Abstract
Bovine tuberculosis (BTB) is a neglected endemic zoonotic disease in Cameroon. The prevalence of human tuberculosis (TB) is also considered very high in the country. The diagnosis of TB in animals is based mostly on detecting the characteristic macroscopic lesions found at slaughter and during meat inspection in abattoirs. The M. bovis strains circulating in animals, the extent of zoonotic TB due to M. bovis as well as M. bovis maintenance hosts, and the role that they play are unknown. The lack of active BTB surveillance in Cameroonian livestock and the close human–animal interactions in the management of herds provide suitable conditions for the emergence and transmission of zoonotic BTB. The potential threat of zoonotic TB due to M. bovis to human health, even at a low prevalence, cannot be overemphasized. Thus, broad multidisciplinary investigations of the sources and identification of TB causing agents, routes of transmission, associated risk factors, and epidemiology of TB among humans and animals as major keys to modelling the control of the disease, are essential. This review is built on records of the high prevalence of BTB in cattle and human TB; various tubercle bacilli strains isolated in cattle and closed human–livestock contacts in agropastoral communities in Cameroon, to highlight the risk factors for exposure and transmission of zoonotic BTB infection to cattle and cattle professionals; and its public health significance. It emphasizes the relevance of the multidisciplinary approach based on the “One Health” philosophy to control zoonotic BTB in Cameroon.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Beef cattle
- Bovine tuberculosis
- Cameroon
- Dairy cattle
- Epidemiology
- Herd management
- Mycobacterium bovis
- Zoonotic tuberculosis
1 Introduction
Human and animal tuberculosis (TB) are well-controlled diseases in most developed countries. However, the rapid population growth, widespread poverty, regional conflict, migration between countries, inappropriate application of disease control measures, and the HIV/AIDS epidemic continue to boost the number of cases and the negative effects of human and animal TB in most of Africa, including Cameroon (WHO 2009). Bovine TB (BTB) is prevalent in cattle in many African countries (Ayele et al. 2004; Zinsstag et al. 2006), but the exact prevalence of BTB in cattle and the extent of zoonotic TB due to M. bovis on the continent are largely unknown. It is also generally unknown which of the M. bovis strains circulate in animals in Africa, the number of existing M. bovis maintenance hosts, and the role that they play in maintaining the infection and contribute to the spread of the infection. However, historical and clinical data, and the sporadic use of the intradermal tuberculin tests, the single cervical (SIT), and the comparative cervical (CCT) tests (Awah-Ndukum et al. 2012b), have clearly established the endemic nature of BTB in cattle in Cameroon (Awah-Ndukum et al. 2012a, b; Egbe et al. 2016). There is, however, a lack of understanding of the magnitude of the infection, and its distribution.
Effective application of the test-and-slaughter policy, the basis of national BTB control programs in developed countries, is not yet practicable in many developing countries because of logistical, political, and financial constraints. Evaluation and application of effective alternative strategies that are technically feasible and economically viable under these circumstances, should be the primary objective in preventing the widespread occurrence of the disease in Africa.
Though BTB is widespread in Cameroon (Awah-Ndukum et al. 2012a, b; Egbe et al. 2016), it remains a neglected zoonotic disease, and its presumptive diagnosis in animals is based mostly on detecting the characteristic macroscopic lesions found at slaughter, and during meat inspection in abattoirs. In many communities in Cameroon, a number of practices increase the risk of contracting zoonotic BTB. In this country, it is common for humans and their animals to share an unhygienic microenvironment and water sources, especially during the dry season. In addition, the food preferences and eating habits of many Cameroonians, which include ingesting fresh animal products such as raw milk and meat, predispose them to contracting zoonotic TB. Other factors that may contribute to zoonotic BTB in Cameroon include the poor implementation of existing disease control legislation, poor monitoring and notification of the presence of the disease, and the lack of collaboration between the different public sector services responsible for controlling zoonotic diseases. In addition, inadequately trained veterinary and medical professionals, a poor diagnostic capability, the lack of public awareness about zoonotic TB, animal husbandry practices that predispose to the occurrence of the disease and human exposure to the infection, further complicate the matter (Ayele et al. 2004; AU/IBAR 2006; Kelly et al. 2016).
Because of the close human-livestock interaction in livestock-rearing communities in Cameroon, opportunities exist for the transmission of M. bovis to humans. Although an estimated median of 2.8% (0–37.7%) of human TB cases in Africa is due to M. bovis, significantly higher prevalences have been reported in some communities, and a similar trend may be expected in Cameroon (Müller et al. 2013).
2 Bovine Tuberculosis in Animals in Cameroon
Cameroon’s human population of over 20 million live in five agroecological zones (AEZ), namely: the (1) Sudano-Sahel (in the North and Far North regions), (2) Guinea High Savannah (in the Adamawa Region), (3) Western Highlands (in the Northwest and West regions), (4) mono-modal humid forests (in the Southwest and Littoral regions), and (5) bimodal humid forests (in the Center, East and South regions). Over 70% of the population lives in rural areas, and their livelihood depends on crop farming and livestock rearing (Tanya 2004).
