Abstract
Genetic engineering, which was first developed in the 1980s, allows for specific additions to animals’ genomes that are not possible through conventional breeding. Using genetic engineering to improve agricultural animals was first suggested when the technology was in the early stages of development by Palmiter et al. (Nature 300:611–615, 1982). One of the first agricultural applications identified was generating transgenic dairy animals that could produce altered or novel proteins in their milk. Human milk contains high levels of antimicrobial proteins that are found in low concentrations in the milk of ruminants, including the antimicrobial proteins lactoferrin and lysozyme. Lactoferrin and lysozyme are both part of the innate immune system and are secreted in tears, mucus, and throughout the gastrointestinal (GI) tract. Due to their antimicrobial properties and abundance in human milk, multiple lines of transgenic dairy animals that produce either human lactoferrin or human lysozyme have been developed. The focus of this review is to catalogue the different lines of genetically engineered dairy animals that produce either recombinant lactoferrin or lysozyme that have been generated over the years as well as compare the wealth of research that has been done on the in vitro and in vivo effects of the milk they produce. While recent advances including the development of CRISPRs and TALENs have removed many of the technical barriers to predictable and efficient genetic engineering in agricultural species, there are still many political and regulatory hurdles before genetic engineering can be used in agriculture. It is important to consider the substantial amount of work that has been done thus far on well established lines of genetically engineered animals evaluating both the animals themselves and the products they yield to identify the most effective path forward for future research and acceptance of this technology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mammary gland is highly efficient at producing protein during lactation, so as genetic engineering (GE) became possible in livestock species, one of the first agricultural applications people proposed was generating transgenic dairy animals that could produce altered or novel proteins in their milk (Jimenez-Flores and Richardson 1988). In particular, lactoferrin and lysozyme were identified as ideal candidate proteins to produce in ruminant milk with the goal of improving milk functionality (Maga and Murray 1995). Human milk provides infants with substances that protect and promote maturation of the gut and the mucosal immune system (Walker 2010), such as lactoferrin and lysozyme, which are antimicrobial and immune modulating proteins that are very abundant in human milk. In human milk, lactoferrin concentrations range from 1 to 3 g/L, while the average concentration of lysozyme is 0.420 g/L (Montagne et al. 2001) (Table 1). In contrast, ruminants including cows and goats produce very low levels of lactoferrin and lysozyme in their milk (Hettinga et al. 2011). Cows average 0.115 g/L of lactoferrin in their milk (Cheng et al. 2008) and goats average 0.0175 g/L (Campanella et al. 2009). For lysozyme, cows produce on average 0.0013 g/L in their milk (Król et al. 2010) and goats average 0.0025 g/L (Chan\dan et al. 1968).
The first transgenic bovine contained a gene for human lactoferrin (Krimpenfort et al. 1991). Expression levels of lactoferrin were low in this line, thus another line of transgenic cattle expressing human lactoferrin in their mammary glands were produced by the same group (van Berkel et al. 2002; Table 2). As a proof of principal transgenic mice producing human lysozyme in their milk were generated (Maga et al. 1995) and in 1999 the Artemis line of transgenic goats that produce human lysozyme in their milk was established (Maga et al. 2006a). Since the generation of these first two herds many other lines of cows and goats producing either lactoferrin or lysozyme have been founded (Yang et al. 2008, 2011; An et al. 2012; Goldman et al. 2012). A great deal of research has been performed to characterize the fitness of these different lines of transgenic animals (Yang et al. 2008; Jackson et al. 2010), as well as the recombinant proteins they produce (Yang et al. 2011; Thomassen et al. 2005; Yu et al. 2011). Recently, many reports have emerged reporting the effects of consumption of milk containing these recombinant proteins in both healthy animal models (Brundige et al. 2010; Zhou et al. 2011; Cooper et al. 2012) and as a treatment in animal models of disease (Wang et al. 2012; Cooper et al. 2013).
