Abstract
A cross-sectional study was conducted to investigate the prevalence and animal level risk factors for bovine tuberculosis (BTB) in indigenous cattle of the livestock/wildlife interface areas in Zambia. A total of 944 cattle from 111 herds were investigated. The comparative intradermal tuberculin test (CIDT) was used to identify reactor animals for BTB. Animal level data on sex, age, parity and body condition score were registered. The overall animal prevalence of BTB as determined by the CIDT was 6.8% (95% CI: 4.2, 9.5%). In Lochinvar and Blue Lagoon areas, animal level prevalence were observed at 5.2% (95% CI: 2.2, 8.2%) and 9.6% (95% CI: 6.1, 13.2%), respectively. Kazungula, an area outside the livestock/wildlife interface, had a prevalence of only 0.8% (95% CI: 0.0, 2.3%). The age of the animal, its body condition score and the type of management system, were predictive of its BTB status. The study revealed that BTB was relatively high in the livestock/wildlife interface areas of Lochinvar and Blue Lagoon compared to Kazungula. These findings should raise a serious public health concern considering the extent to which the communities of the study areas are in contact with their animals and the levels at which they use untreated milk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bovine tuberculosis (BTB) is an important disease of both economic and public health importance (Sudre et al. 1992). In cattle, the disease is mainly caused by the bacteria, Mycobacterium bovis (M. bovis) (Acha and Szyfres 1987). However, occasional occurrence of tuberculosis due to Mycobacterium tuberculosis (M. tuberculosis) species with concurrent tuberculous lesions has been reported in pigs (Lesslie and Brin 1970). In cattle, Mycobacterium tuberculosis accounts for a small proportion of usually sub-clinical cases (Ameni and Erkihun 2007; Pritchard 1988).
Bovine tuberculosis used to be a serious zoonosis and represented a tremendous public health problem in developed countries, mainly through the consumption of unpastuerised milk (Collins and Stollerman 1993). However, after the disease was controlled in cattle, through designed test and slaughter schemes, together with milk pasteurization and public education, these countries drastically reduced and in some cases controlled the transmission of BTB to humans (Dankner et al. 1993).
In low-income countries BTB is still prevalent and is responsible for significant economical loss in animal production through reduced milk yields, abattoir carcass condemnations, low meat yield through emaciation and low reproductive performance. BTB has also been implicated in the increase of human health problems in these countries (Cosivi et al. 1998).
The global prevalence of human tuberculosis due to Mycobacterium bovis has been estimated at 3.1% of all human tuberculosis cases (Cosivi et al. 1998; Sudre et al. 1992). In countries where a majority of poor rural pastoral communities depend on cattle for their livelihoods, BTB is reported to be a constant threat due to lack of pasteurization of milk (Ameni et al. 2003; Cosivi et al. 1998). Unfortunately, lack of information on BTB in many African countries, Zambia inclusive, has reduced the attention given to this infectious agent in terms of prioritizing resources devoted to control of animal diseases (Cosivi et al. 1998).
BTB is known to be a major cause of human extra-pulmonary tuberculosis specifically the gastrointestinal tuberculosis, and is of particular concern in developing countries where bovine milk is often not pasteurized (Sonnenberg et al. 2001). The reason for this is that the major mode of exposure to of M. bovis infection in humans is through drinking of raw milk, and M. bovis in humans mainly manifests as an extra-pulmonary form, particularly causing cervical lymphadenitis.
In Zambia, reports on the Kafue Basin indicate that M. bovis infections could be a problem not only in cattle, but in humans too (Cook et al. 1996; Cosivi et al. 1998). These reports have also indicated the presence of M. bovis both in wildlife (Cook et al. 1996; Cosivi et al. 1998; Munag’andu et al. 2006; Pandey 1998; Sitima 1997). Whilst reports on the existence of tuberculosis in wildlife of the Kafue basin are persistent, no studies have been conducted in cattle sharing grazing land with the Kafue lechwe (Kobus leche Kafuensis). The Kafue lechwe antelopes in Zambia are suspected to be biological reservoir hosts for BTB (Gallagher et al. 1972; Stafford 1991). There is a need therefore, to systematically investigate the levels of BTB in cattle reared in the purported high risk area of the Kafue Basin.