The national livestock population comprises 6 million cattle (mainly indigenous, multipurpose zebu breeds), 4 million sheep, 4.6 million goats, 0.2 million horses, and about 1.8 million pigs (FAOSTAT 2014). There is also a wide range of wildlife, including predators, wild hogs, wild ruminants, and rodents inhabiting the different AEZs. The majority of livestock in Cameroon are traditionally and extensively managed, and the farmers are dependent on limited ranges and feed that are more limited during the dryer seasons and the AEZ in which they live. There is extensive transhumant movement of livestock during the dry season to cope with the feed shortage. The dominant pastoral production and extensive husbandry systems increase their vulnerability to many diseases.
Contact of livestock with wildlife (during seasonal migration) and their congregation during grazing in the field, veterinary interventions, and in livestock markets provide ample opportunity for the transmission of infectious diseases. These diseases have a huge impact on animal productivity and wellbeing; they also threaten public health because of their zoonotic transmission to humans (including BTB), with the poor being particularly vulnerable. Therefore, improved surveillance for BTB and accurate estimations of its magnitude and distribution in cattle are essential to devise appropriate intervention strategies in Cameroon.
Information on the occurrence of BTB in Cameroon is scant especially before the year 2000. Available data extracted from FAO-OIE-WHO Animal Health Yearbooks (1992–1997) and the OIE (1997–2001) confirm that BTB is prevalent in the four sub-Saharan geopolitical regions. However, a definitive regional prevalence could not be estimated due to inadequate data from several key countries such as Cameroon in the Central African region. The disease status in Cameroon is often only documented as “low and sporadic,” “disease reported,” “disease suspected but not confirmed,” and “serological evidence with no clinical disease.” No disease information was available for animals other than cattle in those reports.
Although BTB in cattle occurs widely in Cameroon, there is no structured surveillance for its presence and distribution, and information about its occurrence is mainly dependent on the detection of macroscopic lesions during the postmortal examination of carcasses and meat inspection in abattoirs (Awah-Ndukum et al. 2012b; Egbe et al. 2016). Following the provisional diagnosis of BTB during these events and immunologic reactions to ancillary tests, direct smear microscopy of Ziehl-Neelsen-stained smears of the exudate of suspect lesions, culture (on solid/liquid media), and the results of molecular diagnostic techniques are used to confirm the presence and identification of the Mycobacterium spp. isolated from the lesions (Awah-Ndukum et al. 2013; Koro-Koro et al. 2013; Egbe et al. 2016). Intradermal tuberculin tests, immunochromatographic (lateral-flow-based rapid test), and IFN-γ assays (Bronsvoort et al. unpublished) (Awah-Ndukum et al. 2012a, b) are only occasionally used to diagnose the disease in cattle in Cameroon. In contrast to several reports from other African countries that documented BTB in other domestic animals such as pigs, small ruminants, horses, dogs, and wildlife (Cadmus et al. 2009; Hiko and Agga 2011; Hlokwe et al. 2011; Katale et al. 2012), BTB has not been detected in these animals in Cameroon.
Estimates of the national prevalence of BTB in cattle are based on opportunistic samples obtained from accessible segments of the cattle population. The distribution of the disease appears to be very patchy, and, depending on the sampling and detection methods, the prevalence varies widely from <1% to >50% (Table 12.1). Establishing the true extent of the disease will require representative samples of the total population from across the country. More often too, different prevalence estimates are reported from the same study population because of differing interpretations of the tests used, and there is a need to standardize the application and interpretation of diagnostic tests used in the country (Awah-Ndukum et al. 2012a).
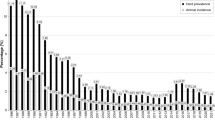
Bovine TB has been detected throughout the course of the year and the monthly prevalence ranges from 0.3 to 0.8% (Fig. 12.1). The observed increase in BTB in recent years seems to be associated with an increase in the number of cattle slaughtered and an increased efficiency of meat inspection (Awah-Ndukum et al. 2012b; Egbe et al. 2016). This increasing trend does not necessarily reflect an actual increase of the disease, but rather an improved diagnostic capability and better meat inspection procedures.
Molecular epidemiological data of BTB in Cameroon reveal a marked heterogeneity of strains with over 46 spoligotypes circulating in the country (Fig. 12.2). Mycobacteriological culture and spoligotyping confirm that though M. bovis is the principal etiological agent of BTB in Cameroon (Egbe et al. 2016; Awah-Ndukum et al. 2013), there is often a mixed and widely diverse infection of M. bovis and other Mycobacterium spp., including M. tuberculosis, M. gordonae, M. phlei, M. fortuitum, M. mucogenicum, and M. scrofulaecum (Egbe et al. 2016). The extent of their contribution to the disease burden is unknown, and confirmation of the cause of tuberculous lesions by mycobacteriological and molecular diagnostic techniques is essential to establish their importance.