Proteins
Lactoferrin
Lactoferrin is an 80 kDa iron binding protein found in various secretions such as milk and tears, as well as in neutrophil granules. Lactoferrin is part of the host defense system and has a wide range of functions including acting as an antimicrobial, immunomodulatory, and antioxidant agent (Wakabayashi et al. 2006). Its role in host immunity predates mammals as birds and reptiles express an ortholog protein known as ovotransferrin which exhibits comparable antimicrobial properties and is abundant in egg white and serum (Ibrahim et al. 1998; Xie et al. 2002). Part of lactoferrin’s antimicrobial activity is due to its highly cationic N-terminal region. This region confers bactericidal action by interacting with the negatively charged part of bacterial membranes, which is lipopolysaccharide (LPS) in Gram-negative bacteria and lipoteichoic acid (LTA) in Gram-positive bacteria (Yen et al. 2009). Lactoferrin can also compete with LPS for binding of CD14, a part of toll like receptor (TLR) 4, thus preventing LPS from activating a pro-inflammatory cascade that can lead to tissue damage (Actor et al. 2009). Lactoferrin’s ability to bind iron not only promotes growth of beneficial low iron requiring bacteria like Lactobacillus and Bifidobacteria (Yen et al. 2009), but sequestering iron also reduces cellular oxidative stress, thus lowering pro-inflammatory cytokines (Actor et al. 2009). Finally, lactoferrin has targeted control of some cellular processes and can act as a transcription factor and regulate granulopoiesis and DNA synthesis in certain cells types (Kanyshkova et al. 2001).
Lactoferrin has distinct properties that make it an ideal molecule for promoting healthy gut maturation and establishment of a beneficial GI-tract microbiota. Lactoferrin is resistant to enzymatic proteolysis in the stomach (Davidson and Lönnerdal 1987), and partial degradation of lactoferrin by stomach pepsin frees the lactoferricin domain, which may be an even more potent antimicrobial (Yen et al. 2009). The lactoferricin domain is also key to lactoferrin’s ability to bind cell surface proteins and DNA (Baker and Baker 2009). During the first hours of life the gut is permeable to many immunologically relevant proteins such as IgA and growth factors necessary for gut development (Commare and Tappenden 2007). After the first few days of life the gut becomes impermeable to most proteins, however there is a 105 kDa lactoferrin receptor (also known as intelectin) that specializes in mediating uptake of lactoferrin into enterocytes and crypt cells (Kawakami and Lönnerdal 1991; Liao et al. 2007, 2012), so infants can transport lactoferrin past gut closure. Once lactoferrin is taken up by enterocytes at the brush border, is internalized into compartments in the apical cytoplasm, where it can have effects on cellular proliferation and directing immune responses (Nielsen et al. 2010).
Lysozyme
The protein lysozyme is an important non-specific antimicrobial factor in many body secretions including milk, saliva, and intestinal mucus (Schenkels et al. 1995), and is very resistant to hydrolysis by acids and proteases in the gut (Eschenburg et al. 1990), allowing it to survive intact all the way through the GI tract (Schanler et al. 1986). Different forms of lysozyme have evolved and are found in organisms ranging from T4 lysozyme in bacteriophages (Jespers et al. 1992) to the various G and C type lysozymes which are found in fish (Irwin and Gong 2003); salamanders (Yu et al. 2013); birds (Short et al. 1996); marsupials (Nicholas et al. 1989; Piotte et al. 1997) rodents (Yeh et al. 1993) and ruminants (Hettinga et al. 2011) to name a few. Lysozyme acts as a N-acetylmuramidase that is able to cleave 1,4-beta-linkages between N-acetylmuramic acid and N-acetyl-D-glucosamine residues found in the peptidoglycan layer of bacterial cells (Wiesner and Vilcinskas 2010). The N-terminal helix portion of lysozyme can kill Gram-negative bacteria by crossing the outer membrane and interfering with membrane potential-dependent respiration (Ibrahim et al. 2011). Lysozyme also possesses the ability to modulate the inflammatory response through several mechanisms (Goldman et al. 1986). Lysozyme binds LPS and LTA, preventing them from interacting with receptors on intestinal epithelial cells (IEC’s) and intestinal macrophages (Ginsburg 2002; Ohno and Morrison 1989a). Sequestration of LPS by lysozyme suppresses pro-inflammatory effects, including production of TNF-α (Ohno and Morrison 1989b; Kurasawa et al. 1996; Takada et al. 1994a, b). Using a porcine model of chemically induced colitis, investigators were able to decrease expression of pro-inflammatory cytokines, including TNF-α and IL-8, as well as increase expression of anti-inflammatory cytokines, including TGF-β1, through oral administration of hen egg white lysozyme (Lee et al. 2009). Lysozyme also has significant immunomodulatory effects on neutrophils, decreasing several responses to pro-inflammatory agents and oxidative metabolism in neutrophils without suppressing phagocytosis (Gordon et al. 1979).
