Abstract
The development of basestock oil derived from renewable sources has become crucial as the world oil reserves are depleting. In this study, samples based on pequi oil (Caryocarbrasiliensis) were obtained by hydrolysis and esterification reactions, using a long-chain alcohol (2-ethylhexanol). Tribological tests in a four-ball configuration were performed to evaluate the lubricating behavior. A comparison of the Stribeck curve of the bio-based sample with that of a mineral hydrotreated basestock oil showed that the samples from pequi oil had a lower friction coefficient at all studied sliding speeds, ranging from 0 to 9.33 × 105 μm/s. The friction coefficient of the bio-based sample was 30.7% lower than that of a mineral hydrotreated basestock oil sample. The wear scar diameters were 0.371 mm for the bio-based samples and 0.195 mm for the mineral oil. This tribological performance indicates that a bio-based sample obtained from pequi oil may be potentially used as a lubricant basestock oil.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Concerns about increasing environmental degradation drive the development of alternatives for fossil oil products. Recent studies have also reported that the world oil reserves would only sustain another fifty years of exploration with the current oil production [1,2,3]. Thus, the world scenario contributes to research studies on the production of basestock oil also derived from vegetable oils. In this way, researchers have developed bio-based lubricants from edible and non-edible vegetable oils, such as sunflower, soybean, jatropha, castor, palm kernel and coconut with satisfactory physicochemical characteristics for use in lubricated mechanical systems [4,5,6,7].
Pequi oil is extracted from the Caryocaraceae family fruit of a native Brazilian tree, occurring in the Amazon, Caatinga, Cerrado and Atlantic Forest regions [8]. The fatty acids composition of pequi oil may vary according to species and geographic location. According to previous studies [9, 10], the chemical composition of triglycerides referring to fatty acids is: oleic acid (47–60%) and palmitic acid (31–40%) as main components, while linoleic acid (1–2.4%) and stearic acid (2.6–4%) are reported in lower contents. Pequi oil production is a value-adding activity for local economies, reaching about 54.7% of its producers' annual income [11]. The low price of the fruit is an advantage in the production of a bio-lubricant and favors socioeconomic aspects, such as family farming and the production of novel products from these oils [12].
Recent studies have reported the use of pequi oil to obtain bio-diesel [13,14,15] and bio-based lubricant [16]. However, they do not report a tribological evaluation of the ester produced from pequi oil. Tribological characterization is the basis for the selection of materials used in machining process in the field of lubricants, as the main task is to reduce friction and wear of mechanical parts in relative motion. Tribological tests are essential to understand the influence of nature and composition of esters on lubrication, film thickness, friction and wear properties [17].
The possibility of using a lubricant depends not only on its physicochemical properties, but also on other operational factors such as load, speed and temperature [18]. These conditions are evaluated through tribological tests that simulate real situations of lubricant operation. A proper use of a lubricant may reduce friction and wear between contacting surfaces.
When evaluating the behavior of a lubricant, also the selection of test geometry has to be taken into account. On the basis of a literature review of available tests for lubricants in industry, the four-ball method is one of the most commonly used. This four-ball configuration has also been applied to evaluate the behavior of synthetic esters when subjected to contact pressures and sliding speed. Recent studies on friction and wear of esters obtained from different vegetable oils (Jatropha, Karanja, Palma, Colza), using a four-ball test, reported good performances in the formation of lubricating films [19,20,21,22,23,24].
Lubricity tests of bio-based lubricant samples (BLS) are usually carried out through the four-ball apparatus as the accessory mounted on a dynamic shear rheometer (DSR). Besides being able to present friction and wear coefficients in tests with a constant sliding speed, the rheometer may also be used to obtain a Stribeck curve, where the friction coefficient is presented in a variable sliding speed condition. From the Stribeck curve, it is possible to evaluate the combined effect of sliding speed and normal load on lubricant properties [25, 26]. The Stribeck curve may be divided into three regimes: boundary lubrication (BL), mixed lubrication (ML) and hydrodynamic lubrication (HL) [27]. In the boundary lubrication regime, the friction between the two contact surfaces is affected by the ability of the sample to adsorb on the contact surfaces thus forming a lubrication film. In this regime, friction is nearly constant and hardly affected by the sliding speed or viscosity of the lubricant. As the sliding speed increases, the Stribeck curve enters the mixed lubrication regime where a hydrodynamic behavior arises and significantly reduces the friction, governed by the viscosity that promotes the lubricant drag. Upon reaching even higher speeds, the Stribeck curve enters the hydrodynamic regime where the hydrodynamic film is fully developed, completely separating the surfaces. In this regime, friction is governed by internal friction and increases linearly with speed, as shown in Fig. 1 [28, 29].