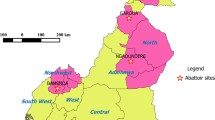
Geographical distribution of the 46 M. bovis spoligotypes isolated in Cameroon. (1) Regions of Cameroon: EN (Far North); N (North); AD (Adamawa); NW (North West); OU (West); SW (South West); LT (Littoral); CE (Centre); SU (South); ES (East); (2) AD = SB0300, SB0893, SB0954, SB0955, SB1027, SB1099, SB1104, SB1418, SB1462, SB2313, SB2316, SB2317, SB2318, SB2319, SB2320, SB2321, SB2323, SB2327, SB2328, SB2330, SB2331, SB0944, SB0951, SB0953, SB1025, SB1460, SB2033, SB2162, SB2324; (3) EN = SB0944, SB0951, SB1460, SB1459, SB2325, SB2332, SB2333, SB0952; (4) N = SB0944, SB0951, SB1025, SB1460, SB2033, SB2324, SB1459, SB0120, SB2329, SB2334, SB2035, SB0952, SB1461, SB1463; (5) NW = SB0944, SB0953, SB2162, SB1026, SB2035, SB2161, SB2163, SB2164, SB2314, SB2315); (6) CE and LT = SB2033, SB2035, SB1419; (7) ?!: No data available (SW, OU, SU and ES are not major cattle producing areas); (8) *: cattle slaughtered in LT and CE mainly originate from AD, N, NW; (9) Spoligotype patterns were named according to www.Mbovis.org International database (Smith and Upton 2012). (Sources: Egbe et al. 2017; Awah-Ndukum et al. 2013; Koro-Koro et al. 2013; Njanpop-Lafourcade et al. 2001)
For effective surveillance of BTB in Cameroon, meat inspection should be supported by tracing-back suspicious cases to the herds of origin to allow appropriate measures such as isolation and slaughter, and restriction of movement to other herds, to be instituted to limit further spread of the infection.
3 Evaluation of Diagnostic Tests Under Local Conditions
Based on the substantial variation in the results reported for the same groups of cattle by different investigators, it appears that there is a marked inconsistency in the application of criteria used for the interpretation of the test results. This implies that the performance of diagnostic tests and interpretation of results at various cut-off values have serious implications for assessing the actual status of BTB in Cameroon.
Since the available diagnostic tests were developed in different settings with different breeds of cattle, it was deemed necessary to assess the accuracy of various diagnostic techniques under Cameroonian conditions. As an example, performing the CCT in Maroua and using ≥2 mm, ≥3 mm, and ≥ 4 mm as cut-off points and the presence of visible BTB lesion as a reference test, the estimated sensitivities were, respectively, 78.5%, 67.8%, and 57.1%, and the specificities were, respectively, 85.9%, 94.7%, and 96.5% (Awah-Ndukum et al. unpublished data). Using the SIT at cut-off points of ≥3 mm and ≥ 4 mm, the respective sensitivity was 82.1% and 71.4%, and the specificity was 91.2% and 96.5%. When the presence of TB lesion in addition to Ziehl-Neelsen smear microscopy was used as the reference test to define the disease status, a sensitivity, respectively, of 100%, 89.4%, and 73.6% and a specificity of 83.3%, 92.4%, and 93.9% at ≥2 mm, ≥3 mm, and ≥ 4 mm cut-off points were obtained. Furthermore, receiver operating characteristic (ROC) analysis showed better performance at the ≥3-mm cut-off (over 91%) compared to the ≥4-mm cut-off point (84%) suggesting that in Cameroon a stricter interpretation of the specific tuberculin skin tests would detect more BTB-positive cattle. Bronsvoort et al. (unpublished data) similarly obtained better values for the sensitivity and specificity of the CCT at a cut-off value of ≥3 mm compared to ≥4 mm. Awah-Ndukum et al. (2012a) previously reported that irrespective of the tuberculin test cut-off values, there is a strong association between seroprevalence using the lateral flow rapid-based (immune-chromatographic) assay and the tuberculin test results.
The detection of lesions that are consistent with those caused by M. bovis during postmortal examination provides most of the information on which the prevalence and distribution of BTB in Cameroon are based. This technique too is flawed; there is ample evidence that under-recording and under-detection are very common, and the information provided is most likely a marked underestimation of the actual situation. Animals demonstrating poor health and diminished productivity are customarily the ones removed from herds and slaughtered for meat production. As they are usually disproportionately old-aged, abattoir inspection may not provide a true estimate of BTB in the local cattle population as the prevalence of BTB is expected to be higher in this group of animals.
Interpretation and the detection of TB-like lesions in slaughtered cattle can be difficult. Routine meat inspection generally only detects lesions in about 50% of carcasses containing tuberculous lesions. These lesions may resemble abscesses (with yellowish pus) or manifest as firm, yellowish, nodular lesions (often “gritty” on cutting), and are commonly detected in the lungs and associated lymph nodes (over 60%), followed by lymph nodes of the head, mesenteric lymph nodes, and the liver. The granulomatous lesions may easily be confused with parasitic granulomas, non-specific inflammatory reactions (Corner 1994; Shitaye et al. 2006; Edwards et al. 1997), and lesions caused by Nocardia, Corynebacterium, and other pyogranuloma-causing organisms (Grist 2008), and wrongly diagnosed as tuberculous unless confirmed by culture. Nonetheless, in slaughtered cattle, TB lesions were 3–5 times more prevalent than similar lesions caused by a different etiological agent.
4 The Epidemiology of BTB in Cattle in Cameroon
There are indications that the prevalence of BTB in Cameroon differs substantially between its regions. The reasons for these differences are not well understood and need further investigation. Generally, the gathering of animals at communal sites, mixing of different age groups (adult and aging), stressors such as adverse environmental factors, and long trekking to grazing and drinking spots are some of the major factors that appear to influence the prevalence and distribution of BTB in cattle in the country. Further factors that may affect its prevalence include the lack of application of quarantine measures; uncontrolled animal movement and smuggling of live animals from neighboring countries like Nigeria, Chad, and the Central African Republic where BTB is widespread (Cadmus et al. 2006; Diguimbaye et al. 2006a; Müller et al. 2009); and the mass influx of refugees and their livestock (displacement of human and animals resources) due to social unrest and civil wars in neighboring countries.