Lactoferrin and lysozyme
Both lactoferrin and lysozyme are found in high concentrations in human milk (Montagne et al. 2001). There are many health benefits that breastfed infants experience and lactoferrin and lysozyme in breast milk help to confer these positive effects (Mountzouris et al. 2002; Newburg and Walker 2007). When lactoferrin and lysozyme are together they have synergistic antimicrobial properties. Lactoferrin has a cationic domain that allows it to increase lysozyme’s ability to kill bacteria. Lactoferrin binds to LPS on the outer membrane which aids in disrupting of the membrane and allows lysozyme better access the peptidoglycan layer underneath in Gram-positive bacteria (Leitch and Willcox 1999), and the proteoglycan matrix of Gram-negative bacteria (Ellison and Giehl 1991). In conjunction lactoferrin and lysozyme demonstrate a synergistic ability to inhibit growth of both Gram-positive and Gram-negative bacteria (van der Linden et al. 2009). They also demonstrate additive effects against amoebas (León-Sicairos et al. 2006). Given the relationship between lactoferrin and lysozyme, transgenic milk containing both antimicrobials has the potential to have an even more pronounced positive effect on health.
Transgenic animals
Lactoferrin
Cows produce relatively little bovine lactoferrin in their milk, however Pharming Group BV, a Dutch-based biotechnology company, used microinjection to genetically engineer a herd of transgenic cows that express approximately 1.5–2.0 g/L recombinant human lactoferrin (rhLF) in their milk, a concentration within the range normally found in human milk (Thomassen et al. 2005). Natural hLF from human milk and rhLF from cows milk had identical iron-binding and -release properties, however natural hLF and rhLF underwent differential N-linked glycosylation (van Berkel et al. 2002). Natural hLF contains complex-type glycans and rhLF contains oligomannose- and hybrid-type N-linked glycans, but the overall structures are identical (Thomassen et al. 2005).
A second group in China employed a different transgenesis technique to produce human lactoferrin transgenic cows. Bovine fibroblast cells were co-microinjected with a 150 kb BAC (bacterial artificial chromosome) carrying the human lactoferrin gene and a marker gene (Yu et al. 2011). Two transgenic cows were generated that secreted rhLF at high levels, 2.5 and 3.4 g/L, respectively. The rhLF had a similar proteolytic susceptibility as the natural human lactoferrin and biochemical analysis revealed that the iron-binding and releasing properties of rhLF were identical to that of native hLF (Yang et al. 2008). Glycosylation patterns between native hLF and rhLF were compared and N-glycans from hLF are comprised entirely of highly branched, highly sialylated and highly fucosylated complex-type structures, while N-glycans from rhLF are of the high mannose-, hybrid- and complex-type structures, with less N-acetylneuraminic acid and fucose. However hLF and rhLF appear to be glycosylated at the same two sites (Yu et al. 2011). A comparison of milk constituents showed that other than rhLF, milk from lactoferrin cattle has the same composition as milk from non-transgenic cows (Zhang et al. 2012).
Multiple lines of goats containing the human lactoferrin transgene have also been generated (An et al. 2012; Goldman et al. 2012; Yu et al. 2012). One line of rhLF transgenic goats was generated in China through somatic cell nuclear transfer using a 3.3 kb hLF minigene and the regulatory elements of the β-casein gene (Yu et al. 2012). One transgenic goat produced more than 30 mg/mL rhLF in its milk, and rhLF expression was stable during the entire lactation cycle. Compared with natural hLF, the rhLF from the transgenic goat had similar a molecular mass, N-terminal sequence, isoelectric point, immunoreactivity and digestive stability (Yu et al. 2012). Another herd of lactoferrin transgenic goats were generated in Russia through microinjection, which produce up to 10 g/L hLF in their milk (Goldman et al. 2012). The hLF was identical to the native protein in its physical and chemical properties including electrophoretic mobility, isoelectric point, and recognition by polyclonal and monoclonal antibodies (Goldman et al. 2012).