In this study, a bio-based lubricant sample is obtained from a Brazilian natural vegetable oil—pequi oil—which, due to its characteristics, seems to be potentially useful for applications as lubricant basestock oil. The main chemical and physicochemical properties for the bio-based lubricant sample (BLS) were measured, and tribological properties were evaluated using the four-ball method.
2 Experimental section
2.1 Materials
Pequi oil was purchased as a natural product at a street market in the city of São Luís, MA, Brazil. All reagents, 2-ethylhexanol (> 99% wt.), ethanol (> 99% wt.), hydrochloric acid (37% wt.), sodium hydroxide (≥ 97% wt.), potassium bromide and deuterated chloroform (CDCl3 99.8%) were purchased from Sigma-Aldrich (USA). Also Amberlyst 15 resin was supplied by Sigma-Aldrich (USA). Commercial nitrogen (> 99.5%) was supplied by White Martins Praxair (Brazil). A sample of hydrotreated mineral oil (HMO) was supplied by Petrobras (Brazil). The main physicochemical properties of the HMO are presented in Table 1.
2.2 Synthesis procedures
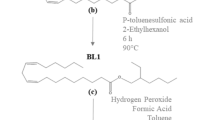
The synthesis procedures were based on those previously reported by [30]. Initially a hydrolysis reaction was carried out in a three-tube round bottom flask by adding 100 g of pequi oil to 180 ml of ethanol and 18 g of sodium hydroxide (NaOH) to obtain a free fatty acids (FFA) solution. The reaction was performed under continuous reflux for four hours at 80 °C with agitation of 900 rpm. After that, the FFA solution was cooled by slowly adding 450 mL of distilled water. Hydrochloric acid (HCl) was then added to the FFA solution gradually until the solution reached pH 4 and then transferred to a separating funnel, where the supernatant acids solution was treated three times with hot water.
Following the hydrolysis, an esterification reaction was carried out in a glass reactor by adding 30 g of the FFA solution, 115 g of 2-ethylhexanol and 1.5 g of the Amberlyst 15 resin, previously treated at 100 °C for one hour. In an inert atmosphere (N2), the esterification reaction lasted six hours at 85 °C. At the end of the reaction, the solution was distilled using a Kugelrohr system under vacuum (3∙10−2 mbar) at 110 °C to remove the excess alcohol (2-ethylhexanol).
2.3 Chemical and physicochemical characterization
The free fatty acids (FFA) resulting from the hydrolysis reaction were characterized using a gas chromatograph (Varian, USA) with a CP–Sil 5 CB capillary column, 25 m × 0.25 mm × 0.25 μm (Agilent, USA). The GC oven temperature was initially set at 80 °C, kept for three minutes, then increased by 6 °C/min up to 340 °C and then kept for another six minutes. The injector and detector temperatures were 300 and 360 °C, respectively. The carrier gas was hydrogen, and the flow rate was 26.7 mL/min. The split ratio was established at 1:1, and 2.0 μL of the FFA sample was injected into the gas chromatograph [31].
FTIR measurements were taken on a potassium bromide (KBr) tablet using a Shimadzu IRTracer-100 (Japan) within the range 400–4,000 cm−1. [14] The pressure used to make the tablet was 8KN. Thirty-two scans were taken with a resolution of 4 cm−1.
The samples were also evaluated using one-dimensional proton nuclear magnetic resonance (1H NMR and 13C). The spectra were obtained in a Bruker spectrometer instrument model Avance DRX-500 operating at 125 MHz for NMR 13C and 500 MHz for 1H, using deuterated chloroform (CDCl3 99.8%) as solvent [16].
Density and kinematic viscosities at 40 °C and 100 °C were evaluated using the ASTM D7042 and the ASTM D455 methods, respectively, with an Anton Paar's SVM 3000 equipment (Austria) [32, 33]. The viscosity index (VI) could then be calculated using the ASTM D2270 method.
The total acidity number (TAN) was obtained using the AOCS Cd 3d-63 method [34]. The oxidative stability of the samples was measured using a Rancimat 893 equipment (Metrohm, Switzerland). The bio-based samples (ca. 3 g) were oxidized at 110 °C under air flow of 10 L/h. The induction period (IP) was calculated from the curve of conductivity in a deionized water cell (see EN14112). For automatic determination of the IP value, the second derivative of the measured conductivity was used, which exhibits a maximum at the IP time.