In Cameroon, many conditions favor the occurrence and spread of BTB in cattle (Awah-Ndukum et al. 2014; Kelly et al. 2016). For example, over 84% of cattle live to a very old age, and over 70% of cattle are kept in moderate to large herds, and in traditional, extensive (38%) or semi-extensive (58%) management systems. Many cattle trek at least 5 km daily for grazing and drinking (60%), and there are ample inter-herd intermingling and animal-animal contact (99%) when individual herds of the same or different owners come together (Table 12.2).
There are perceptions of apparent resistance of some of the indigenous African breeds in Cameroon to contracting BTB (Inangolet et al. 2008; Ameni et al. 2006; Oloya et al. 2006), although the prevalence of BTB in the predominantly zebu breeds of Cameroonian cattle still appears to be high. A lower prevalence of BTB was recorded in the Namchi, Gudali, and White Fulani breeds, compared to the Red Bororo cattle, suggesting that some of the local breeds may have a degree of innate resistance or tolerance to the disease. There are similar differences between the Gudali and Fulani breeds (Egbe et al. 2016). The reasons for the perceived resistance to BTB of local breeds may be more complex and include the possibility of reduced virulence and infectiousness of the causative agent, perhaps because of M. bovis being exposed to the effects of the harsh tropical climate (Oloya et al. 2006).
The burden of BTB is higher in the Northern Regions than in the Northwest (Fig. 12.1; Table 12.1), the odds of detecting gross TB lesions in carcasses being 4 times higher in cattle originating from the Northern Region compared to those from the Northwest Region (Egbe et al. 2016). A number of factors may affect this distribution, such as the uncontrolled movement of cattle in the Northern Regions, including those from neighboring countries that are BTB-infected. Elsewhere, such as in the Western Highlands and Far Northern Regions, continuous close contact between animals due to increasing animal and human population densities, and limited grazing, influence the prevalence. A lower prevalence has been recorded in regions with lower population densities, abundant natural pasture, and low herd-herd (animal-animal) contact (Awah-Ndukum et al. unpublished data).
Though intensive husbandry practices create favorable conditions for BTB transmission by providing opportunities for closer and prolonged contact between animals than those in extensive management systems (Ayele et al. 2004; Inangolet et al. 2008), in Cameroon, more BTB-positive reactions to tuberculin tests were recorded in animals kept under semi-extensive management compared to the other systems. In the extensive and semi-extensive systems, increased transmission of BTB can result from close contact, particularly during periods of drought, between animals sharing common grazing, water, and salt licks. Additionally, the intermingling of animals from different regions at cattle markets or during veterinary interventions may also enhance transmission of the infection (Ayele et al. 2004).
An important feature of BTB in Cameroon is the higher prevalence of the disease recorded in beef compared to dairy cattle, and in small compared to large herds (Fig. 12.3). This seems to result from fewer sources of M. bovis, or the absence of diseased cattle in dairy herds, and reduced contact between animals in the large herds that are usually managed in extensive farming systems. The characteristics of the traditional pastoral systems in the tropics, such as close contact between animals in shared microenvironments, as animals gather at the few watering points and salt-licks, or congregate under trees or shaded areas to protect them from the intense tropical ambient temperatures, mimic conditions created by intensive management systems (Ayele et al. 2004). These conditions often create increased opportunities for nose-to-nose and mouth-to-mouth contact between cattle, and enhance transmission of the disease.
Another important factor that favors the spread of BTB in the traditional pastoral husbandry system, is the high mobility of cattle herds, crisscrossing the regions during transhumance. Environmental stress associated with this movement and the ensuing mixing of transhumant and semi-intensive resident herds in the village, wildlife, and other domestic species create ideal conditions for exposure to and widespread transmission of M. bovis.
Although a wide diversity of wildlife and a vast livestock-wildlife interface exist in Cameroon, no data are available about the occurrence of BTB in its wildlife. Neither is there any information about the disease in the other economically important domestic species such as pigs, sheep, and goats. Several of these species, including wildlife, have been reported to serve as maintenance hosts for BTB in other countries (Courtenay et al. 2006). The need for a comprehensive investigation of the status of BTB in Cameroon, including establishing specific herd- and animal-level risk factors and identifying the maintenance hosts of the infection, and its zoonotic importance cannot be overemphasized.
5 Control of BTB in Cameroon
Although BTB has enormous public health significance, it is a neglected zoonosis in Cameroon. Governmental resources are inadequate for monitoring animal diseases including the zoonoses, and the private sector lacks the necessary capacity and resources to assume or to share the responsibility.
Implementation of existing legislature governing BTB is poor. The existing control programs are poorly applied, and the control of BTB is mainly dependent on the regulation of animal movements and by the postmortal examination of carcasses for the presence of tuberculous lesions. Further constraints include the poor execution of a number of activities: regular tuberculin testing and the removal of positive reactors, strict meat inspection and removal of suspected lesions, tracing tuberculous cases to farms or areas of origin, and restricting the movements of infected animals. There is also an absence of the application of broad hygienic and biosecurity measures reflecting deep-seated problems with respect to inter-institutional veterinary and medical collaboration causing them to not best serve the needs of society.