Non-traditional dairy animals have also been generated that produce lactoferrin in their mammary glands. A recombinant adenovirus vector carrying human lactoferrin cDNA was injected into rabbit mammary glands (Han et al. 2008). After viral vector infection rabbits exhibited a high level of expression of human lactoferrin in their milk, reaching up to 2.3 mg/mL. However this injected adenoviral vector method is more suited for transient high-level expression of recombinant proteins.
Effect of lactoferrin milk
Multiple studies have been conducted investigating the effects of milk containing recombinant hLF produced in the mammary glands of transgenic animals. Zhang et al. (2001) showed in an experiment with neonatal mice that feeding rhLF-containing milk from a transgenic mouse strain improved intestinal growth. When comparing rhLF-milk fed pigs to non-transgenic milk fed pigs, rhLF-milk fed pigs had beneficial changes in systemic health and GI villi architecture (Cooper et al. 2012). There was a significant decrease in neutrophils and increase in lymphocytes, which is an indicator of decreased systemic inflammation. There were also changes in intestinal villi architecture and rhLF-milk fed pigs had taller villi, deeper crypts, and a thinner lamina propria.
During a 90 day rat feeding trial, no negative effects were observed from consuming a diet that included rhLF milk powder compared to standard milk powder (Zhou et al. 2011). Higher mean ferritin and Fe(+) concentrations were observed in both male and female rats fed the rhLF milk powder diets, as compared to rats fed non-transgenic milk diets or the commercial diet. Another study investigated the effects of rhLF on anemia and found that rhLF can improve iron status of rats with anemia more than supplementation with ferrous lactate alone (Wang et al. 2012).
Lactoferrin is an antimicrobial protein with in vitro bactericidal activity against Escherichia coli and Listeria monocytogenes, and fungicidal activity against Candida albicans, as well as the ability to increase in the activity of antibiotics when used in combination in vitro (Goldman et al. 2012). Using an in vivo model, rhLF beneficially modulated the intestinal flora composition and improved the growth of young pigs (Hu et al. 2012). Pigs fed rhLF had decreased Salmonella spp. in the colon and E. coli throughout the intestine and increased concentrations of Bifidobacterium spp. in the ileum and of Lactobacillus spp. throughout the intestine. These pigs also had improved average daily weight gain. Experimental infection models have also been tested. Transgenic cows producing rhLF in their milk and non-transgenic cows were infected with Staphylococcus chromogenes and all transgenic cows became infected but showed no clinical signs, while the control cows developed mild clinical mastitis (Simojoki et al. 2010). Transgenic cows producing rhLF in their milk eliminated bacteria faster from their quarters than did the controls and seemed to be protected from clinical disease and from prolonged inflammatory reaction caused by intramammary infection induced by S. chromogenes.
Lysozyme
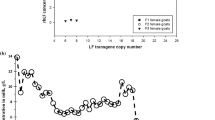
A herd of transgenic goats that produced hLZ in their milk was produced through pro-nuclear microinjection of a 23 KB construct that included the bovine αs1 casein promoter and 5′UTR and human lysozyme cDNA. These goats produce 270 mg/L of hLZ in their milk, which is approximately 65 % of what humans produce. Other than the hLZ protein milk from transgenic and non-transgenic goats had the same fat and protein composition (Maga et al. 2006a). The overall fitness of these transgenic goats was evaluated (Jackson et al. 2010). In males, none of the parameters of semen quality, including semen volume and concentration, total sperm per ejaculate, sperm morphology, viability and motility, were significantly different between hLZ transgenic bucks and non-transgenic full-sib controls. The hLZ transgenic females did not significantly differ in the reproductive traits of gestation length and litter size compared to their non-transgenic counterparts. Neither the presence of the transgene nor the consumption of milk from transgenic animals significantly affected birth weight, weaning weight, overall gain and post-wean gain. This indicates that reproductive and growth traits are not impacted by the presence or expression of the transgene (Jackson et al. 2010). Furthermore, the presence and expression of the transgene had no off-target effects on lactating animals of the transgenic line as indicated by lack of differences in circulating metabolites (Clark et al. 2014).
