Abstract
The effect of dietary aflatoxin B1 (AFB1) and Salmonella Enteritidis infection on intestinal permeability was investigated. Two hundred 1-day-old male Ross 308 broiler chickens were randomly divided into 4 treatments of 5 replicates each (10 birds per replicate), which were fed ad libitum for 3 weeks with the following treatments: control, chickens fed an AFB1-free diet; AF, chickens fed an AFB1-contaminated diet at 470 ng/g; SE, chickens fed an AFB1-free diet and challenged with 108 cfu of S. Enteritidis per bird at 18 days old; AF + SE, chickens fed an AFB1-contaminated diet and challenged with 108 cfu of S. Enteritidis per bird at 18 days old. At day 21 of age, chicks received an oral gavage dose of fluorescein isothiocyanate dextran (FITC-dextran) to evaluate gastrointestinal leakage. Blood and intestinal samples were collected to evaluate serum biochemistry and total intestinal IgA secretion, respectively. Liver tissues were aseptically collected to assess bacterial invasiveness and for histomorphological studies. The results showed that chickens receiving AFB1 presented a significant increment (up to 2.4-fold) in serum FITC-dextran concentration (p < 0.05). Nevertheless, S. Enteritidis infection had no additional effect on gastrointestinal leakage. Furthermore, the ingestion of AFB1 had no impact on the invasive potential of S. Enteritidis. These results suggest that moderate-dose AFB1 adversely affects intestinal barrier function resulting in increased gut permeability in broiler chickens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aflatoxins (AFs) are the most investigated assemblage of mycotoxins (del Pilar Monge et al. 2012); these toxins are synthesized by toxigenic species of fungi of the Aspergillus genus, among them A. flavus Link, A. parasiticus Speare, and A. nomius Kurtzman et al. (Asao et al. 1963; Feibelman et al. 1998). Four major toxins are produced by these fungi: aflatoxin B1 (AFB1), aflatoxin B2 (AFB2), aflatoxin G1 (AFG1), and aflatoxin G2 (AFG2). AFB1 is by far the most powerful hepatotoxic, carcinogenic, teratogenic, and mutagenic compound from natural origin; consequently, it has been classified the International Agency of Research on Cancer as a human Group 1 carcinogen (Ostry et al. 2017).
Mexican regulations established the maximum levels of AFs allowed in cereals intended for human and animal consumption. For total AFs, action levels are set to 20 ng/g, and when this content is exceeded, the cereal can only be utilized for animal feed. In this case, the maximum limit for poultry is 100 ng/g (NOM-188-SSA1-2002 n.d.). It is well known that AFB1 is toxic to a wide range of animal species. In poultry, AFs cause extensive toxic effects resulting in millions of dollars in annual losses due to poor performance, immunosuppression, and many other adverse effects (Rawal et al. 2010). AFs are also able to compromise fundamental functions of the gastrointestinal tract, including loss of barrier function (Gratz et al. 2007; Chen et al. 2016). Disruption of the intestinal epithelial barrier results in a leaky gut, which contributes to bacterial translocation (Ilan 2012; Grenier and Applegate 2013). Similarly, several studies have proven that Salmonella spp. induce disruption of tight junctions and leaky gut (Awad et al. 2017). Reports on the effects of AFB1 on gut health in broiler chickens are very limited and contradictory (Tejada-Castaneda et al. 2008; Chen et al. 2016; Galarza-Seeber et al. 2016). Many of the discrepancies among the findings may be attributable to differences in avian species, gender, and age, as well as dose and time of aflatoxin exposure. Furthermore, in the abovementioned studies, animal responses were evaluated using higher doses of AFs, from 1000 to 2000 ng/g (up to 20 times of the upper legal limit in Mexico). So far, there have been no investigations focusing on intestinal permeability in broiler chickens fed moderate doses of AFB1 and subsequently challenged with S. Enteritidis. Consequently, this research aimed to evaluate the effect of dietary AFB1 and S. Enteritidis infection on intestinal permeability in broiler chickens.
Materials and methods
Animal ethics
Birds were managed as prescribed by the Internal Committee for Care and Use of Experimental Animals (CICUAE, from its abbreviation in Spanish) of the Postgraduate Program in Animal Production and Health Sciences of the National Autonomous University of Mexico. Ethical approval code: CICUAE-C17_2.