There are attitudinal problems by stock owners too in controlling the disease. Many cattle owners endeavor to increase their livestock numbers (increasing the size of their “living banks”) but are unaware of the negative impact of BTB on animal productivity and health such as economic loss, lower production, and poor animal health (Table 12.3). Not many stockmen (≈30%) can recognize the presence of BTB in their or adjacent herds, and most cattle owners (>86%) report that they do not implement known control measures to deal with the disease in their communities that are predominantly rural in “seminatural” or “semi-wild” areas (Table 12.4). They do, however, accept condemnations at abattoirs as an acceptable governmental measure to remove BTB-infected animals from the food chain.
Tuberculin skin testing and the elimination of infected animals (test-and-slaughter) that have been used effectively in other parts of the world (Good 2006; Pavlik 2006) are not practicable in Cameroon because of the lack of compensation when infected animals are slaughtered. Instead, testing and segregation of positive reactors, with phased slaughtering of the infected animals, could be economically and technically achievable as an alternative to the conventional test-and-slaughter method (WHO 1994). In the interim, there is a need to intensify meat inspection for the detection of BTB, to maintain reliable abattoir records, and to validate the various diagnostic tests used for screening live cattle for the presence of BTB in Cameroon.
There is an urgent need for a multidisciplinary approach in Cameroon, based on the “One Health” philosophy (Kahn et al. 2007; Vallat 2009), to control BTB. Specific activities in this regard include enhancing public awareness through continuing education of cattle owners, medical and veterinary professionals, and the general public on the potential risk of BTB, proper food (animal products) handling, good husbandry practices, personal hygiene, and maintaining a healthy environment. Restricting movements of BTB-infected herds, cooperative efforts by veterinary and medical personnel to maximize TB detection rates, engaging the populations at risk, and good health surveillance systems are essential activities that should be implemented to ensure effective control of the disease in humans and animals.
6 Current Status of Human Tuberculosis in Cameroon
Human tuberculosis (TB) is highly prevalent in Cameroon, and it has a current annual incidence of over 200 cases per 100,000 population (WHO 2009) (Table 12.5). The unfavorable local socioeconomic conditions, the close association of TB with the HIV epidemic, and the widespread mycobacterial resistance to drugs used in the treatment of TB hamper its control (Kuaban et al. 2000a). Because of the close association between the re-emergence of TB and the emerging HIV/AIDS epidemic in Cameroon (WHO 2009) (Table 12.5), the seroprevalence of HIV in TB patients serves as an accurate indicator of the prevalence of HIV in the general population (Noeske et al. 2004). In addition to HIV/AIDS, other factors such as poverty, malnutrition, stress, and smoking are important risk factors predisposing to contracting the disease (Pešut et al. 2008).
The prevalence of human TB in Cameroon is still increasing, especially in the economically active age group (21–40 years) and in immunocompromised individuals, such as those suffering from HIV/AIDS (WHO 2009; Noeske et al. 2004; Ane-Anyangwe et al. 2006). About 40% of all TB patients in the country are also HIV-positive, and the prevalence of HIV both in the general adult population and in adults with TB is steadily increasing. More recent studies of HIV/AIDS in TB cases of all ages in the general population indicated an even higher prevalence (ranging from 31 to 43%) (WHO 2009). Due to the magnitude and the increase of the two infections in Cameroon, it is expected that even after the HIV epidemic in the general population reached its peak and starts to decline, TB/HIV coinfection would probably continue to occur since TB can be contracted at any time during the course of an HIV infection (Noeske et al. 2004).
7 Zoonotic (M. bovis) TB in Humans in Cameroon
Tuberculosis (TB) is globally a leading cause of human death by a single causal infectious agent. The extent of human TB caused by M. bovis is unknown, but it seems to account for only a small percentage of the cases of TB reported in humans. Thus, in the developed countries, M. bovis accounts for less than 0.5–7.2% of the cases of human TB, while in developing countries, 10–15% or more are estimated to be caused by the infection (de la Rua-Domenech 2006). Reliable data are unavailable about the number of human cases of TB caused by M. bovis in Cameroon, due to the lack of attention to the problem by policy makers, and the limited number of available diagnostic facilities able to identify M. bovis. In most of the rural areas, for TB, the main diagnostic approach is the detection of acid-fast bacilli (AFB) in the sputum of suspected cases by direct microscopy, and only urban reference laboratories attempt to isolate the organism. These referral laboratories, however, make little or no attempt to identify the isolates to the strain/species level, thus probably missing many M. bovis cases. Their argument is that the same drug regimen would be used to treat all TB cases, although resistance of M. bovis to some of the first-line anti-TB drugs has been reported worldwide (Diguimbaye et al. 2006b; Gibson et al. 2004), including in Cameroon (Kuaban et al. 2000a, b). Currently, the level of public awareness of the zoonotic risk of BTB, and the lack of animal and human health surveillance programs to control TB, is very limited; this is in spite of an established epidemiologic association between tuberculin-positive cattle and human TB in several countries, such as in Ethiopia and Zambia (Regassa et al. 2008).