The overall aim of this study was to determine the prevalence of BTB in cattle sharing grazing land with Kafue lechwe antelopes in the livestock/wildlife interface areas of the Kafue Basin of Zambia, and identify animal level risk factors of its occurrence.
Materials and methods
Study areas
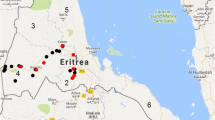
The Kafue Basin is a floodplain along the Kafue River of about 6,000 km2 located in the South-Central region of Zambia, (Fig. 1)- (Sheppe 1985). It lies between the lines of latitude 26°-28°E and 15°20′-15°55′ S. It is comprised of Lochinvar National Park (410 Km2; 15°43′-16°01′S, 27°11′-27°19′ E), Blue Lagoon National Park (420 Km2; 15°21′-43′S, 27°15′-27°32′ E) and the Kafue flats Game Management Areas (5 175 Km2) (Mwima 1995). The comparative area of Kazungula District lies in the most Southern part of the country, located by coordinates 17°05′S to 17°45′S and 25°38′E to 25°59′E along the Zambezi Basin. Cattle reared in the Kazungula District have no apparent contact with wild animals as they are outside the livestock/wildlife interface areas.
Study design
A cross-sectional study was conducted between August 2003 and February 2004. Due to lack of comprehensive information on the cattle herds in the study areas, a base line study was conducted to estimate the population of cattle herds (N) in the areas. Based on the baseline study, we estimated that there were approximately 110 cattle herds in the Blue Lagoon area, 100 in Lochnivar and 50 in Kazungula. This population of herds constituted the study population from which actual testing was conducted (sample population).
Assuming low heterogeneity between herds, we used a detection power (1-β) of 90%, the level of significance (α) at 95% and the desired absolute precision at 5 %. We further assumed the sensitivity and specificity of the comparative intradermal tuberculin test (CIDT) to be 80% and 100%, respectively (Monaghan et al. 1994; Quirin et al. 2001). The BTB prevalence previously reported for cattle in Zambia varies from 10% to 20% at animal level. The average herd size was assumed to be at 100 animals. We thus planned to sample individual cattle from herds at a 10% testing fraction. Based on these assumptions, we used Herdacc™ Version 3 (Jordan 1995) to estimate the herd specificity (HSp) and herd sensitivity (HSe). Our predicted HSp and HSe were 100% and 73.9%, respectively, at 10% testing fraction when a herd was classified positive if at least one animal tested positive on CIDT. Thus applying the estimates in the sample size calculation formula for simple random testing, and correcting for a finite population we planned to sample a total of 125 herds, represented by 53, 48 and 24 herds for Blue Lagoon, Lochinvar and Kazungula, respectively. To select this number of herds and to avoid selection bias, a simple random mechanism of choosing farms was designed using a lottery system. In each study area, the farms were given numbers on a piece of paper. These numbers were then put in a suitable receptacle from which random selection of farms were made. Testing of farms from the receptacle was done without replacement. In areas where herd owners were un-cooperative, other herds sharing similar exposure factors to those excluded, such as herd size, grazing strategy etc were chosen for the study. At animal level, the situation was slightly different. For those animals that were tested from crush pens, we used a systematic random testing, were as true random testing was difficult to attain in animals that were restrained by casting in the kraals. In these study areas, three grazing strategies were practised. Those animals that were grazed within village perimeters without migration in search of pasture were known as village resident herds (VRH), while those that went in search of pasture during drier times coming back to the villages in the rainy season were known as transhumant herds (TH). However, some herds of cattle remain permanently resident in the plains and these are known as interface herds (IFH).
Epidemiological data
Information about each animal such as age, sex, pregnancy status, lactating status, parity, body condition score etc, were collected And entered into a data sheet.
Intradermal skin test
For the determination of prevalence of BTB in cattle, the comparative intradermal cervical tuberculin test (CIDT) was applied. The procedure was conducted as described in the manual of Office International Des Epizooties (OIE 2004). Briefly, two areas of about 2 cm diameter of the cervical skin were clipped (or short cropped), washed with soap and disinfected with 70% ethanol. The initial skin thickness was measured followed by intradermal injection of 0.1 ml of bovine and avian purified protein derivatives (PPD), respectively. The results of hyper-sensitization were read after 72 hours by measuring again the skin thickness at the inoculation sites. A strict standard level of interpretation was used to classify reactors according to the OIE manual (OIE 2004). Negative reactors were indicated by a differential increase in skin thickness of less than 2 mm after subtracting the increase of skin thickness at the avian PPD injection site from the one at the bovine PPD injection site (OIE 2004). Inconclusive reactors were indicated by skin thickness increases of between 2 mm and 4 mm. A positive reaction was indicated by an increase in skin thickness of more than 4 mm.