Fungal isolate and aflatoxin analysis
Aflatoxins were produced in maize according to the technique suggested by Méndez-Albores et al. (2005) using a highly toxigenic strain of Aspergillus flavus Link (UNIGRAS-1231, Culture Collection of the Grain and Seed Research Unit of the National Autonomous University of Mexico). This fungus has a high ability to synthesize AFB1 (Hernández-Meléndez et al. 2018). AFs were analyzed following the recommendations of Jardon-Xicotencatl et al. (2015) using Ultra Performance Liquid Chromatography (UPLC) with a Waters ACQUITY H-Class System which included a quaternary solvent manager, an ACQUITY UPLC BEH C18 phase reverse column (2.1 × 100 mm, 1.7 μm), and an UPLC-optimized fluorescence detector (Waters, MA, USA). The limits of detection were 2.01 and 0.58 ng/kg for AFB1 and AFB2, respectively. The mean recovery for this methodology was 92% with a standard error of 1.2 and a coefficient variation value of 4.7%.
Preparation of the aflatoxin-contaminated diet
The aflatoxin-contaminated maize was milled (Molinos Pulvex, Mexico City, Mexico) using a hammer head and a 0.5-mm mesh screen to provide ground material. The ground maize was mixed in a starter feed formulated to approximate the nutritional requirements of broiler chickens (Table 1) as recommended by the National Research Council (NRC 1994). Levels of AFs (AFB1, AFB2, AFG1, AFG2), T-2 toxin/HT-2 toxin, total fumonisins (FB1, FB2, and FB3), and deoxynivalenol (DON) were determined in the feed using monoclonal antibody-based affinity columns and subsequent analysis by UPLC with fluorescence or photodiode array detection. No antibiotic nor anticoccidial drugs, or even growth promoters, were added to the diet. Feed batches (15 kg) were artificially contaminated with AFB1 (470 ng/g) using 36 g of the aflatoxin-contaminated milled maize per kilogram of feed. In order to assure the proper distribution of the AFs, feed was mixed for 15 min in a Ribbon Blender Mixer model MH-7050 (Molinos Pulvex, Mexico City, Mexico). The control feed was also conditioned with 3.6% of aflatoxin-free maize.
Experimental birds and housing
Two hundred 1-day-old male Ross 308 broiler chicks (obtained from a commercial hatchery) were individually weighted and randomly distributed in four pens at the Poultry Research Station of the National Autonomous University of Mexico. Five replicates of 10 birds (n = 50 per treatment) were grouped based on the following four dietary treatments: control, chickens fed an AFB1-free diet; AF, chickens fed an AFB1-contaminated diet; SE, chickens fed an AFB1-free diet challenged with 108 cfu of S. Enteritidis per bird at 18 days old; AF + SE, chickens fed an AFB1-contaminated diet challenged with 108 cfu of S. Enteritidis per bird at 18 days old. The temperature, lighting, and ventilation programs were followed according to standard recommendations of the supplier. Feed and water were provided ad libitum during the whole period of the experiment (21 days).
Bacterial challenge strain and experimental infection
The Salmonella enterica serovar Enteritidis strain kindly supplied by the USDA National Veterinary Services Laboratory (Ames, IA, USA) was used. A spontaneous nalidixic acid (20 μg/mL) and novobiocin (25 μg/mL)-resistant mutant of this strain was used for challenge purposes. Briefly, 100 μL of S. Enteritidis from a frozen aliquot was added to 10 mL of tryptic soy broth, incubated at 37 °C for 8 h, and passed 3 consecutive times every 8 h to ensure that all of the bacteria were in log phase. Subsequently, bacterial cells were washed 3 times with sterile 0.9% saline by centrifugation at 1864×g for 10 min, reconstituted in saline, quantified spectrophotometrically, and diluted to 1 × 108 cfu/mL. Chickens were orally challenged with 108 cfu of S. Enteritidis per bird at 18 days old.