2.4 Tribological assessment
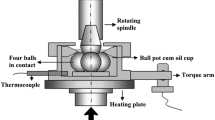
The tribological properties of the bio-based samples were evaluated using a DHR-3 rheometer (TA Instruments, USA) with an accessory for four-ball tribological testing. This accessory allows the measurement of the friction coefficient between two solid surfaces under dry or lubricated conditions. Its design secures uniform solid-to-solid contact and axial force distribution, controlling rotational speed and temperature in a wide range of friction measurements. Data are collected through the TRIOS software. The monitored variables are friction coefficient, loading force and friction force. This test consists of assembling three spheres fixed in a support at the bottom. The upper holder has one rotating steel ball, which is loaded against three stationary lower steel balls. All contact areas are submerged in a lubricant bath, as shown in Fig. 2.
Each ball is made of chrome alloy steel (AISI 52,100), with diameter of 12.7 mm. A lubricant sample of 4.5 ml was used in each tribological experiment using two standard conditions as detailed in Table 2. The first condition is used to obtain the Stribeck curves, varying the sliding speed of the balls, at constant load and temperature for 10 min. The second one evaluates the friction coefficient during one hour with constant sliding speed (4.59 × 105 μm/s), load and temperature, with an evaluation of the wear of the balls at the end of the test.
A sample of hydrotreated mineral oil (HMO) was used as a reference to compare the tribological performance of the bio-based lubricant sample. The wear scar diameter (WSD) and the morphology of the worn surface were determined using an optical microscope (Zeiss, Germany).
3 Results and discussion
3.1 Chemical and physicochemical characterizations
The fatty acid composition of the product of the hydrolysis reaction, evaluated by GC analysis, is shown in Table 3. The highest contents were those of oleic fatty acid (59.19%) and palmitic acid (35.52%), similarly to what was reported by Pessoa et al. [35]. It may also be observed that the total composition of unsaturated fatty acids was 61.64%.
The physicochemical properties of the BLS sample obtained after the esterification reaction are shown in Table 4, along with the properties of the fresh pequi oil and its hydrolysis product (FFA). The density values at 20 °C showed a slight decrease, when compared to the original pequi oil (0.90 g/cm3), for the FFA and BLS samples (0.89 and 0.86 g/cm3, respectively). This indicates that the reaction has a limited influence on the mass and volume ratio. The kinematic viscosity of the bio-lubricant at 40 °C and 100 °C decreased due to the ability of fatty acids to form a hydrogen bond through the (-COOH) group and to have intermolecular interactions of dipole–dipole type and London dispersion [36]. Therefore, the FFA will present a higher viscosity compared to that of the bio-lubricant. Viscosity is the most important characteristic when defining a lubricant application in mechanical systems. The BLS may be used in automotive transmission systems, due to its low viscosity, where its efficiency in separating parts would be more evident [37]. The viscosity index is the property that evaluates how much the viscosity changes when temperature changes. The higher the viscosity index, the lower the influence of temperature on viscosity [38]. The viscosity index of FFA and the BLS showed high values. However, the bio-based sample showed a better result. The viscosity index of BLS was within the limit for mineral-based lubricants (group III), according to the API classification [39].
The oxidative stability of the BLS (5.44 ± 0.19 h) reflects the sudden change in the acidity index of FFA compared to that of esters. The oxidative stability of the pequi bio-lubricant sample showed better results than similarly synthesized bio-lubricants obtained from other plant species, as previously reported and summarized in Table 5 [36, 40,41,42]. The oxidative stability of the BLS product is highly influenced by the fatty acids profile and the amount and position of unsaturations in the chain. The tendency to oxidation increases with increasing degree of unsaturation in the free fatty acids and the presence of carboxylic acid functional groups [43].