Data analyses
The database was maintained in Excel® before transferring to Stata 9 for Windows (Stata Corp. College Station, TX, USA). This included bio-data about each animal such as sex, age, parity and body condition score. Animal level prevalence estimate for BTB with confidence intervals were computed using the survey command estimates in Stata with adjustments for strata (study area) as described by Dohoo et. al. (2003). At animal level, the effect of age, sex, and other factors with a plausible (causal-effect) relationship to BTB status were investigated. Independent effect of categorical variables on BTB status were assessed using the two sided Fisher exact test while those of continuos variables were assessed using the Kruskal_Walli’s tests. Only variables that had p-values (two-sided) ≤0.25 and those without many missing values (>15) were retained for multivariable analysis in a logistic regression model. The model was manually constructed using forward selection according to details of logistic model building as described by Dohoo and others (Dohoo et al. 2003). The model’s goodness of fit was assessed using the Hosmer - Lameshow goodness-of-fit test, while its significance was assessed by using the likelihood Ratio Test with sensitivity and specificity being assessed using the ROC analysis.
Results
A total of 944 cattle were tested from 111 herds under investigation. Median herd size was 39 (95% CI: 36, 41) animals across all the three study areas (Table 1). In Blue Lagoon and Kazungula District, the targeted numbers of herds that were to be tested were attained, while in Lochinvar only 34 out of a targeted number of 48 herds were tested. Out of 944 animals tested, 52 tested positive on tuberculinisation, giving a BTB prevalence of 6.8% (95% CI: 4.2, 9.5) after adjusting for sampling weights (Table 1). Table 1 also shows the distribution of reactor animals both at individual and herd level according to area of study which formed the primary study strata.
Table 2 shows the animal BTB prevalence in cattle by sex, age and grazing strategy. The results showed that animals from the livestock/wildlife interface areas (IFH) had by far the highest BTB prevalence (11.4%, 95% CI: 7.4, 15.4), followed by cattle that practiced the transhumance grazing strategy (TH) (5.3%, 95% CI: 1.9, 8.7). Village resident cattle had a markedly lower BTB prevalence of (1.6%, 95% CI: 0.3, 2.9). The prevalence of BTB was shown to increase proportionately with age as indicated in Table 2.
A number of factors were found to be associated (p < 0.05) with increased risk of testing positive for a tuberculin test (Table 3). Significant differences were observed between animals in the study area (the livestock/wildlife interface) and those in the control area of Kazungula in relation to body condition score, age, sex, grazing strategy and size of the herd. Survey logistic regression analysis results showed that there was a strong association between being BTB positive and having a poor body condition score (OR = 15.6) (Table 3). The model also showed significant association between being BTB positive and age as well as type of grazing strategy under which animals where managed (Table 3). The results also showed that animals that were reared in the interface areas were almost seven times more likely to test positive for BTB than village resident herds (OR = 6.7) (Table 3). The model also showed an increasing prevalence with increasing age, although the association was statistically significant only for animals older than seven years (P < 0.001).