Collection of samples and measurements
Broilers were weighed individually on a weekly basis, feed consumption for each replicate was also measured weekly, and mortality was recorded as it occurred. Feed intake and feed conversion ratio were adjusted for mortalities when necessary. At 21 days of age, blood was drawn by cardiac puncture under anesthesia (chicks were exposed for 1 min to 40% carbon dioxide, 30% oxygen, and 30% nitrogen) from 15 randomly selected birds from each treatment (3 chickens per replicate), and serum prepared. The following analyses were performed spectrophotometrically using commercially available kits (BioSystems, Barcelona, Spain): total protein, albumin, glucose, and cholesterol. The serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities were also determined spectrophotometrically. The bled chickens were then exposed to 80% carbon dioxide, 5% oxygen, and 15% nitrogen for euthanasia (Coenen et al. 2000). Liver, kidney, spleen, and bursa of Fabricius were excised and washed in cold saline and their relative weight (mg/100 g body weight) determined. For histopathological studies, liver specimens were fixed in 10% neutral-buffered formalin for 48 h, routinely embedded in paraffin, cut into 4-μm thick sections, and processed for hematoxylin and eosin (H&E) staining. Histopathological evaluation was accomplished in a double-blind study and the severity of lesions were scored from 0 (no lesions) to 3 (most severe). Additionally, the Gram staining technique was used to study bacterial invasion. For this purpose, the red-stained bacteria (Gram-) were computed from digital images taken with a × 100 objective using ImageJ 1.52 version software (U. S. National Institutes of Health). A minimum of 25 digital images per treatment were considered.
Total intestinal immunoglobulin A levels
Total intestinal immunoglobulin A (IgA) levels were determined in gut rinse samples as described by Merino-Guzmán et al. (2017). Briefly, an intestinal section of 5 cm distal to Meckel’s diverticulum was collected and rinsed 3 times with 5 mL (0.9%) saline; then, the rinse was centrifuged at 1864×g at 4 °C for 10 min and the supernatant collected. A commercial indirect ELISA kit was used to quantify IgA according to the manufacturer’s instructions (Bethyl Laboratories Inc., TX, USA). Samples were measured at 450 nm using an ELISA plate reader (BioTek Instruments Inc., VT, USA).
Serum determination of fluorescein isothiocyanate dextran leakage
Fluorescein isothiocyanate dextran with molecular weight of 3–5 kDa (Merck KGaA, Darmstadt, Germany) was used as a marker of paracellular transport and mucosal barrier dysfunction (Vicuña et al. 2015). Following the recommendation of Baxter et al. (2017), 1 h before the chicks were euthanized, 15 chicks of each group (3 per replicate) were given an oral gavage dose of 8.32 mg fluorescein isothiocyanate dextran (FITC-dextran) per kilogram. The concentration of FITC-dextran was determined using a fluorescence LS-55 spectrophotometer (Perkin Elmer, MA, USA). Spectra were acquired in the 350–600-nm range using a 96-well plate reader accessory. The fluorescence emission spectra were collected at an excitation wavelength of 365 nm. FITC-dextran concentration was calculated using a standard reference with a calibration curve.
Experimental design and statistical analysis
Data were subjected to analysis of variance (ANOVA) as a 2 × 2 factorial using the general linear model (GLM) procedure in Statistical Analysis System software version 8.0 (SAS 2002), and means were separated by the Dunnett procedure and judged to be significantly different if p < 0.05. The Kruskal–Wallis nonparametric test was performed to assess the histopathological analysis with a level of significance set at p < 0.05.
Results
Analysis of dietary aflatoxins
The analysis of the artificially contaminated feed by UPLC indicates the presence of AFB1 (470 ± 27 ng/g) and AFB2 (30 ± 4 ng/g). In this work, the presence of AFB2 was considered to be negligible, since this toxin is approximately 200-fold less toxic than AFB1 (Méndez-Albores et al. 2005). The control diet had no detectable levels of AFs, T-2 toxin/HT-2 toxin, and total fumonisins. Assayed contents of these toxins were below the detection limits of the immunoaffinity column techniques employed (AFs < 1 ng/g, T-2 toxin/HT-2 toxin < 100 ng/g, total fumonisins < 0.016 mg/kg). DON was present at a level of 0.05 mg/kg.
