Philadelphia).
Johnson, J. and Reid, W.M., 1970. Anticoccidial drugs: Lesion scoring techniques in battery and floor-pen experiments with chickens, Experimental Parasitology, 28, 30–36.
Karaman, M., Basmacioglu, H., Ortatatli, M. and Oguz, H., 2005. Evaluation of the detoxifying effect of yeast glucomannan on aflatoxicosis in broilers as assessed by gross examination and histopathology, British Poultry Science, 46, 394–400.
Kind, P.R. and King, E.J., 1954. Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine, Journal of Clinical Pathology, 7, 322–326.
Lillehoj, H.S. and Trout, J.M., 1993. Coccidia: A review of recent advances on immunity and vaccine development, Avian Pathology, 22, 3–31.
Long, P.L., Millard, B.J., Joyner, L.P. and Norton, C.C., 1976. A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis, Folia Veterinaria Latina, 6, 201–217.
Mani, K., Narhari, D. and Kumara, J.R., 1993. Ramamoorthy N. Influence of dietary aflatoxin B1 on certain haematological and biochemical characters of broiler chicken, Indian Veterinary Journal, 70, 801–804.
Miazzo, R., Rosa, C.A.R., De Queiroz Carvalho, E.C., Magnoli, C., Chiacchiera, S.M., Palacio, G., Saenz, M., Kikot, A., Basaldella, E. and Dalcero, A., 2000. Efficacy of synthetic zeolite to reduce the toxicity of aflatoxin in broiler chicks, Poultry Science, 79, 1–6.
Nabney, J. and Nesbitt, B.F., 1965. A spectrophotometric method for determining the aflatoxins, The Analyst, 90, 155–160
Oğuz, H. and Kurtoglu, V., 2000. Effect of clinoptilolite on performance of broiler chickens during experimental aflatoxicosis, British Poultry Science, 41,512–517.
Oğuz, H., Keçeci, T., Birdane, Y.O., Önder, F. and Kurtoǧlu, V., 2000. Effect of clinoptilolite on serum biochemical and haematological characters of broiler chickens during aflatoxicosis, Research in Veterinary Science, 69, 89–93.
Ortatatli, M., Oğuz, H., Hatipoǧlu, F. and Karaman, M., 2005. Evaluation of pathological changes in broilers during chronic aflatoxin (50 and 100 ppb) and clinoptilolite exposure, Research in Veterinary Science, 78, 61–68.
Pinard-van der Laan, M.H., Bed’hom, B., Coville, J.L., Pitel, F., Feve, K., Leroux, S., Legros, H., Thomas, A., Gourichon, D., Repérant, J.M. and Rault, P., 2009. Microsatellite mapping of QTLs affecting resistance to coccidiosis (Eimeria tenella) in a Fayoumi × White Leghorn cross, BMC Genomics, 10, 31.
Rastogi, R., Srivastava, A.K. and Rastogi, A.K., 2001. Biochemical changes induced in liver and serum of aflatoxin B1-treated male Wistar rats: Preventive effect of picroliv, Pharmacology and Toxicology, 88, 53–58.
Redmond, S.B., Chuammitri, P., Andreasen, C.B., Palić, D. and Lamont, S.J., 2009. Chicken heterophils from commercially selected and non-selected genetic lines express cytokines differently after in vitro exposure to Salmonella enteritidis, Veterinary Immunology and Immunopathology, 132, 129–134.
Saif, Y.M., 2003. Diseases of Poultry. 11th Ed, (Iowa State, Blackwell Publishing Company).
Sakhare, P.S., Harne, S.D., Kalorey, D.R., Warke, S.R., Bhandarkar, A.G. and Kurkure, N.V., 2007. Effect of Toxiroak® polyherbal feed supplement during induced aflatoxicosis, ochratoxicosis and combined mycotoxicoses in broilers, Veterinarski Arhiv, 77, 129–146.
SAS., 2001. Statistical Analysis System. Users Guide: Statistics, (SAS Institute, Cary North Carolina)
Shareef, A.M., 2010. Concurrent aflatoxicosis and caecal coccidiosis in broilers, Iraqi Journal of Veterinary Science, 24, 11–16.
Shirley, M.W., Smith, A.L. and Blake, D.P., 2007. Challenges in the successful control of the avian coccidian, Vaccine, 25, 5540–5547.
Stoev, S.D., Koynarsky, V. and Mantle, P.G., 2002. Clinicomorphological studies in chicks fed ochratoxin A while simultaneously developing coccidiosis, Veterinary Research Communications, 26, 189–204.
Sur, E. and Celik, I., 2003. Effects of aflatoxin B1 on the development of the bursa of Fabricius and blood lymphocyte acid phosphatase of the chicken, British Poultry Science, 44, 558–566.
Tipu, M.A., Pasha, T.N. and Ali, Z., 2002. Comparative efficacy of salinomycin sodium and neem fruit (Azadirachta indica) as feed additive anticoccidials in broilers, International Journal of Poultry Science, 1, 91–93.
Toulah, F.H., 2007. Effect of aflatoxin on the coccidial infection in broilers, Journal of the Egyptian Society of Parasitology, 37, 785–792.
Tung, H.T., Donaldson, W.E. and Hamilton, P.B., 1972. Altered lipid transport during aflatoxicosis, Toxicology and Applied Pharmacology, 22, 97–104
Varley, H., Gowenlock, A.H. and Bell, M., 1980. Determination of serum lactate dehydrogenase activity. In: Practical clinical biochemistry. 5th Ed, (Williams Hieinemann Medical Books Ltd, London).
West, S., Wyatt, R.D. and Hamilton, P.B., 1973. Improved yield of aflatoxin by incremental increases of temperature, Journal of Applied Microbiology, 25, 1018–1019.
Williams, R.B., Carlyle, W.W.H., Bond, D.R. and Brown, I.A.G., 1999. The efficacy and economic benefits of Paracox®, alive attenuated anticoccidial vaccine, in commercial trials with standard broiler chickens in the United Kingdom, International Journal for Parasitology, 29, 341–55.
Zulpo, D.L., Peretti, J., Ono, L.M., Longhi, E., Oliveira, M.R., Guimarães, I.G., Headley, S.A., Junior, J.G. and Garcia, J.L., 2007. Pathogenicity and histopathological observations of commercial broiler chicks experimentally infected with isolates of Eimeria tenella, Semina: Ciências Agrárias, Londrina, 28, 97–104.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ellakany, H.F., Abuakkada, S.S., Oda, S.S. et al. Influence of low levels of dietary aflatoxins on Eimeria tenella infections in broilers. Trop Anim Health Prod 43, 249–257 (2011). https://doi.org/10.1007/s11250-010-9685-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-010-9685-0