Discussion
This study focused on tuberculosis in indigenous cattle breeds in the Zambian livestock/wildlife interface areas across different cattle grazing strategies. It also explored the relationship between cattle sharing grazing land with wildlife (mainly Kafue lechwe antelopes). In this type of set up, BTB infection was found to be prevalent in the livestock/wildlife interface areas. The animal prevalence of BTB varied among the 3 grazing strategies identified in the study areas. Significant differences were recorded between those in the interface areas and those outside. Animal BTB prevalence in Lochinvar was recorded at 5.2% and Blue Lagoon 9.6%, both found in the wildlife/livestock interface areas whilst Kazungula which is outside the interface area had a prevalence of 0.8%. These findings hint on the probable existence of a likely focus of infection around this geographical zone of the country (Sitima 1997). However, the reasons for such spatial distribution of infection are not well known, although it is suspected that the lechwe antelope is the biological reservoir host of BTB in the Kafue Basin area (Cook et al. 1996; Pandey 1998; Sitima 1997). Cattle raised in the livestock/wildlife interface areas of the Kafue Flats were more likely to have a positive tuberculin reaction on CIDT than those found in Kazungula District although relatively larger herd sizes were recorded in Kazungula (Table 1) and the mixing of animals and other characteristics were similar to those found in the Kafue Flats, with the exception of lack of wildlife contact. Animals that were reared in the interface areas (IFH), were almost seven times more likely to have a positive test reaction than those reared in the villages (VRH) (OR = 6.7). These results strengthened the earlier suggestion of an existing concentric focus of infection towards the wildlife sanctuary in the Kafue Basin as postulated by Pandey (Pandey 1998). The Kafue Basin has been identified as an area with favourable ecological conditions for the spread of infectious diseases between livestock and wild animals due to the interaction that exists between cattle and wildlife, coupled by the favourable marshy environmental conditions (Munag’andu et al. 2006). The gregarious nature of lechwe antelopes with higher herd densities obtaining in drier seasons is thought to have had facilitated the survival of M. bovis in these wetlands (Gallagher et al. 1972; Siamudaala et al. 2003; Stafford 1991). Yearly seasonal flooding may also help in the propagation of micro-organisms in the environment, while overcrowding of animals during the dry season at watering points may enhance the direct animal to animal transmission of the disease (Corner 2006). Thus, from the biological stand point of spread and maintenance of M. bovis, both environmental, host and agent attributes may be regarded as being optimal in the Kafue Basin region (Corner 2006; Zieger et al. 1998).
Data from this study indicated that sex had no effect on BTB status. This is in accordance with findings from other studies (Oloya et al. 2006; Omer et al. 2001). According to the biology of the disease, tuberculosis positive cattle go through a period of desensitization before and after calving and as many as 30% may give false negative reactions returning to a positive status 4 to 6 weeks later (Radostist et al. 1994). In our study less than 0.5 % of the animals had given birth within a month or two prior to being tested, which imply that the number of false negatives due to this aspect could not have an effect on the validity of the results.
In our study, age was found to be related to the distribution of BTB reactors across all areas and grazing strategies with low prevalence values being recorded in younger age groups and high values in older ones. These results agree with the established observation by other researchers (Ameni et al. 2003; Asseged et al. 2000; Cook et al. 1996) Cattle of all ages are susceptible to TB, but the probability of becoming infected generally increases with age due to the proportionate increase in exposure time, with older animals being said to have a greater risk on account of their longer life spans (O’Reilly and Daborn 1995).
Results from the model indicated that body condition score, age and grazing strategy as important predictors for cattle BTB. It is likely that emaciation was an indicator of tuberculosis occurring in an animal. It is also possible that tuberculosis positive animals have poor body condition score as a result of being infected, i.e. a clinical sign that typically follows an active infection with M. bovis (Kazwala et al. 2001; Pritchard 1988).
Grazing strategy apart from being a major predictor variable for BTB status, it is also a proxy variable for other risk factors. For example, interface cattle herds are known to be in constant contact with lechwe herds, previously described as sources of residual infection of tuberculosis (Cook et al. 1996; Pandey 1998; Stafford 1991). Interface herds are also known to be large thus facilitating for close contact within and between herds and thus favouring the transmission of respiratory diseases including tuberculosis (Radostist et al. 1994)
It was also noted that there was more than one animal per herd that gave a positive result, giving rise to a certain degree of clustering. However, these numbers were very small and it was considered very unlikely that a clustering effect would influence the results of the study, and it was not included in the analysis.
This study has shown that BTB is relatively high in cattle in the livestock/wildlife interface areas of Lochinvar and Blue Lagoon compared to Kazungula raising concerns in terms of animal productivity and public health. The study further showed that old and emaciated animals raised in the interface areas where more likely to test positive for BTB. Overall, the study has been able to establish geographical area attributable differences in prevalence coupled with potential risk factors of its occurrence at animal level.
References
Acha, P.N., Szyfres, B. 1987. Zoonotic tuberculosis. In: Zoonoses and communicable diseases common to man and animals (Washington: Pan American Health Organization/World Health Organization).