Performance parameters
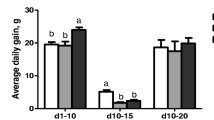
Data on performance parameters are summarized in Table 2. At the end of week 1 (7 days old), there were no significant differences in weight gain (WG) among the four treatment groups. However, by the end of week 2 (14 days old), WG was significantly reduced (p < 0.05) in chickens of the AF and AF + SE groups when compared with the control and SE groups, respectively. By the end of week 3, chickens receiving the AFB1-contaminated diets have 31.4% and 29.6% reductions in WG, respectively. Similarly, by the end of weeks 2 and 3, feed conversion (FC) was significantly affected in the aflatoxin treatments. The observed mortality during the 21-day period was as follows: 8 chickens in the AF group, 10 chickens in the AF + SE group, and 0 mortalities in the control and SE groups, respectively. Although there was no statistically significant effect on performance parameters in chickens challenged with S. Enteritidis, a slight reduction (2.5%) in WG was observed during the last week (Table 2). It is important to note that S. Enteritidis infection had the only effect during 3 days of the last treatment week.
Plasma biochemistry and intestinal IgA levels
The results of the effects of dietary aflatoxins and S. Enteritidis infection on plasma biochemistry and total intestinal IgA levels in broiler chickens at 21 days are summarized in Table 3. Significant differences in plasma concentrations of total protein, albumin, globulin, glucose, and cholesterol were observed between birds fed control and AFB1-contaminated diets. In general, AFs caused a significant decrease in plasma biochemistry profiles among the different dietary groups. Clear indications of aflatoxin toxicity were detected in chickens of the AF and AF + SE groups by the serum aspartate aminotransferase (AST) activity level, which increased by 1.8-fold in comparison with the control group. Furthermore, the ratio of AST:ALT increased 1.7 times in chickens fed with the AFB1-contaminated feed as compared with the control group. Infection by S. Enteritidis did not cause alterations in the plasma biochemistry profile of the SE and AF + SE groups. Moreover, no significant differences in the total intestinal IgA levels were observed among the AF, AF + SE, and control groups. However, a significant increment in the total intestinal IgA level was detected in the SE group, showing values up to 9394 ng/mL (Table 3).
Relative organ weight
Table 4 shows the effects of dietary aflatoxins and S. Enteritidis infection on relative organ weight (mg/100 g body weight) in broiler chickens at 21 days. When compared with the control group, relative weights of the liver and kidney increased significantly in chickens fed with the AFB1-contaminated diets. Moreover, no significant differences were noted among all treatments in the spleen relative weight. However, when compared with the control group, the relative weight of the bursa of Fabricius increased up to 2.3-fold, in chickens of the AF, SE, and AF + SE groups. Table 4 also shows the bursa of Fabricius:spleen ratio; the AF and AF + SE groups reached the highest values. Chickens challenged with S. Enteritidis also showed a slightly larger bursa of Fabricius:spleen ratio (but not significantly) when compared with the control group (Table 4).
Macroscopic findings, histopathology, and bacterial invasiveness
At the end of the trial, the major gross lesions were observed in the liver of chickens fed with the AFB1-contaminated diets. In general, livers of the AF and AF + SE groups were yellowish, friable, and appeared much larger in size compared with those of the control and SE groups, respectively. These lesions were also accompanied by hemorrhagic streaks. Furthermore, histopathological studies confirmed extensive liver damage; lesions observed were hepatic steatosis, massive bile duct proliferation, congestion, hemorrhage, inflammation, and fibrosis (Fig. 1, profiles b and d). In contrast, only a minimal degree of hepatic degeneration and minimal inflammation was seen in the livers of the SE group (Fig. 1, profile c). Infection by S. Enteritidis did not cause additional alterations in liver histology of the AF + SE group (Fig. 1, profile d). Primary lesions in the liver that showed significant differences at 21 days are summarized in Table 5. Furthermore, no bacteria were seen in the Gram-stained liver tissue sections of the control and AF groups (Fig. 2, profiles a and b). However, chickens challenged with S. Enteritidis presented a significant increment in bacterial invasion of the liver, showing values up to 2381 ± 364 relative counts (Fig. 2, profile c). No increase in bacterial invasion related to AFB1 intake was observed (2548 ± 308 relative counts).
