Abstract
The focus of this study was the development of a new lubricating grease, using surface-modified attapulgite clay as thickener and synthetic oil (PAO 40) as the base oil. The tribological sensitivity of the new grease was investigated by studying the effect of adding three solid additives [KB3O5, MoS2, graphite and a graphite/MoS2 mixture (mass ratio 3:2)]. Its tribological behavior was compared with that of traditional bentone grease by adding MoS2. The dropping point and the cone penetration of the new grease were also investigated and analyzed. The wear scar diameter of the base grease was measured on an MRS-1 J (G) four-ball tester, and the tribological sensitivity of solid lubricating additives to attapulgite clay base grease was evaluated using an Optimol SRV reciprocating friction and wear tester. The addition of MoS2 and the graphite/MoS2 mixture to the new lubricating grease improved its friction-reducing ability, while the addition of KB3O5 improved its antiwear ability. The additives MoS2 and the graphite/MoS2 mixture also increased the load-carrying capacity of the base grease. The attapulgite clay grease containing MoS2 had a better friction-reducing ability than the traditional bentone grease containing MoS2.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Lubricating greases are generally highly structured colloidal dispersions that consist of a thickener dispersed in mineral or synthetic oil. The most commonly used thickeners are fatty acid soaps of lithium, calcium, sodium, aluminum, and barium [1]. However, in recent years, the use of the non-metallic mineral materials to synthesize lubricating grease has attracted much attention. One such example is bentone grease, which is usually synthesized by mixing the base oil and surface-modified bentonite. Bentone grease is famous for its excellent physical–chemical properties and extraordinary performance.
Many researchers have recently focused on a kind new non-metallic mineral material, attapulgite clay. This clay, with the structural formula Si8O20Mg5(Al)(OH)2(H2O)4·4H2O, is a type of natural fibrillar silicate clay consisting of two double chains of the pyroxene-type (SiO3)2−-like amphibole (Si4O11)6− running parallel to the fiber axis [2]. This unique structure confers many special surface and interface properties to attapulgite clay, including a larger specific surface area, surface activity, porous and adsorption ability, ion exchange, and salt resistance. There are reports of attapulgite being used in rubbers, adhesives, thermoplastic polymers, silicate/epoxy nanocomposite, catalyst supports, and environmental absorbents [3–7], among others. Attapulgite clay has a structure similar to that of bentonite. However, to date, there have been no reports on the use of organically modified attapulgite to synthesize lubricating grease.
In the study reported here, we used the method standardly used to synthesize bentone grease and adopted a wet way of manufacturing to synthesize the lubricating grease based on the organically modified attapulgite. Different from bentonite, attapulgite can be directly modified by hexadecyltrimethylammonium bromide (HTMAB). Therefore, in comparison to bentonite, using the organically modified attapulgite to synthesize lubricating grease involves a predigestion step in the manufacturing process. In our study, we first used HTMAB to modify the attapulgite and then used the wet way of manufacturing to synthesize lubricating grease. We also characterized the physical properties of the new lubricating grease, investigated the effect of different solid additives on the tribological behavior of the new lubricating grease and compared the tribological behaviors of the new grease and traditional bentone grease.
2 Experimental
2.1 Materials
The attapulgite clay was obtained from the DAIREN Chemical Corporation (Jiangsu, China). The cation exchange capacity (CEC) was calculated to be 50.88 meq/100 g using the formaldehyde method. HTMAB was purchased from the Sinopharm Chemical Reagent Co. (Shanghai, China).The commercially available organo-bentonite surface modified with HTMAB was obtained from the Zhejiang Anji Tianlong Organic Bentonite Co. Synthetic PAO 40 was purchased from the Lanzhou Refinery company (Lanzhou, China). The additives of KB3O5, MoS2, and graphite were also commercially obtained. All chemical reagents were of analytical grade and used without further purification.
2.2 Preparation of Organo-attapulgite
The organo-attapulgite was prepared according to a previously described method [8]. To ensure that the attapulgite was sufficiently modified, we mixed 10 g of attapulgite in 100 ml HTMAB aqueous solution containing the amount of surfactant equivalent ratios to 3.0 of the CEC of attapulgite.
2.3 Characterization of the Organo-Attapulgite
Infrared spectroscopic measurements were carried out on a FTS-165 spectrophotometer (Bio-Rad, Hercules, CA) using the KBr pellets method. Thermogravimetric analysis (TGA) was carried out on a ZRY-2P thermogravimetric analyzer at a heating rate of 10°C min−1 in flowing air.