The FTIR spectra for FFA and for the ester are displayed in Fig. 3. A comparison between these spectra shows that the wavelength indicating the presence of the carbonyl group -OH (3,300–3,100 cm−1) is absent in the ester spectrum. This indicates that the -OH bond of 2-ethylhexanol totally reacted with the free fatty acids to form the ester. In the spectrum, the band at 2870 cm−1 appears in the range of stretching vibrations characteristic of C–H groups There is also a shift in wavelength from 1,710 cm−1 of the FFA spectrum, characteristic of the carbonyl group (C = O) frequent in long-chain fatty acids, to wavelength of 1,740 cm−1 for the spectrum of ester. The absorption band around 1,740 cm−1 is attributed to the C = O group of the ethyl esters [44]. This indicates that the functional groups of carboxylic acids present in the spectrum of fatty acids after esterification transformed into esters. In addition, a wavelength of 1,170 cm−1 appears in the ester spectrum, which characterizes the presence of the functional C = O group, which results from the formation of the ester [45, 46]. The band identified between 1,400 and 1,500 cm−1 (1462 cm−1) corresponds to the CH3 group of the ethyl ester [47].
The spectrum of 1H and 13C NMR for BLS is shown in Fig. 4a,b. The esterification reaction was confirmed in Fig. 4a with the presence of the peak (a) since this peak refers to the hydrogen bound to the carbon next to the sp3 oxygen of the ester functional group [16, 48,49,50]. The esterification reaction showed a high conversion value (92 mol%) through the evaluation of acidity measurement [34]. The lower acidity values for BLS are due to the fact that the esterification reaction modifies the end of the carboxylic acid, thus decreasing the amount of ionizable atoms in the ester molecule. The 13C NMR spectrum in Fig. 4b showed the peak (b) characteristic of the carboxylic ester. The peaks between (c and d) are characteristic of the presence of unsaturation in the fatty acid carbon chain [16, 49,50,51].
3.2 Tribological evaluation
The friction behaviors of the BLS and HMO lubricant samples using a Stribeck diagram are shown in Fig. 5. The friction coefficients range between 0.11 and 0.05 (at low and high sliding speeds, respectively) for the mineral lubricant sample. For the bio-lubricant sample, the friction coefficients range between 0.08 and 0.037, at the same sliding speed conditions. The sudden drop in the friction coefficient at low sliding speeds of the Stribeck curve for HMO may be due to the dry contact and difficult initial formation of a lubricant film [52]. The experimental data shown in Fig. 5 indicate that both HMO and BLS samples reached the boundary and mixed lubrication regimes for the sliding speeds that were studied. The transition from the boundary to the mixed lubrication regime may be observed through the decrease in friction coefficients at sliding speeds around 6.0 × 105 µm/s. The tribological tests indicate that both BLS and HMO were effective at reducing the friction coefficients in the four-ball method. The Stribeck curves indicate that BLS has lower friction coefficients than HMO across all sliding speed ranges. As previously reported [53, 54], the esters contained in the BLS sample seem to facilitate the lubricant adhesion to the surface of the spheres in the used method.
The values for the friction coefficients using a constant sliding speed (4.59 × 105 μm/s) for one hour are shown in Fig. 6 for both BLS and HMO samples. As seen before in the Stribeck curves, the friction coefficients of BLS were lower than those of HMO during the whole test. However, the average wear scar diameters (WSD) at the end of the tests are higher for the bio-lubricant sample than for the HMO sample (also reported in Fig. 6). The wear morphology of the balls after these tests is shown in Fig. 7. It may be noted that the wear may be associated with the grooves in the direction of the application, which means that the wear mechanism was probably abrasive and adhesive [55]. These results indicate that the friction and wear performances may not be directly related to each other. In this study, lower friction of the bio-lubricant oil caused higher wear. This could be due to the molecular structure, polar groups and also the type of esters (unsaturation) present in the oil [56]. In addition, bio-lubricants with unsaturated compounds in their composition are usually less stable at higher temperatures [57]. A factor that contributes to minimizing wear is the capacity of the molecules to orient themselves with the polar end directed toward the metal surface, making a close-packed monomolecular (or multimolecular)-layered structure [58]. This packing results in a surface film that may inhibit metal-to-metal contact and roughness on the metal surface. Both strength of the fluid film and extent of adsorption on the metal surface contribute to the efficiency of bio-lubricant performance [56].
However, assessing the visual aspect of the wears, it may be observed that the balls lubricated with the BLS sample present a smoother scar when compared to the HMO sample, proving that the lubrication film of BLS, formed by the functional ester groups, seems to be more effective in the creation of a monolayer to separate the metallic surfaces. However, it seems that it might not be able to remain as effective throughout the whole test, which would explain higher values of WSD [55, 59].