Histological findings in liver tissues (× 40, H&E stain). Control, chickens fed an AFB1-free diet; AF, chickens fed an AFB1-contaminated diet; SE, chickens fed an AFB1-free diet challenged with 108 cfu of S. Enteritidis per bird at 18 days old; AF + SE, chickens fed an AFB1-contaminated diet challenged with 108 cfu of S. Enteritidis per bird at 18 days old. The normal structure in the control group (profile a). Severe hepatic steatosis, massive bile duct proliferation, and inflammation are clear in the AF (profile b) and AF + SE (profile d) groups. Minimal hepatic degeneration and minimal inflammation in the SE group (profile c). Scale bar = 100 μm
Chicken liver tissue treated with Gram’s method of staining (× 100). Control, chickens fed an AFB1-free diet; AF, chickens fed an AFB1-contaminated diet; SE, chickens fed an AFB1-free diet challenged with 108 cfu of S. Enteritidis per bird at 18 days old; AF + SE, chickens fed an AFB1-contaminated diet challenged with 108 cfu of S. Enteritidis per bird at 18 days old. Scale bar = 50 μm
FITC-dextran leakage
There were no differences in serum levels of FITC-dextran between control and S. Enteritidis-challenged chickens (0.17 ± 0.01 μg/mL serum vs. 0.15 ± 0.01 μg/mL serum). However, a significant increment (2.4-fold) in serum FITC-dextran concentration was detected in chickens fed the AFB1-contaminated diets. In those birds, the serum FITC-dextran concentration reached values up to 0.49 ± 0.02 μg/mL serum. S. Enteritidis infection had no additional effect on gastrointestinal leakage (0.47 ± 0.03 μg/mL serum).
Discussion
The starter feed was mixed with the aflatoxin-contaminated maize to produce significant toxicity to broiler chickens. The feed also contained T-2 toxin/HT-2 toxin, fumonisins, and DON at levels that were not as toxic as the AFB1 content (470 ± 27 ng/g); consequently, these mycotoxins should have a negligible effect on chicks. Several previous reports have indicated that contents > 75 mg FB1/kg, > 4 mg T-2 toxin/kg, and > 16 mg DON/kg are necessary to induce significant toxicity in young broiler chickens (Weibking et al. 1993; Kubena et al. 1989).
During the 21-day period, significant alterations in body weight gain, feed conversion, mortality, blood biochemistry, relative weights of the liver, kidney, bursa of Fabricius, and liver histology were observed due to the addition of AFB1 to the diet. These findings are in close agreement with the results found by Raju et al. (2005) and Sapcota et al. (2006), who reported that an experimental diet containing 300 ng AFB1/g feed produced adverse effects on body weight gain, feed intake, and serum concentrations of proteins and cholesterol in broiler chickens. In general, the results obtained in this research in relation to performance (Table 2), serologic analysis (Table 3), relative organ weight (Table 4), and histopathology (Fig. 1, Table 5) indicate that these deleterious effects were caused by dietary AFB1.
In this experiment, AFs (470 ng AFB1/g feed) were able to induce significant effects on intestinal permeability, since birds receiving AFB1 presented a substantial increment (up to 2.4-fold) in serum FITC-dextran concentration. Our results are consistent with two previous in vivo studies using higher doses of AFB1 (up to 1500 ng AFB1/g feed). Tejada-Castaneda et al. (2008) in a 3-week study, where Ross 308 broiler chickens were fed a diet with 1200 ng AFB1/g feed, reported that microvilli were uniformly affected by dietary AFs. Scanning electron microscopy investigations showed that microvilli were shorter and combined, the tight junction completely disappeared, and only irregular masses of denatured proteins were observed in the duodenum, jejunum, and ileum sections. The authors concluded that AFs induce loss of epithelial polarity. Chen et al. (2016) determined the impact of 1500 ng AFB1/g feed on gut health in Ross 708 broiler chickens. On day 20, using the dual-sugar gut permeability test, authors found significant increments in the serum lactulose:rhamnose ratio indicating impaired intestinal barrier of chickens that were fed AFB1-contaminated diet. Conversely, Galarza-Seeber et al. (2016) evaluated the effect of 1000, 1500, and 2000 ng AFB1/g feed on gastrointestinal leakage in Cobb-Vantress broiler chickens. Authors reported that AFB1 did not increase gut leakage as evidenced by the lack of increase in permeability of FITC-dextran in the serum. The researchers concluded that the integrity of gut epithelial barrier was not compromised after exposure to the three different AFB1 contents.