2.4 Synthesis of the Greases
Synthetic PAO 40 has a kinematic viscosity (at 100 °C) of 40 mm2 s−1. The lubricating grease was prepared using the following procedures. First, 50 wt% of the total base oil was put into a vessel under stirring and stirring was continued. The organo-attapulgite was then slowly added to the base oil in the vessel and vigorously stirred to obtain a homogenous mixture. A certain amount of acetone was added to ensure that the organo-attapulgite was thoroughly dispersed throughout the base oil, with continued stirring for about 30 min. The mixture was then heated up to 60°C and maintained at this temperature for about 30 min to remove the acetone. Finally, the remaining base oil was stepwise added to the vessel in batches. The new lubricating grease was obtained after five separate fine grinding/homogenization steps in a three-roller mill. Bentone grease was prepared in the same manner as just described.
To investigate the effect of different solid additives on the tribological behavior of the new grease, we added routine solid additives, such as MoS2 (2H layered), KB3O5, graphite, graphite, and a mixture of graphite and MoS2 (2H layered; mass ratio 3:2; abbreviated as graphite/MoS2), into the grease at a 5.0 wt% concentration; these mixtures were mixed by mechanical stirring and finely ground three times in a three-roller mill.
2.5 Characterization the Physical Properties of the New Lubricating Grease
The dropping point and penetration of the lubricating grease were measured according to national standards GB/T 3498 and GB/T 269, similar to ASTM D2265 and ASTM D217, respectively.
2.6 Friction and Wear Tests
The wear scar diameter of the base grease was measured on an MRS-1 J (G) four-ball tester (MRS Seitter, Holzmaden, Germany). The four-ball test was performed at a rotating speed of 1450 rpm (linear speed 33.38 m min−1) and load of 392 N, for a test duration of 60 min. The wear scar diameters on the three lower balls were measured using an optical microscope to an accuracy of 0.01 mm. The result shows that the wear scar diameter of the lubricating grease based on attapulgite clay is about 0.94 mm.
An Optimol (Munich, Germany) SRV oscillating friction and wear tester (Fig. 1) was used to evaluate the tribological sensitivity of the attapulgite clay base grease following the addition of the additives. The upper ball, with a diameter of 10 mm, was made of AISI 52100 steel (hardness 710 HV). In all the tests, the ball slides reciprocally at an amplitude of 1 mm against the stationary disks (AISI 52100 steel, hardness 700 HV). Friction and wear tests were all conducted at the load of 200 N with a testing duration of 30 min. The friction coefficient was recorded automatically by a computer connected to the SRV tester. The volume loss of the lower disk due to wear was determined using a MicroXAM-3D surface mapping microscope profilometer. The results reported here are the average of duplicate tests.
2.7 Characterization of the Worn Surface
The morphologies of the wear scars were observed using a JSM-5600LV scanning electron microscope (SEM) (JEOL, Tokyo, Japan). The binding energies of some typical elements in the worn surfaces were analyzed using a PHI-5702 multi-functional X-ray photoelectron spectroscope (XPS, X-ray photoelectron). Al–Ka radiation was used as the excitation source, and the binding energy of carbon (C1s: 284.6 eV) was used as the reference.
3 Results and Discussion
3.1 Characterization of the Organo-attapulgite
The Fourier transform infrared (FT-IR) spectra of attapulgite and organo-attapulgite are shown in Fig. 2. Compared to the spectra of attapulgite, strong absorption bands at 2930 and 2856 cm−1 are visible in the spectra of the organo-attapulgite. These latter bands indicate the presence of –CH3 and –CH2 groups and suggest that the natural attapulgite has been modified by the surfactant. Figure 3 shows that the black curve is thermogravimetric (TG) curve and the blue one is differential scanning calorimetry (DSC) cure of the organo-attapulgite. The organo-attapulgite began to decompose at about 190 °C.
3.2 Physical Properties of the New Lubricating Grease
The dropping point and the penetration of the base grease and the grease containing three solid additives at 5.0 wt% concentration are listed in Table 1. From Table 1, it can be directly seen that each of the three solid additives, particularly KB3O5, had an effect on the penetration of the attapulgite grease. However, the additives had little effect on the dropping point of the grease, with only the addition of MoS2 slightly decreasing the dropping point of the base grease. Table 1 also clearly shows that the dropping point of the attapulgite clay base grease is higher than that of the bentone base grease.