4 Conclusions
Bio-based lubricant samples from pequi oil (BLS) were obtained using hydrolysis and esterification reactions. The ester product presented better viscosity index when compared to the original FFA. In comparison with other bio-lubricants synthesized in an equivalent way using other vegetable oils, the pequi bio-lubricant showed better oxidative stability. Tribological assessments using Stribeck curves showed boundary and mixed lubrication regimes for BLS and HMO. The friction coefficients obtained for BLS were lower than those for HMO in both regimes. Experiments performed to evaluate the wear of the balls (using a four-ball apparatus) showed that the bio-based sample (BLS) presented a lower value of the average friction coefficient (0.0588 ± 0.0011) when compared to the mineral sample HMO (0.0849 ± 0.0020), but with a higher value of the wear scar (0.3710 ± 0.1836 versus 0.1950 ± 0.0151, for BLS and HMO, respectively). This may be explained by the presence of unsaturated molecules in the BLS sample. The visual evaluation of the worn surfaces suggests that the wear is smoother in the ball lubricated with the bio-based lubricant indicating a better formation of the protective layer by the ester compounds. However the larger diameter of the wear scar in the bio-based sample indicates difficulty in maintaining the lubricating film throughout the duration of the experiment (1 h). In general, from these tribological evaluations, there may be potential applications for bio-based lubricants obtained from pequi oil.
References
BP (2021) Statistical Review of World Energy - 70th edition. https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2021-full-report.pdf
Energy Information Agency (2019) Annual energy outlook 2019 with projections to 2050 https://www.eia.gov/outlooks/aeo/pdf/aeo2019.pdf
Pillot B, Muselli M, Poggi P, Dias JB (2019) Historical trends in global energy policy and renewable power system issues in Sub-Saharan Africa: the case of solar PV. Energy Policy 127:113–124. https://doi.org/10.1016/j.enpol.2018.11.049
Attia NK, El-Mekkawi SA, Elardy OA, Abdelkader EA (2020) Chemical and rheological assessment of produced biolubricants from different vegetable oils. Fuel 271:117578. https://doi.org/10.1016/j.fuel.2020.117578
Tulashie SK, Kotoka F (2020) The potential of castor, palm kernel, and coconut oils as biolubricant base oil via chemical modification and formulation. Therm Sci Eng Prog 16:100480. https://doi.org/10.1016/j.tsep.2020.100480
Fernandes KV, Cavalcanti ED, Cipolatti EP, Aguieiras EC, Pinto MC, Tavares FA, Assunção CL (2020) Enzymatic synthesis of biolubricants from by-product of soybean oil processing catalyzed by different biocatalysts of Candida rugosa lipase. CatalToday. https://doi.org/10.1016/j.cattod.2020.03.060
Aguieiras ÉC, Cavalcanti ED, da Silva PR, Soares VF, Fernandez-Lafuente R, Assunção CLB, Freire DM (2020) Enzymatic synthesis of neopentyl glycol-bases biolubricants using biodiesel from soybean and castor bean as raw materials. Renew Energy 148:689–696. https://doi.org/10.1016/j.renene.2019.10.156
Caryocaraceae (2017) In: Flora do Brasil 2020 em construção Jardim Botânico do Rio de Janeiro Available fromhttp://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB47900
Guedes AMM, Antoniassi R, de Faria-Machado AF (2017) Pequi: a Brazilian fruit with potential uses for the fat industry. OCL 24(5):D507
Johner JCF, Hatami T, Meireles MAA (2018) Developing a supercritical fluid extraction method assisted by cold pressing for extraction of pequi (Caryocar brasiliense). J Supercrit Fluids 137:34–39
Oliveira MEBD, Guerra NB, Maia ADHN, Alves RE, Xavier DDS, Matos NMDS (2009) Caracterização física de frutos do pequizeiro nativos da chapada do Araripe-CE. Rev Bras Frutic 31(4):1196–1201. https://doi.org/10.1590/S0100-29452009000400038