Several in vitro studies also showed that exposure of human colon carcinoma cells (Caco-2) to mycotoxins such as AFB1, FB1, ochratoxin A (OTA), T2-toxin (T-2), and DON resulted in impaired intestinal barrier (Kasuga et al. 1998; McLaughlin et al. 2004; Sergent et al. 2006; Gratz et al. 2007; Pinton et al. 2009; Romero et al. 2016). Impaired gut epithelial integrity—due to alterations in tight junction proteins—may also be the pathological mechanism underlying bacterial translocation (Ilan 2012; Seki and Schnabl 2012). In this work, all of the liver sections from the SE and AF + SE groups were found to be positive for bacteria by the Gram staining technique (Fig. 2), confirming the invasive feature of the used bacterial strain. However, AFB1 (470 ng/g feed) had no additional effect on the invasive potential of S. Enteritidis. Our results are in accordance with those of Burel et al. (2013), who reported that 8.6 mg FB1/kg feed had no impact on Salmonella spp. translocation or seroconversion in inoculated pigs.
Studies have demonstrated that Salmonella spp. also induce disruption of tight junctions (Awad et al. 2017), and the expression of inflammatory cytokines (Overman et al. 2012). In this study, the total intestinal IgA levels were determined as a biomarker to evaluate intestinal inflammation, since this immunoglobulin has been previously used to evaluate local humoral immunity in broiler chickens challenged with this pathogen (Husáková et al. 2015). The results showed that IgA expression increased up to 9394 ng/mL in chickens challenged with S. Enteritidis in a short period of time after challenge, while control chickens had a basal level of 5770 ng/mL (Table 3). The higher total intestinal IgA levels may be directly related to the severity of S. Enteritidis infection (Hernandez-Patlan et al. 2019). For AF + SE group, intestinal IgA level did not differ significantly between AF and control groups, showing that AFB1 has an immunosuppressive effect, probably attributed to a significant decrease in the number of IgA+ cells in the duodenum, jejunum, and ileum, and a reduced expression of IgA, pIgR, IgM, and IgG mRNA in the small intestine (Jiang et al. 2015). Thus, the humoral local antibody response against S. Enteritidis was slightly reduced.
The intestinal absorption of AFB1 may be accomplished by several possible routes: (i) a portion of the AFB1 passes intact through the epithelial layer; (ii) AFB1 penetrates the enterocyte by passive transport and molecules are converted to the active epoxide by the Cytochrome P450 forming an adduct with proteins, and subsequently, adducts arrive to the liver through the portal vein; (iii) due to their lipophilic nature, AFB1 is absorbed via paracellular route damaging tight junctions, and consequently, AFB1 has a direct impact on gut epithelium. Besides, leaky gut could be a result of an indirect effect of AFB1 toxicity, since increased intestinal permeability has been also associated with the pathogenesis of both liver and kidney (Cesaro et al. 2011).
Taken together, these results suggest that AFB1 exerts direct and indirect effects on the gut epithelium, and may be partially responsible for the physiological and metabolic disorders in poultry during aflatoxicosis. To the best of our knowledge, this is the first report on the effect of moderate-dose AFB1 (470 ng/g feed) on gut barrier in broiler chickens. However, a more comprehensive knowledge of these effects will improve our understanding of the link between moderate intake of AFB1, gut barrier, and bacterial invasiveness in poultry. Further studies to evaluate gene expression of tight junction proteins are currently being evaluated.