Because of a number of practices that may enhance the transmission of the disease, there is concern that zoonotic M. bovis infections may be more prevalent in Cameroon than anticipated. Given the close association between livestock and humans because of prevailing farming practices in Cameroon, the high prevalence of BTB in the indigenous cattle could contribute to an increase in the prevalence of human TB. Opportunities exist for the transmission of M. bovis to humans because of the very close human-livestock interaction (Table 12.4) favoring aerosol transmission, and the habit of consuming unpasteurized milk (Thoen et al. 2006) and raw meat (Table 12.3). Other people at risk, such as butchers, abattoir workers, and those with low levels of education, appear not to be well informed about the risk posed by and the modes of transmission of zoonotic TB. Of TB patients surveyed in Bamenda (Awah-Ndukum et al. 2014), only 17.3% were aware of zoonotic TB, and Kelly et al. (2016) too reported a low level of awareness by both dairy farmers and cattle owners/pastoralists that consuming milk could cause zoonotic TB. A large segment of the population (32.1%) drink fresh milk (pasteurized or unpasteurized), 19.8% drink unpasteurized milk, 2.5% eat raw meat, and 61.3% eat Suya that is meat briefly roasted over hot charcoal or fire. The practice of pooling milk from cows of several owners could further increase the risk to a larger number of people consuming the raw product (Awah-Ndukum et al. 2014). Under these conditions, particularly those suffering from HIV/AIDS (Noeske et al. 2004) are at risk (Berg et al. 2009).
PCR-based genomic deletion analyses showed evidence of M. bovis in human and M. tuberculosis in cattle samples (Awah-Ndukum et al. 2011) suggesting possible animal-to-human and human-to-animal transmission cycles. Molecular analyses confirmed the presence of M. bovis in humans with pulmonary TB in the Western and Northwestern regions of Cameroon (Niobe-Eyangoh et al. 2003; Egbe et al. 2017). This indicates the possibility of human-to-human transmission of M. bovis by the respiratory route. Similarly, a range of mycobacteria, including M. tuberculosis and several mycobacteria other than tubercle (MOTT) bacilli, has been isolated from cattle (Egbe et al. 2016).
The epidemiologic link between TB in humans and BTB in cattle, with the possibility of a cattle-human-cattle cyclical transmission, is of serious concern in Cameroon due to issues related to drug resistance. Mycobacterium bovis strains isolated from human TB cases also have different drug susceptibility profiles compared to M. tuberculosis strains (Diguimbaye et al. 2006b; Gibson et al. 2004).
The contribution of BTB to the overall TB morbidity and mortality in Cameroon thus needs a broad investigative approach. This would involve establishing the epidemiology of the disease in cattle and human populations, identification of TB-causing agents and their respective sources, maintenance hosts, possible routes of transmission, and associated risk factors.
The public health threat of M. bovis in Cameroon therefore requires the urgent attention of veterinary and medical professionals, biomedical and ecological experts, social workers, and policy makers within the context of the “One Health” approach.
8 Conclusion
The prevalence of BTB in Cameroon is high and it poses a major zoonotic threat to the human population. Most herdsmen and livestock handlers, small-scale farmers, nomads, and wage laborers in Cameroon are poorly educated about the presence and the consequences of BTB. Currently, urban and peri-urban livestock farming is growing fast, bringing animals closer to the large urban populations, thus exposing more and more people to the zoonotic risk of the disease. These changes are occurring in the absence of any meaningful BTB control policy, including restriction of cattle movement within the country, and from neighboring countries. Due to the significant public health implications, prompt action to control BTB in both animals and humans in Cameroon is required.
Establishing basic biosecurity measures (such as farm and personal hygiene, good husbandry practices, movement control, regular health inspection, and proper food handling) and public education programs to raise awareness about zoonotic TB are the activities that need immediate attention. For the long-term control of BTB in animals, assessment of the full extent of the problem in Cameroon is required. Therefore, a comprehensive molecular epidemiological study is needed to provide accurate data about cattle-cattle transmission and the role of wildlife (and domestic animals) reservoirs in the maintenance and transmission of TB in animals and humans. Only once the full extent of the problem has been determined, can the most appropriate and cost-effective control measures for Cameroon be planned and implemented.
References
Ameni G, Aseffa A, Engers H et al (2006) Cattle husbandry in Ethiopia is a predominant factor affecting the pathology of bovine tuberculosis and gamma interferon responses to mycobacterial antigens. Clin Vacc Immunol 13:1030–1036