Ameni, G., Erkihun, A., 2007, Bovine tuberculosis on small-scale dairy farms in Adama Town, central Ethopia, and farmer awareness of the disease. Revue Scientifique et technique office International des Epizooties 26, 711–719.
Ameni, G., Ameni, K., Tibbo, M., 2003, Bovine tuberculosis:Prevelance and risk factor assessment in cattle and cattle owners in Wuchale-Jida district central Ethopia. Journal of applied research in veterinary medicine, 1, 1–4.
Asseged, B., Lubke-Becker, A., Lemma, E., Taddele, K., Britton, S., 2000, Bovine tuberculosis:a crosssectional and epidemiological study in and arround Addis Ababa. Bulletin of Animal health production in Africa, 48, 71–80.
Collins, M.M., Stollerman, G.H., 1993, Disseminated tuberculosis: a presumptive diagnosis. Hospital Practice (Office Edition) 28, 63–66, 71, 75–69.
Cook, A.J., Tuchili, L.M., Buve, A., Foster, S.D., Godfrey-Fausett, P., Pandey, G.S., McAdam, K.P., 1996, Human and bovine tuberculosis in the Monze District of Zambia–a cross-sectional study. British Veterinary Journal, 152, 37–46 DOI 10.1016/S0007-1935(96)80084-4.
Corner, L.A.L., 2006, The role of wild animal populations in the epidemiology of tuberculosis in domestic animals: How to assess the risk. Veterinary Microbiology 112, 303–312 DOI 10.1016/j.vetmic.2005.11.015.
Cosivi, O., Grange, J.M., Daborn, C.J., Raviglione, M.C., Fujikura, T., Cousins, D., Robinson, R.A., Huchzermeyer, H.F., de Kantor, I., Meslin, F.X., 1998, Zoonotic tuberculosis due to Mycobacterium bovis in developing countries. Emerging Infectious Diseases 4, 59–70.
Dankner, W.M., Waecker N.J., Essey E.M., Moser K., Thompson M., Davis C.E., 1993, Mycobacterium bovis infection in San Diego: a clinicoepidemiological study of 73 patients and a historical review of a forgotten pathogen. Medicine (Baltimore) 72, 11–37 DOI 10.1097/00005792-199301000-00002.
Dohoo, I., Martin, W., Stryhn, H., 2003, Veterinary Epidemiologic Research. AVC inc, Charlottetown, Cananda, 35–42 pp.
Gallagher, J., MacAdam, I., Sayer, J., & van Lavieren, L.P., 1972, Pulmonary tuberculosis in free-living Lechwe antelope in Zambia. Tropical Animal Health and Production 4, 204–213 DOI 10.1007/BF02360112.
Jordan, D. 1995. Herdacc: a program for calculating herd level (aggregate) sensitivity and specificity (Guelph, O. N., Canada, N1G2W1., Department of population medicine, University of Guelph).
Kazwala, R.R., Kambarage, D.M., Daborn, C.J., Nyange, J., Jiwa, S.F., Sharp, J.M., 2001, Risk factors associated with the occurrence of bovine tuberculosis in cattle in the Southern Highlands of Tanzania. Veterinary Research Communication 25, 609–614 DOI 10.1023/A:1012757011524.
Lesslie, I.W., Brin K.J, 1970, Mycobacterium avium infections in cattle and pigs in Great Britain. Tubercle 51, 446–451 DOI 10.1016/0041-3879(70)90011-5.
Monaghan, M.L., Doherty, M.L., Collins, J.D., Kazda, J.F., Quinn, P.J., 1994, The tuberculin test. Veterinary Microbiology 40, 111–124 DOI 10.1016/0378-1135(94)90050-7.
Munag’andu, H.M., Siamudaala, V.M., Nambota, A., Bwalya, J.M., Munyeme, M., Mweene, A.S., Takada, A., Kida, H., 2006, Disease constraints for utilization of the African buffalo (Syncerus caffer) on game ranches in Zambia. Japanese Journal of Veterinary Research 54, 3–13.
Mwima, H.K., 1995. Wildlife research and managent in Zambia with special reference to some protected areas where wild and domestic animals co-exist. In: The effects of enlargement of domestic animal pasture on the wildlife in Zambia, Lusaka, Zambia, pp. 44–64.