Transgenic cows expressing recombinant human lysozyme (hLZ) were generated using somatic cell nuclear transfer (Yang et al. 2011). These cows produce up to 25.96 mg/L hLZ in their milk, which is approximately twice the amount of endogenous bovine lysozyme produced; a concentration that is still only around 6 % of what is found in human milk (Montagne et al. 2001; Table 1). The purified recombinant hLZ showed the same physicochemical properties, such as molecular mass and bacterial lysis, as its natural counterpart and both recombinant and natural lysozyme had similar pH and temperature stability during in vitro simulations. The gross composition of transgenic and non-transgenic milk, including levels of lactose, total protein, total fat, and total solids was the same (Yang et al. 2011), and a more complete assessment of milk composition found that other than the hLZ, transgenic and non-transgenic milk have the same composition (Zhang et al. 2012).
Effects of lysozyme milk
Goats producing hLZ-milk have a significantly lower somatic cell counts than control goats, indicating an overall healthier udder environment and less stimulation of the immune system by pathogens (Maga et al. 2006a). Looking at the relative abundance of microbial populations in milk from hLZ transgenic goats and control goats across the course of lactation, lysozyme in milk had some positive and some neutral effects on the bacteria present in the milk throughout the course of lactation, and overall the presence of the lysozyme does not appear to be disrupting the natural evolution of the microbiota during the course of lactation (Mcinnis et al. 2015). In vitro, hLZ-milk slows the growth of mastitis-causing strains of E. coli and Staphylococcus aureus as well as the cold-spoilage organism Pseudomonas fragi (Maga et al. 2006b). The growth of an organism involved in cheese-making, Lactococcus lactis, was not affected by the presence of lysozyme in milk. Cheese made from hLZ goats’ milk had significantly stronger curd and less clotting time, attributed to hLZ’s positive charge because the addition of polycations can promote faster curd precipitation through the increased number of positive–negative bonds forming (Maga et al. 2006b).
When fed to young pigs the hLZ goat milk had a variety of effects. These effects include reducing numbers of coliforms and E. coli in the intestine (Maga et al. 2006c; Brundige et al. 2008). The hLZ goat milk also increases the abundance of bacteria associated with gut health (Bifidobacteriaceae and Lactobacillaceae) and decreases abundance of those associated with disease (Mycobacteriaceae, Streptococcaceae, Campylobacterales) (Maga et al. 2012). The hLZ goat milk also positively impacts intestinal health, increasing villi surface area (Brundige et al. 2008), decreasing lamina propria thickness, and increasing expression of the anti-inflammatory cytokine TGFβ1 (Cooper et al. 2011). Systemic effects have also been observed including increased levels of serum metabolites that are associated with health including myo-inostitol, co-enzyme Q10, and taurine (Brundige et al. 2010).
The hLZ goat milk has also been tested in in vitro and in vivo experimental infection models. In an in vitro model, intestinal epithelial cell migration was significantly decreased in the presence of enteroaggregative E. coli (EAEC) alone but was restored in the presence of milk (Carvalho et al. 2012). Milk from hLZ transgenic goats improved migration significantly more than control milk. Both milks significantly reduced EAEC adhesion to Caco-2 cells and transgenic milk resulted in less colonization than control milk using a HEp-2 cell line assay. While normal goat milk is able to repair intestinal barrier function damage induced by EAEC the hLZ transgenic goat milk provides further protection (Carvalho et al. 2012). In an in vivo model of diarrhea using young pigs infected with enterotoxigenic E. coli (ETEC), pigs consuming hLZ-milk recovered from clinical signs of infection faster than pigs consuming control milk, with significantly improved fecal consistency and activity level (Cooper et al. 2013). Circulating monocytes, neutrophils, and lymphocytes returned faster to pre-infection proportions in hLZ-milk fed pigs, while control-fed pigs had significantly higher hematocrit, indicating continuing dehydration. In the ileum, pigs fed hLZ-milk had significantly lower expression of pro-inflammatory cytokine IL-8, longer intestinal villi, deeper crypts, and a thinner lamina propria. Thus consumption of hLZ-milk helped pigs recover from infection faster, making hLZ-milk an effective treatment of E. coli-induced diarrhea (Cooper et al. 2013).