References
Asao T, Buchi G, Abdel-Kader M, Chang S, Wick EL, Wogan G (1963) Aflatoxins B and G. J Am Chem Soc 85:1706–1707
Awad W, Hess C, Hess M (2017) Enteric pathogens and their toxin-induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins 9:60
Baxter MF, Merino-Guzman R, Latorre JD, Mahaffey BD, Yang Y, Teague KD, Graham LE, Wolfenden AD, Hernandez-Velasco X, Bielke LR, Hargis BM, Tellez G (2017) Optimizing fluorescein isothiocyanate dextran measurement as a biomarker in a 24-h feed restriction model to induce gut permeability in broiler chickens. Front Vet Sci 4:56
Burel C, Tanguy M, Guerre P, Boilletot E, Cariolet R, Queguiner M, Postolec G, Pinton P, Salvat G, Oswald IP, Fravalo P (2013) Effect of low dose of fumonisins on pig health: immune status, intestinal microbiota and sensitivity to Salmonella. Toxins 5:841–864
Cesaro C, Tiso A, Del Prete A, Cariello R, Tuccillo C, Cotticelli G, del Vecchio Blanco C, Loguercio C (2011) Gut microbiota and probiotics in chronic liver diseases. Dig Liver Dis 43:431–438
Chen X, Naehrer K, Applegate T (2016) Interactive effects of dietary protein concentration and aflatoxin B1 on performance, nutrient digestibility, and gut health in broiler chicks. Poult Sci 95:1312–1325
Coenen A, Smit A, Zhonghua L, Van Luijtelaar G (2000) Gas mixtures for anaesthesia and euthanasia in broiler chickens. Worlds Poult Sci J 56:226–234
del Pilar Monge M, Magnoli CE, Chiacchiera SM (2012) Survey of Aspergillus and Fusarium species and their mycotoxins in raw materials and poultry feeds from Córdoba, Argentina. Mycotoxin Res 28:111–122
Feibelman TP, Cotty PJ, Doster M, Michailides T (1998) A morphologically distinct strain of Aspergillus nomius. Mycologia 90:618–623
Galarza-Seeber R, Latorre JD, Bielke LR, Kuttappan VA, Wolfenden AD, Hernandez-Velasco X, Merino-Guzman R, Vicente JL, Donoghue A, Cross D, Hargis BM, Tellez G (2016) Leaky gut and mycotoxins: aflatoxin B1 does not increase gut permeability in broiler chickens. Front Vet Sci 3:10
Gratz S, Wu Q, El-Nezami H, Juvonen R, Mykkänen H, Turner P (2007) Lactobacillus rhamnosus strain GG reduces aflatoxin B1 transport, metabolism, and toxicity in Caco-2 cells. Appl Environ Microbiol 73:3958–3964
Grenier B, Applegate T (2013) Modulation of intestinal functions following mycotoxin ingestion: meta-analysis of published experiments in animals. Toxins 5:396–430
Hernández-Meléndez D, Salas-Téllez E, Zavala-Franco A, Téllez G, Méndez-Albores A, Vázquez-Durán A (2018) Inhibitory effect of flower-shaped zinc oxide nanostructures on the growth and aflatoxin production of a highly toxigenic strain of Aspergillus flavus Link. Materials 11:1265
Hernandez-Patlan D, Solis-Cruz B, Adhikari B, Pontin KP, Latorre JD, Baxter MF, Hernandez-Velasco X, Merino-Guzman R, Méndez-Albores A, Kwon YM, Hargis BM, Lopez-Arellano R, Arreguin-Nava MA, Tellez-Isaias G (2019) Evaluation of the antimicrobial and intestinal integrity properties of boric acid in broiler chickens infected with Salmonella enteritidis: proof of concept. Res Vet Sci 123:7–13
Husáková E, Bobíková K, Stašová D (2015) Total IgA in spleen, bursa and intestine of chickens pretreated with E. faecium AL41 and challenged with Salmonella Enteritidis PT4. Food Agric Immunol 26:366–370
Ilan Y (2012) Leaky gut and the liver: a role for bacterial translocation in nonalcoholic steatohepatitis. World J Gastroenterol 18:2609
Jardon-Xicotencatl S, Díaz-Torres R, Marroquín-Cardona A, Villarreal-Barajas T, Méndez-Albores A (2015) Detoxification of aflatoxin-contaminated maize by neutral electrolyzed oxidizing water. Toxins 7:4294–4314
Jiang M, Fang J, Peng X, Cui H, Yu Z (2015) Effect of aflatoxin B1 on IgA+ cell number and immunoglobulin mRNA expression in the intestine of broilers. Immunopharmacol Immunotoxicol 37:450–457
Kasuga F, Hara-Kudo Y, Saito N, Kumagai S, Sugita-Konishi Y (1998) In vitro effect of deoxynivalenol on the differentiation of human colonic cell lines Caco-2 and T84. Mycopathologia 142:161–167
Kubena LF, Huff WE, Harvey RB, Phillips TD, Rottinghaus GE (1989) Individual and combined toxicity of deoxynivalenol and T-2 toxin in broiler chicks. Poult Sci 68:622–626
McLaughlin J, Padfield PJ, Burt JP, O'Neill CA (2004) Ochratoxin A increases permeability through tight junctions by removal of specific claudin isoforms. Am J Phys Cell Physiol 287:C1412–C1417
Méndez-Albores A, Arambula-Villa G, Loarca-Piña M, Castano-Tostado E, Moreno-Martínez E (2005) Safety and efficacy evaluation of aqueous citric acid to degrade B-aflatoxins in maize. Food Chem Toxicol 43:233–238
Merino-Guzmán R, Latorre JD, Delgado R, Hernandez-Velasco X, Wolfenden AD, Teague KD, Graham LE, Mahaffey BD, Baxter MFA, Hargis BM, Tellez G (2017) Comparison of total immunoglobulin A levels in different samples in Leghorn and broiler chickens. Asian Pac J Trop Biomed 7:116–120
Nacional Research Council, (NRC) (1994) Nutrient requirements of chickens. In: Nutrient requirements of poultry. 8th rev. National Academy Press, Washington DC, pp 11–15
Norma Oficial Mexicana NOM-188-SSA1-2002 Productos y Servicios: Control de aflatoxinas en cereales para consumo humano y animal, especificaciones sanitarias. Diario Oficial de la Federacion. 2002:11 de Marzo de 1999.