OIE, 2004, Manual of diagnostic tests and vaccines for terrestial animals Office International Des Epizooties, Paris, France.
Oloya, J., Opuda-Asibo, J., Djonne, B., Muma, J.B., Matope, G., Kazwala, R., Skjerve, E., 2006, Responses to tuberculin among Zebu cattle in the transhumance regions of Karamoja and Nakasongola district of Uganda. Tropical Animal Health and Production 38, 275–283 DOI 10.1007/s11250-006-4322-7.
Omer, M.K., Skjerve, E., Woldehiwet, Z., Holstad, G., 2001, A cross-sectional study of bovine tuberculosis in dairy farms in Asmara, Eritrea. Tropical Animal Health and Production 33, 295–303 DOI 10.1023/A:1010531802372.
O’Reilly, L.M., Daborn, C.J., 1995, The epidemiology of Mycobacterium bovis infections in animals and man: a review. Tubercle and Lung Disease 76, 1–39 DOI 10.1016/0962-8479(95)90591-X.
Pandey, G.S., 1998. Studies of the infectious diseases of the Kafue lechwe (Kobus leche kafuensis) with particular reference to tuberculosis in Zambia. Azabu University, Tokyo.
Pritchard, D.G., 1988, A centuary of bovine tuberculosis. journal of comarative pathology 99, 357–399.
Quirin, R., Rasolofo, V., Andriambololona, R., Ramboasolo, A., Rasolonavalona, T., Raharisolo, C., Rakotoaritahina, H., Chanteau, S., Boisier, P., 2001, Validity of intradermal tuberculin testing for the screening of bovine tuberculosis in Madagascar. Onderstepoort Journal of Veterinary Research 68, 231–238.
Radostist, O.M., Blood, D.C., Gay, C.C., 1994, Veterinary Medicine, 8th Edition. Bailliere Tndall, London.
Sheppe, W.A., 1985, Effects of human activities on Zambia’s Kafue flats ecosystmes. Environmental conservation Journal 12, 49–57.
Siamudaala, V.M., Muma, J.B., Munag’andu, H.M., Mulumba, M., 2003. Veterinary challenges regardding the utilisation of the Kafue lechwe (Kobus leche kafuensis) in Zambia. In: Conservation and development interventions: at the wildlife/wildlife interface: implication for wildlife, livestock and human health, Durban, South Africa, 14th to 15th August, pp. 75–80.
Sitima, A.C., 1997. Variability of Mycobacterium bovis in traditionally processed sour milk and the prevalence of bovine tuberculosis in Namwala district of Zambia. University of Zambia, Lusaka.
Sonnenberg, P., J, M., JR, G., S, S., B, K., P, G.-F., 2001, HIV-1 and recurrence, relapse, and reinfection of tuberculosis after cure: a cohort study in South African mineworkers. Lancet 358, 1687–1693 DOI 10.1016/S0140-6736(01)06712-5.
Stafford, K.J., 1991, A review of diseases of parasites of the Kafue lechwe (Kobus leche kafuensis). Journal of Wildlife Diseases 27, 661–667.
Sudre, P.G., Ten Dam., Kochi, A., 1992, Tuberculosis: a global overview of the situation today. Bulletin of the Wild Health Organisation 70, 149–159.
Zieger, U., Pandey, G.S., Kriek, N.P., Cauldwell, A.E., 1998, Tuberculosis in Kafue lechwe (Kobus leche kafuensis) and in a bushbuck (Tragelaphus scriptus) on a game ranch in central province, Zambia. Journal of South African Veterinary Association 69, 98–101.
Acknowledgements
We are grateful for the financial support from the Norwegian Programme for Development, Research and Education (NUFU) to this project. We also acknowledge the financial support through the University of Zambia and the Flemish Inter University Council (VLIR) Project under the Belgium Inter University Cooperation at the University of Zambia (UNZA-VLIR IUC programme). We are very grateful for the support and cooperation we received from the cattle owners and many individuals who facilitated this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Munyeme, M., Muma, J.B., Samui, K.L. et al. Prevalence of bovine tuberculosis and animal level risk factors for indigenous cattle under different grazing strategies in the livestock/wildlife interface areas of Zambia. Trop Anim Health Prod 41, 345–352 (2009). https://doi.org/10.1007/s11250-008-9195-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-008-9195-5