dos Santos Alves CE, Belarmino LC, Padula AD (2017) Feedstock diversification for biodiesel production in Brazil: using the policy analysis matrix (PAM) to evaluate the impact of the PNPB and the economic competitiveness of alternative oilseeds. Energy Policy 109:297–309. https://doi.org/10.1016/j.enpol.2017.07.009
Silva TA, de Assunção RMN, Vieira AT, de Oliveira MF, Batista ACF (2014) Methylic and ethylic biodiesels from pequi oil (CaryocarbrasilienseCamb.): production and thermogravimetric studies. Fuel 136:10–18. https://doi.org/10.1016/j.fuel.2014.07.035
dos Santos RCM, Gurgel PC, Pereira NS, Breves RA, de Matos PRR, Silva LP, Lopes RDVV (2020) Ethyl esters obtained from pequi and macaúba oils by transesterification with homogeneous acid catalysis. Fuel 259:116206. https://doi.org/10.1016/j.fuel.2019.116206
Cardoso CMM, Zavarize DG, Vieira GEG (2019) Transesterification of Pequi (CaryocarbrasiliensisCamb) bio-oil via heterogeneous acid catalysis: catalyst preparation, process optimization and kinetics. Indus Crops Prod 139:111485. https://doi.org/10.1016/j.indcrop.2019.111485
Ferreira EN, Arruda TBMG, Rodrigues FEA, Arruda DTD, da Silva Junior JH, Porto DL, Ricardo NMPS (2019) Investigation of the thermal degradation of the biolubricant through TG-FTIR and characterization of the biodiesel–Pequi (Caryocarbrasiliensis) as energetic raw material. Fuel 245:398–405. https://doi.org/10.1016/j.fuel.2019.02.006
Biresaw G, Bantchev G (2008) Effect of chemical structure on film-forming properties of seed oils. J Synth Lubr 25(4):159–183. https://doi.org/10.1002/jsl.58
Suresha B, Hemanth G, Rakesh A, Adarsh KM (2019) Tribologicalbehaviour of pongamia oil as lubricant with and without halloysite nanotubes using four-ball tester. In AIP Conference Proceedings (Vol. 2128, No. 1, p. 030011) AIP Publishing LLC https://doi.org/10.1063/1.5117954
Zulkifli NWM, Masjuki HH, Kalam MA, Yunus R, Azman SSN (2014) Lubricity of bio-based lubricant derived from chemically modified jatropha methyl ester. JurnalTribologi 1:18–39
Sharma UC, Sachan S (2019) Friction and wear behavior of karanja oil derived biolubricant base oil. SN Appl Sci 1(7):668. https://doi.org/10.1007/s42452-019-0706-y
Dodos GS, Karonis D, Zannikos F, Lois E (2015) Renewable fuels and lubricants from Lunaria annua L. Ind Crops Prod 75:43–50. https://doi.org/10.1016/j.indcrop.2015.05.046
Zulkifli NWM, Kalam MA, Masjuki HH, Shahabuddin M, Yunus R (2013) Wear prevention characteristics of a palm oil-based TMP (trimethylolpropane) ester as an engine lubricant. Energy 54:167–173. https://doi.org/10.1016/j.energy.2013.01.038
Wu X, Zhang X, Yang S, Chen H, Wang D (2000) The study of epoxidized rapeseed oil used as a potential biodegradable lubricant. J Am Oil Chem Soc 77(5):561–563. https://doi.org/10.1007/s11746-000-0089-2
Zulkifli NWM, Azman SSN, Kalam MA, Masjuki HH, Yunus R, Gulzar M (2016) Lubricity of bio-based lubricant derived from different chemically modified fatty acid methyl ester. Tribol Int 93:555–562. https://doi.org/10.1016/j.triboint.2015.03.024
Stribeck R (1901) Ball bearings for any stress. Z des VDI 45:664
Stribeck R (1902) Die wesentlichenEigenschaften der Gleit-und Rollenlager (The basic properties of plain and roller bearing). Z VerDtschIng 46(36):1342–1348
Hamdan SH, Chong WWF, Ng JH, Ghazali MJ, Wood RJK (2017) Influence of fatty acid methyl ester composition on tribological properties of vegetable oils and duck fat derived biodiesel. Tribol Int 113:76–82. https://doi.org/10.1016/j.triboint.2016.12.008
Nguyen PT, Kravchuk O, Bhandari B, Prakash S (2017) Effect of different hydrocolloids on texture, rheology, tribology and sensory perception of texture and mouthfeel of low-fat pot-set yoghurt. Food Hydrocolloids 72:90–104. https://doi.org/10.1016/j.foodhyd.2017.05.035
Luo Y, Liu X, Pang Z (2019) Tribo-rheological properties of acid milk gels with different types of gelatin: effect of concentration. J Dairy Sci 102(9):7849–7862. https://doi.org/10.3168/jds.2019-16305
Mukherjee S, Ghosh M (2017) Studies on performance evaluation of a green plasticizer made by enzymatic esterification of furfuryl alcohol and castor oil fatty acid. Carbohyd Polym 157:1076–1084