Ane-Anyangwe IN, Akenji TN, Mbacham WF et al (2006) Seasonal variation and prevalence of tuberculosis among health seekers in the South Western Cameroon. East Afr Med J 83:588–595
AU/IBAR (2006) Pan African Animal Health Yearbook 2006. African Union/Inter-African Bureau for Animal Resources Nairobi, Kenya
Ayele WY, Neill SD, Zinsstag J et al (2004) Bovine tuberculosis: an old disease but a new threat to Africa. Int J Tuberc Lung Dis 8:924–937
Awah-Ndukum J, Kudi AC, Bradley G (2010) Prevalence of bovine tuberculosis in abattoirs of the Littoral and Western highland regions of Cameroon: a cause for public health concern. Vet Med Int 2010:8. https://doi.org/10.4061/2010/495015
Awah-Ndukum J, Kudi AC, Bradley G et al (2011) Preliminary report on the zoonotic significance of tuberculosis in cattle in Cameroon. In: Kofer J, Schobesberger H (eds) Animal health and sustainable livestock production. Proceedings of the 15th International Congress on animal hygiene organized by the International Society of animal hygiene, Vienna, Austria. Austria, 3–7 Jul 2011, Tribun EU 1, pp 193–195
Awah-Ndukum J, Kudi AC, Bah GS et al (2012a) Bovine tuberculosis in cattle in the highlands of Cameroon: seroprevalence estimates and rates of tuberculin skin test reactors at modified cut-offs. Vet Med Int 2012(Article ID 798502):13. https://doi.org/10.1155/2012/798502
Awah-Ndukum J, Kudi AC Bradley B et al (2012b) Prevalence of bovine tuberculosis in cattle in the highlands of Cameroon based on the detection of lesions in slaughtered cattle and tuberculin skin tests of live cattle. Vet Medicina 57:59–76
Awah-Ndukum J, Kudi AC, Bradley G et al (2013) Molecular genotyping of Mycobacterium bovis isolated from cattle tissues in the North West region of Cameroon. Trop Anim Health Prod 45:829–836
Awah-Ndukum J, Kudi AC, Bah CS (2014) Risk factors analysis and implications for public health of bovine tuberculosis in the highlands of Cameroon. Bull Anim Health Prod Afr 62:353–376
Berg S, Firdessa R, Habtamu M et al (2009) The burden of mycobacterial disease in Ethiopian cattle: Implications for public health. PLoS One 4(4):e5068
Cadmus S, Palmer S, Okker M et al (2006) Molecular analysis of human and bovine tubercle bacilli from a local setting in Nigeria. J Clin Microbiol 44:29–34
Cadmus SI, Adesokan HK, Jenkins AO et al (2009) Mycobacterium bovis and M. tuberculosis in goats, Nigeria. Emerg Infect Dis 15:2066–2067
Corner LA (1994) Post mortem diagnosis of Mycobacterium bovis infection in cattle. Vet Microbiol 40:53–63
Courtenay O, Reilly LA, Sweeney FP et al (2006) Is Mycobacterium bovis in the environment important for the persistence of bovine tuberculosis? Biol Lett 2:460–462
de la Rua-Domenech R (2006) Human Mycobacterium bovis infection in the United Kingdom: Incidence, risks, control measures and review of the zoonotic aspects of bovine tuberculosis. Tuberculosis 86:77–109
Diguimbaye DC, Hilty H, Ngandolo R et al (2006a) Mycobacterium bovis isolates from tuberculous lesions in Chadian Zebu carcasses. Emerg Infect Dis 12:769–771
Diguimbaye DC, Hilty M, Ngandolo R et al (2006b) Molecular characterization and drug resistance testing of M. tuberculosis isolates from Chad. J Clin Microbiol 44:1575–1577
Edwards DS, Johnston AM, Mead GC (1997) Meat inspection: an overview of present practices and future trends. Vet J 154:135–147
Egbe NF, Muwonge A, Ndip L et al (2016) Abattoir-based estimates of mycobacterial infections in Cameroon. Sci Rep 6:24320. https://doi.org/10.1038/srep24320
Egbe NF, Muwonge A, Ndip L, Kelly RF, Sander M, Tanya V, Ngu Ngwa V, Handel IG, Novak A, Ngandalo R, Mazeri S, Morgan KL, Asuquo A, Bronsvoort BMdeC (2017) Molecular epidemiology of Mycobacterium bovis in Cameroon. Sci Rep 7:4652. https://doi.org/10.1038/s41598-017-04230-6
FAOSTAT (2014) Cameroon livestock population. http://www.fao.org/faostat/en
Gibson AL, Hewinson G, Goodchild T et al (2004) Molecular epidemiology of disease due to Mycobacterium bovis in humans in the United Kingdom. J Clin Microbiol 42:431–434
Good M (2006) Bovine tuberculosis eradication in Ireland. Ir Vet J 59:154–162
Grist A (2008) Bovine Meat Inspection—Anatomy, physiology and disease conditions, 2nd edn. Nottingham University Press, Nottingham, p 296
Hiko A, Agga G (2011) First-time detection of Mycobacterium species from goats in Ethiopia. Trop Anim Health Prod 43:133–139
Hlokwe TM, Jenkins AO, Streicher EM et al (2011) Molecular characterization of Mycobacterium bovis isolated from African buffaloes (Syncerus caffer) in Hluhluwe-iMfolozi Park in KwaZulu-Natal, South Africa. Onderstepoort J Vet Res 78:232
Inangolet F, Demelash B, Oloya J et al (2008) A cross-sectional study of BTB in the transhumant and agro-pastoral cattle herds in the border areas of Katakwi and Moroto districts, Uganda. Trop Anim Health Prod 40:501–508
Kahn LH, Kaplan B, Steele JH (2007) Confronting zoonoses through closer collaboration between medicine and veterinary medicine (as ‘one medicine’). Vet Italia 43:5–19
Katale BZ, Mbugi EV, Kendal S et al (2012) Bovine tuberculosis at the human-livestock-wildlife interface: Is it a public health problem in Tanzania? A review. Onderstepoort J Vet Res 79:84–97