3.3 The Tribological Sensitivity of Solid Lubricating Additives to Attapulgite Clay Base Grease
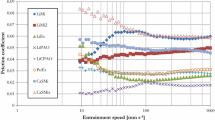
Figure 4 shows the variations in the friction coefficient with sliding frequency. The introduction of MoS2 and graphite/MoS2 as additives significantly decreased the friction coefficient of the attapulgite clay base grease, with the friction coefficients of the base grease containing MoS2 being the lowest. The friction coefficients also tended to decrease slightly following the addition of graphite and KB3O5 into the base grease. The relationship between the wear rate and the sliding frequency with the lubrication of the base grease containing four different solid additives is shown in Fig. 5. Lower wear rates were observed with lubrication by the base grease containing KB3O5 and MoS2 than with the attapulgite clay base grease. The addition of graphite/MoS2 slightly reduced the wear rate, except at the sliding frequency of 10 Hz. However, the addition of graphite has a counteracting effect on the base grease, dramatically increasing its wear rate.
These results show that although the KB3O5 did not improve the friction coefficient of the base grease, its use as an additive provided effective antiwear properties. The addition of MoS2 effectively reduced the friction and wear. Therefore, we conclude that the tribological properties of KB3O5 and MoS2 are closely related with their structures. The structure of KB3O5 is a web [9]; as such it does not decrease the friction coefficient but does provide effective antiwear ability. MoS2 has a layered structure and can effectively improve friction-reducing and antiwear abilities.
We also investigated the load-carrying capacity of the base grease and the grease containing the different solid additives. The applied loads were set at 400, 600, and 800 N, at a constant sliding frequency of 30 Hz. The results show that the base grease seized at 600 N, while the base grease containing KB3O5 and graphite, respectively, seized at 400 and 600 N. However, relatively stable friction coefficients were observed up to load of 800 N with the lubrication by grease containing MoS2 and the graphite/MoS2, respectively (Fig. 6). Overall, as additives, KB3O5 and graphite did not increase the load-carrying capacity of the new grease, while MoS2 and graphite/MoS2 were able to effectively increase the load-carrying capacity. These results are consist with those reported in the literature [10]. Graphite had a counteracting effect on the base grease, but the MoS2 and the graphite/MoS2 provided similar results. Therefore, we can conclude that graphite works together with MoS2 in the sliding process. An important point to note is that graphite is much less expensive than MoS2.
The tribological behaviors of the attapulgite clay base grease containing 5.0 wt% MoS2 were compared with those of the bentone base grease containing 5.0 wt% MoS2 (Fig. 7). Figure 7 clearly shows that at all frequencies the friction coefficients of the attapulgite clay base grease containing 5.0 wt% MoS2 were lower than those of the bentone base grease containing 5.0 wt% MoS2. However, at lower sliding speeds (10 and 20 Hz), the wear rates of the attapulgite clay base grease containing 5.0 wt% MoS2 were higher than those of the bentone base grease containing 5.0 wt% MoS2. At the higher sliding speed, the wear rates of the two greases containing 5.0 wt% MoS2 were similar.
Figure 8 shows the friction coefficient as a function of time at 40 Hz and 200 N. The friction coefficient for the base grease continually increased with increasing time. In comparison, the friction coefficient for the bentone base grease containing KB3O5 or graphite lubricant was relatively stable. The friction coefficients for the grease containing MoS2 or graphite/MoS2 increased at the beginning of the lubrication trial but remained relatively stable at a low level thereafter. The explanation for this behavior is the formation of a stable tribo-chemical film on the contact surface with increasing time.
3.4 SEM and XPS Analysis of the Worn Surface
The morphologies of the worn surfaces lubricated with the different lubricant systems at 200 N and 30 Hz are shown in Fig. 9. All of the SEM images were obtained at the same magnification. The worn surface lubricated with the base grease can be seen to be quite rough, with some desquamated layers near the wear grooves. However, the worn surfaces lubricated with the grease containing the different solid additives show only slight grooves and smooth surfaces, thereby demonstrating the anti-wear properties of these additives.
In order to investigate the friction and wear mechanism of the additives, we analyzed the worn surfaces by XPS. Table 2 shows the elemental composition (at%) of the worn surfaces lubricated with the different lubricant systems. Figure 10 gives the XPS spectra of the typical elements on the rubbed surface lubricated with the grease containing different solid additives. The broad O1s peak indicates that the worn surfaces have complex oxide species [11].The weak single of B1s peak at 192.6 eV (Fig. 10b) was assigned to B3O5 [12]. Figure 10c, d shows the XPS spectra of Mo3d and S2p on the worn surface lubricated with the base grease containing MoS2 and the graphite/MoS2 mixture, respectively. Mo exists as MoO3 at 231.8 eV and as MoS2 at 228.90 eV, while S exists as SO4 2− at 168.50 eV and MoS2 at 161.30 eV. Therefore, it is reasonable to assume that with the lubrication of the base grease containing MoS2 and the graphite/MoS2, one part of MoS2 was physically absorbed onto the surface of the steel friction pair, while the other part of MoS2 was oxidized into MoO3 in the friction process. The peak of Fe2p at 710.50 eV was attributed to FeSO4 [13]. These results suggest that KB3O5 can improve the anti-wear properties of the base grease because a tribofilm containing B, Fe and O was formed on the worn surface during the sliding process. The reasons why MoS2 and the graphite/MoS2 can effectively reduce the friction are: (1) the MoS2 is physically absorbed on the rubbing surface; (2) the absorbed MoS2 is transferred into a chemical film containing MoO3 and FeSO4 by the tribo-chemical reaction; (3) the graphite is mainly physically absorbed on the worn surface; (4) the graphite can separate the rubbing surfaces, preventing direct contact.