Yunus R, Lye OT, Fakhru’l-Razi A, Basri S (2002) A simple capillary column GC method for analysis of palm oil-based polyol esters. J Am Oil Chem Soc 79(11):1075–1080. https://doi.org/10.1007/s11746-002-0606-3
ASTM International (2014) Standard test method for dynamic viscosity and density of liquids by Stabinger viscometer (and the calculation of kinematic viscosity) ASTM International
ASTM International (2006) Standard test method for kinematic viscosity of transparent and opaque liquids:(and Calculation of Dynamic Viscosity) ASTM International
Rade LL, Lemos COT, Barrozo MAS, Ribas RM, Monteiro RS, Hori CE (2018) Optimization of continuous esterification of oleic acid with ethanol over niobic acid. Renew Energy 115:208–216. https://doi.org/10.1016/j.renene.2017.08.035
Pessoa AS, Podesta R, Block JM, Franceschi E, Dariva C, Lanza M (2015) Extraction of pequi (Caryocarcoriaceum) pulp oil using subcritical propane: determination of process yield and fatty acid profile. J Supercrit Fluids 101:95–103. https://doi.org/10.1016/j.supflu.2015.03.006
Rios ÍC, Cordeiro JP, Arruda TB, Rodrigues FEA, Uchoa AF, Luna FMT, Ricardo NM (2020) Chemical modification of castor oil fatty acids (Ricinuscommunis) for biolubricant applications: an alternative for Brazil’s green market. Ind Crops Prod 145:112000. https://doi.org/10.1016/j.indcrop.2019.112000
Owuna FJ, Dabai MU, Sokoto MA, Dangoggo SM, Bagudo BU, Birnin-Yauri UA, Jibrin MS (2019) Chemical modification of vegetable oils for the production of biolubricants using trimethylolpropane: a review. Egypt J Pet. https://doi.org/10.1016/j.ejpe.2019.11.004
Duggal B (2013) Rheology – viscosity index In: Wang QJ, Chung YW (eds) Encyclopedia of tribology Springer, Boston, MA https://doi.org/10.1007/978-0-387-92897-5_961
Pirro DM, Daschner E (2001) Lubrication fundamentals. CRC Press, USA
Marques JPC, Rios IC, Arruda TBMG, Rodrigues FEA, Uchoa AFJ, de Luna FMT, Ricardo NMPS (2019) Potential bio-based lubricants synthesized from highly unsaturated soybean fatty acids: physicochemical properties and thermal degradation. Ind Eng Chem Res 58(38):17709–17717. https://doi.org/10.1021/acs.iecr.9b03038
Marques JPC, Rios ÍC, ParenteJr EJ, Quintella SA, Luna FMT, Cavalcante CL Jr (2019) Synthesis and characterization of potential bio-based lubricant basestocks via epoxidation process. J Am Oil Chem Soc. https://doi.org/10.1002/aocs.12317
Sripada PK, Sharma RV, Dalai AK (2013) Comparative study of tribological properties of trimethylolpropane-based biolubricants derived from methyl oleate and canola biodiesel. Indus Crops Prod 50:95–103. https://doi.org/10.1016/j.indcrop.2013.07.018
Melo MAR, Melo MAM, da Silva EV, Carvalho Filho JR, de Souza AG (2014) Study of the oxidative stability of oils vegetables for production of Biodiesel. Revista Verde de Agroecologia e Desenvolvimento Sustentável 9(1):13
Zhou J, Xiong Y, Gong Y, Liu X (2017) Analysis of the oxidative degradation of biodiesel blends using FTIR, UV–Vis, TGA and TD-DES methods. Fuel 202:23–28. https://doi.org/10.1016/j.fuel.2017.04.032
Arbain NH, Salimon J (2010) Synthesis and characterization of ester trimethylolpropane based Jatropha curcas oil as biolubricant base stocks. J Sci Technol 2(2). Retrieved from https://publisher.uthm.edu.my/ojs/index.php/JST/article/view/245
Pavia DL, Lampman GM, Kriz GS (2009) Introduction to spectroscopy. Thomson Learning, USA
Bhardwaj RM (2016) Aims and objectives In Control and prediction of solid-state of pharmaceuticals (pp 29–30) Springer, Cham https://doi.org/10.1007/978-3-319-27555-0
Kamalakar K, Rajak AK, Prasad RBN, Karuna MSL (2013) Rubber seed oil-based biolubricant base stocks : a potential source for hydraulic oils. Ind Crop Prod 51:249–257. https://doi.org/10.1016/j.indcrop.2013.08.058