Ostry V, Malir F, Toman J, Grosse Y (2017) Mycotoxins as human carcinogens—the IARC monographs classification. Mycotoxin Res 33:65–73
Overman EL, Rivier JE, Moeser AJ (2012) CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-α. PLoS One 7:e39935
Pinton P, Nougayrède JP, Del Rio JC, Moreno C, Marin DE, Ferrier L, Bracarense AP, Kolf-Clauw M, Oswald IP (2009) The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression. Toxicol Appl Pharmacol 237:41–48
Raju M, Rao S, Radhika K, Chawak M (2005) Dietary supplementation of Spirulina and its effects on broiler chicken exposed to aflatoxicosis. Indian J Poult Sci 40:36–40
Rawal S, Kim JE, Coulombe R Jr (2010) Aflatoxin B1 in poultry: toxicology, metabolism and prevention. Res Vet Sci 89:325–331
Romero A, Ares I, Ramos E, Castellano V, Martínez M, Martínez-Larrañaga MR, Anadón A, Martínez MA (2016) Mycotoxins modify the barrier function of Caco-2 cells through differential gene expression of specific claudin isoforms: protective effect of illite mineral clay. Toxicology 353:21–33
Sapcota D, Islam R, Upadhyaya T (2006) Dietary supplementation of Emblica officinalis for amelioration of experimental aflatoxicosis in commercial broilers. Anim Nutr Feed Technol 6:65–71
SAS Institute (2002) SAS/STAT User’s guide release 9.0 edition. SAS institute, Cary
Seki E, Schnabl B (2012) Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J Physiol 590:447–458
Sergent T, Parys M, Garsou S, Pussemier L, Schneider YJ, Larondelle Y (2006) Deoxynivalenol transport across human intestinal Caco-2 cells and its effects on cellular metabolism at realistic intestinal concentrations. Toxicol Lett 164:167–176
Tejada-Castaneda Z, Avila-Gonzalez E, Casaubon-Huguenin M, Cervantes-Olivares R, Vásquez-Peláez C, Hernandez-Baumgarten E, Moreno-Martínez E (2008) Biodetoxification of aflatoxin-contaminated chick feed. Poult Sci 87:1569–1576
Vicuña EA, Kuttappan VA, Tellez G, Hernandez-Velasco X, Seeber-Galarza R, Latorre JD, Faulkner OB, Wolfenden AD, Hargis BM, Bielke LR (2015) Dose titration of FITC-D for optimal measurement of enteric inflammation in broiler chicks. Poult Sci 94:1353–1359
Weibking TS, Ledoux DR, Bermudez AJ, Turk JR, Rottinghaus GE, Wang E, Merrill AH Jr (1993) Effects of feeding Fusarium moniliforme culture material, containing known levels of fumonisin B1, on the young broiler chick. Poult Sci 72:456–466
Acknowledgments
J.O. Hernández-Ramírez acknowledges CONACyT for the Ph.D. scholarship (245747).
Funding
This work was partially supported by Programa Interno de Apoyo para Proyectos de Investigacion (PIAPI) Grant number PIAPI-1806.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hernández-Ramírez, J.O., Nava-Ramírez, M.J., Merino-Guzmán, R. et al. The effect of moderate-dose aflatoxin B1 and Salmonella Enteritidis infection on intestinal permeability in broiler chickens. Mycotoxin Res 36, 31–39 (2020). https://doi.org/10.1007/s12550-019-00367-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12550-019-00367-7