4 Conclusions
We have synthesized a new lubricating grease with a relatively high dropping point based on surface-modified attapulgite clay. The addition of additives MoS2 and the graphite/MoS2 mixture to the new lubricating grease resulted in the new lubricating grease having a better friction-reducing ability. As additive, the KB3O5 showed a relatively better anti-wear ability. Moreover, the additives of MoS2 and the graphite/MoS2 mixture were able to increase the load-carrying capacity of the new grease. XPS analyses indicated that tribofilms consisting of MoS2 and MoO3 compounds were formed on the worn surface under the grease containing MoS2, resulting in better tribological performance. Also, compared to the traditional bentone grease containing 5.0 wt% MoS2, the attapulgite clay grease containing MoS2 showed a better friction-reducing ability.
References
Martín-Alfonso, J.E., Valencia, C., Sánchez, M.C., Franco, J.M., Gallegos, C.: Development of new lubricating grease formulation using recycled LDPE as rheology modifier additive. Eur. Polym. J. 43, 139–149 (2007)
Liu, Y., Wang, W.B., Wang, A.Q.: Adsorption of lead ions from aqueous solution by using carboxymethyl cellulose-g-poly (acrylic acid)/attapulgite hydrogel composites. Desalination 259, 258–264 (2010)
Wang, L.H., Sheng, J.: Preparation and properties of polypropylene/org-attapulgite nanocomposites. Polymer 46, 6243–6249 (2005)
Liu, Y.S., Liu, P., Su, Z.X.: Core-shell attapulgite@polyaniline composite particles via in situ oxidative polymerization. Synth. Met. 157, 585–591 (2007)
Yang, C., Liu, P.: Core-shell attapulgite@polypyrrole composite with well-defined corn cob-like morphology via self-assembling and in situ oxidative polymerization. Synth. Met. 159, 2056–2062 (2009)
Qiu, J.H., Feng, H.: Preparation and properties of PAn/ATTP/PE conductive composites, Trans. Nonferr. Met. Soc. 16, 444–448 (2006)
Lei, X.P., Liu, Y.S., Su, Z.X.: Synthesis and Characterization of organo-attapulgite/polyaniline-dodecylbenzenesulfonic acid based on emulsion polymerization method. Polym. Composite 29, 239–244 (2008)
Chen, H., Zhao, J.: Adsorption study for removal of Congo red anionic dye using organo-attapulgite. Adsorption 15, 381–389 (2009)
Youngman, R.E., Zwanziger, J.W.: On the formation of tetracoordinate boron in rubidium borate glasses. J. Am. Chem. Soc. 117, 1397–1402 (1995)
Gansheimerand, J., Holinsky, R.: A study of solid lubricants in oils and greases under boundary conditions. Wear 19, 439–449 (1972)
Zhang, H.B., Yao, M.H., Jia, Z.F., Liu, Z.L.: The influences of methyl group at C2 position in imidazolium ring on tribological properties. Tribol. Lett. 36, 105–111 (2009)
Jia, Z.F., Xia, Y.Q., Li, J.L., Pang, X.J., Shao, X.: Friction and wear behavior of diamond-like carbon coating on plasma nitrided mild steel under boundary lubrication. Tribol. Int. 43, 474–482 (2009)
Hua, K.H., Liu, M., Wang, Q.J., Xu, Y.F., Schraube, S., Hu, X.G.: Tribological properties of molybdenum disulfide nanosheets by monolayer restacking process as additive in liquid paraffin. Tribol. Int. 42, 33–39 (2009)
Acknowledgments
This work is supported by Hundreds Talent Program of Chinese Academy of Science.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s11249-011-9782-x
Rights and permissions
About this article
Cite this article
Wang, Z., Xia, Y. & Liu, Z. Study the Sensitivity of Solid Lubricating Additives to Attapulgite Clay Base Grease. Tribol Lett 42, 141–148 (2011). https://doi.org/10.1007/s11249-011-9754-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11249-011-9754-1