Marques JPC, Rios ÍC, ParenteJr EJ, Quintella SA, Luna FMT, CavalcanteJr CL (2020) Synthesis and characterization of potential bio-based lubricant basestocks via epoxidation process. J Am Oil Chem Soc 97(4):437–446. https://doi.org/10.1002/aocs.12317
Rios ÍC, Cordeiro JP, Parente EJ, Quintella SA, Alemán J, Cavalcante CL, Luna FMT (2020) Biodegradable base stock oils obtained from ricinoleic acid using C 8 alcohols and process integration into a biodiesel industry. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-00942-4
Padmaja KV, Rao BVSK, Reddy RK, Bhaskar PS, Singh AK, Prasad RBN (2012) 10- Undecenoic acid-based polyol esters as potential lubricant base stocks. Ind Crop Prod 35:237–240. https://doi.org/10.1016/j.indcrop.2011.07.005
Tercariol WT (2018) Tribo-rheometry: quantifying friction between surfaces on rotational rheometers In VIII Brazilian conference on rheology
Ruggiero A, D’Amato R, Merola M, Valašek P, Müller M (2017) Tribological characterization of vegetal lubricants: comparative experimental investigation on Jatrophacurcas L. oil, rapeseed methyl ester oil hydrotreated rapeseed oil. Tribol Int 109:529–540. https://doi.org/10.1016/j.triboint.2017.01.030
Quinchia LA, Delgado MA, Reddyhoff T, Gallegos C, Spikes HA (2014) Tribological studies of potential vegetable oil-based lubricants containing environmentally friendly viscosity modifiers. Tribol Int 69:110–117. https://doi.org/10.1016/j.triboint.2013.08.016
Noorawzi N, Samion S (2016) Tribological effects of vegetable oil as alternative lubricant: a pin-on-disk tribometer and wear study. Tribol Trans 59(5):831–837. https://doi.org/10.1080/10402004.2015.1108477
Bahari A (2017) Investigation into tribological performance of vegetable oils as biolubricants at severe contact conditions (Doctoral dissertation, University of Sheffield)
Ing TC, Mohammed Rafiq AK, Azli Y, Syahrullail S (2012) The effect of temperature on the tribological behavior of RBD palm stearin. Tribol Trans 55(5):539–548. https://doi.org/10.1080/10402004.2012.680176
Buyanovskii IA (2010) Boundary lubrication by an adsorption layer. J Frict Wear 31(1):33–47
Afifah AN, Syahrullail S, Azlee NIW, Sidik NAC, Yahya WJ, Rahim EA (2019) Biolubricant production from palm stearin through enzymatic transesterification method. Biochem Eng J 148:178–184. https://doi.org/10.1016/j.bej.2019.05.009
Acknowledgements
Financial support from CNPq (Conselho Nacional de Pesquisa e Desenvolvimento Científico) is gratefully acknowledged. Ribeiro-Filho thanks the FAPEMA (Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão) and Universidade Estadual do Maranhão (UEMA) for financial support.
Funding
The authors received financial support from CNPq (Conselho Nacional de Pesquisa e Desenvolvimento Científico). Ribeiro-Filho received personal financial support from Scientific and Technological Research and Development Support Foundation of Maranhão (FAPEMA) and Universidade Estadual do Maranhão (UEMA). The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper. The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions. The authors have read and approved the final version of the manuscript. Paulo Roberto Campos Flexa Ribeiro Filho took the experimental measurements, carried out the data analysis and wrote the original draft; Matheus Rocha do Nascimento and Silvia Shelly Otaviano da Silva assisted in the experimental measurements; and Sandra de Aguiar Soares, Francisco Murilo Tavares de Luna and Célio Loureiro Cavalcante Jr. supervised the work and critically reviewed the results reported in this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Additional information
Technical Editor: by João Marciano Laredo dos Reis.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ribeiro Filho, P.R.C.F., da Silva, S.S.O., do Nascimento, M.R. et al. Tribological properties of bio-based lubricant basestock obtained from pequi oil (Caryocar brasiliensis). J Braz. Soc. Mech. Sci. Eng. 44, 51 (2022). https://doi.org/10.1007/s40430-021-03358-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40430-021-03358-x