Kelly RF, Hamman SM, Morgan KL et al (2016) Knowledge of bovine tuberculosis, cattle husbandry and dairy practices amongst pastoralists and small-scale dairy farmers in Cameroon. PLoS One 11:e0146538. https://doi.org/10.1371/journal.pone.0146538
Koro-Koro F, Bouba FE, Ngatchou AFS et al (2013) First insight into the current prevalence of bovine tuberculosis in cattle slaughtered in Cameroon: the case of main abattoirs of Yaoundé and Douala. Br Microbiol Res J 3:272–279
Kuaban C, Bercion R, Jifon G et al (2000a) Acquired anti-tuberculosis drug resistance in Yaounde, Cameroon. Int J Tuberc Lung Dis 4:427–432
Kuaban C, Bercion R, Noeske J et al (2000b) Anti-tuberculosis drug resistance in the West Province of Cameroon. Int J Tuberc Lung Dis 4:356–360
Martrenchar A, Njanpop BM, Yaya A et al (1993) Problems associated with tuberculosis and brucellosis skin-test methods in northern Cameroon. Prev Vet Med 15:221–229
Merlin P, Tsangueu P (1985) Incidence de la tuberculose bovin dans le nord ouest du Cameroun. Rev SciTech (Organisation mondiale de la santé animale - OIE) 1:89–93
Muchaal PK (2002) Assessment of Bovine Tuberculosis (Mycobacterium bovis) and risk factors of transmission in the peri-urban centres of Bamenda, Northwest Province (Cameroon). Urban Agriculture and Zoonoses in West Africa: an assessment of the potential impact on public health. CFP Report 35. The International Development Research Centre (IDRC), Ottawa
Müller B, Hilty M, Berg S et al (2009) African 1, an epidemiologically important clonal complex of Mycobacterium bovis dominant in Mali, Nigeria, Cameroon, and Chad. J Bacteriol 191:1951–1960
Müller B, Dürr S, Alonso SJ et al (2013) Zoonotic Mycobacterium bovis-induced tuberculosis in humans. Emerg Infect Dis 19:899–908
Nfi AN, Ndi C (1997) Bovine tuberculosis at the animal research antenna (ARZ) Bangangte, Western province, Cameroon. Bull Anim Prod Health Afr 45:1–3
Niobe-Eyangoh SN, Kuaban C, Sorlin P et al (2003) Genetic biodiversity of Mycobacterium tuberculosis complex strains from patients with pulmonary tuberculosis in Cameroon. J Clin Microbiol 41:2547–2553
Njanpop-Lafourcade BM, Inwald J, Ostyn A, Durand B, Hughes S, Thorel MF, Hewinson G, Haddad N (2001) Molecular typing of Mycobacterium bovis isolates from Cameroon. J Clin Microbiol 39:222–227
Noeske J, Kuaban C, Cunin P (2004) Are smear-positive pulmonary tuberculosis patients a ‘sentinel’ population for the HIV epidemic in Cameroon? Int J Tuberc Lung Dis 8:346–351
Oloya J, Opuda-Asibo J, Djønne B et al (2006) Responses to tuberculin among Zebu cattle in the transhumance regions of Karamoja and Nakasongola district of Uganda. Trop Anim Health Prod 38:275–283
Pavlik I (2006) The experience of new European Union Member States concerning the control of bovine tuberculosis. Vet Microbiol 112:221–230
Pešut DP, Gledović ZB, Grgurević AD et al (2008) Tuberculosis incidence in elderly in Serbia: Key trends in socioeconomic transition. Croatian Med J 49:807–812
Regassa A, Medhin G, Ameni G (2008) Bovine tuberculosis is more prevalent in cattle owned by farmers with active TB in central Ethiopia. Vet J 178:119–125
Shitaye JE, Getahun B, Alemayehu T et al (2006) A prevalence study of bovine tuberculosis by using abattoir meat inspection and tuberculin skin testing data, histopathological and IS6110 PCR examination of tissues with tuberculous lesions in cattle in Ethiopia. Vet Medicina 51:512–522
Smith NH, Upton P (2012) Naming spoligotype patterns for the RD9-deleted lineage of the Mycobacterium tuberculosis complex; www.Mbovis.org. Infect Genet Evol 2:873–876
Tanya VN (2004) The contribution of animal and fisheries research to poverty alleviation in Cameroon. In: Mbiapo FT, Etoa FX (eds) Proceedings of the 11th Annual Conference of Bioscience: Animal production and poverty alleviation, 10th edn. The Cameroon Bioscience Society, Yaoundé, pp 1–6
Tanya VN, Sallah JNS, Tayou KR (1985) Screening for bovine tuberculosis at Wakwa. Rev Sci Tech OIE Ser Sci Agronomiques et Zootechniques (Cameroon) 1:65–68
Thoen CO, LoBue PA, de Kantor I (2006) The importance of Mycobacterium bovis as a zoonosis. Vet Microbiol 112:339–345
Vallat B (2009) One World, One Health. OIE Bull 2:1–2. http://www.oie.int/en/for-the-media/editorials/detail/article/one-world-one-health/
WHO (1994) Zoonotic tuberculosis (Mycobacterium bovis) memorandum from a WHO meeting (with the participation of FAO). Bull World Health Org 72:851–857
WHO (2009) Global Tuberculosis Control: Epidemiology, Strategy, Financing. WHO Report: WHO/HTM/TB/2009, vol 411. World Health Organization, Geneva
Zinsstag J, Kazwala RR, Cadmus I et al (2006) Mycobacterium bovis in Africa. In: Thoen CO, Steele JH, Gilsdorf MJ (eds) Mycobacterium bovis infection in animals and humans, 2nd edn. Blackwell, Iowa, pp 199–210
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Awah-Ndukum, J., Egbe, N.F., Ngu-Ngwa, V. (2019). The Status of Bovine Tuberculosis in Cameroon. In: Dibaba, A., Kriek, N., Thoen, C. (eds) Tuberculosis in Animals: An African Perspective. Springer, Cham. https://doi.org/10.1007/978-3-030-18690-6_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-18690-6_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-18688-3
Online ISBN: 978-3-030-18690-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)