Effects of lactoferrin and lysozyme in conjunction
There are no transgenic ruminants that produce both human lactoferrin and human lysozyme in their mammary gland. However a study has been conducted combining milk from lactoferrin transgenic cows and milk from lysozyme transgenic goats (Cooper et al. 2014). Pigs fed a combination of rhLF and hLZ milk had significantly thinner lamina propria layer and significantly deeper crypts. Hematological parameters were also changed. Pigs fed hLZ with control milk, rhLF with control milk and rhLF with hLZ had significantly reduced mean corpuscular volume (MCV) and increased red blood cells compared to control milk only fed pigs. These results support previous research that shows milk containing rhLF induces proliferation of intestinal crypt cells, and that pigs fed milk containing rhLF and hLZ had decreased intestinal inflammation, particularly in the lamina propria. The increased RBC’s shows animals fed these antimicrobials had higher numbers of RBC’s and that the RBC’s were more mature since as RBC’s mature the volume (MCV) decreases (Cooper et al. 2014).
Future research
There are now many transgenic dairy animals producing either human lactoferrin or lysozyme, and a growing body of research about the basic effects of these types of milk, both in vitro and in vivo. As research continues the effects of various milk processing techniques, such as powdering, on the bioactivity of lactoferrin and lysozyme in milk will likely be investigated, since powdering allows for extended shelf life and easier shipment of the milk. Also, it has already been shown that milk containing different concentrations of lactoferrin and lysozyme can induce different physiological changes (Cooper et al. 2014), thus more in-depth dose–response studies could be done to determine the threshold concentrations needed to induce specific changes. Additionally investigations into the effects of long term consumption as well as monitoring how long after the cessation of consumption the effects on parameters such as intestinal architecture, immune response, and microbiota persist. Knowledge about these factors will be vital in enabling the design of future studies, as more research teams transition from testing the general effects of lactoferrin or lysozyme milk, to testing the efficacy of these milks against specific diseases such as E.coli infections (Cooper et al. 2013), and other GI tract disturbances.
Lactoferrin and lysozyme are the most abundant antimicrobial proteins in human milk, but many other antimicrobial proteins besides these two exist in human milk, and there are groups in the process of engineering dairy animals to make other antimicrobial proteins in their milk, such as β-defensin (Jia et al. 2001; Liu et al. 2013). As knowledge about the role of endogenously produced antimicrobial proteins in innate and adaptive immunity increases it is possible that transgenic dairy animals will be used to produce a wider variety of antimicrobial proteins. Another trend in antimicrobial protein research is the design of specific proteins and peptides that target specific bacteria and viruses (Polcyn et al. 2009; Mao et al. 2013; Ma et al. 2013), and genetically engineered dairy animals represent a possible production system for these novel proteins and peptides.
While there are many possible uses for genetically engineered dairy animals, as well as genetically engineered animals in agriculture in general, a key to continuing progress in the field of transgenic animal research is the streamlining of the regulatory process. Many lines of transgenic agricultural animals have been engineered to help solve specific problems around the world including bacterial diseases (Cooper et al. 2013), environmental pollution (Golovan et al. 2001), and food security (Du et al. 1992; Wheeler et al. 2001). Despite decades of research into both the safety and benefits that these animal that could provide, no transgenic animals have been approved for use in agriculture (Murray and Maga 2010). To effectively face current and future issues around the globe, we must be willing to objectively evaluate and implement new technologies, including genetic engineering of animals used in agriculture.
References
Actor JK, Hwang SA, Kruzel ML (2009) Lactoferrin as a natural immune modulator. Curr Pharm Des 15:1956–1973
An LY, Yuan YG, Yu BL, Yang TJ, Cheng Y (2012) Generation of human lactoferrin transgenic cloned goats using donor cells with dual markers and a modified selection procedure. Theriogenology 78:1303–1311
Baker EN, Baker HM (2009) A structural framework for understanding the multifunctional character of lactoferrin. Biochimie 91:3–10
Brundige DR, Maga EA, Klasing KC, Murray JD (2008) Lysozyme transgenic goats’ milk influences gastrointestinal morphology in young pigs. J Nutr 138:921–926
Brundige DR, Maga EA, Klasing KC, Murray JD (2010) Consumption of pasteurized human lysozyme transgenic goats’ milk alters serum metabolite profile in young pigs. Transgenic Res 19:563–574
Campanella L, Martini E, Pintore M, Tomassetti M (2009) Determination of lactoferrin and immunoglobulin g in animal milks by new immunosensors. Sensors (Basel) 9:2202–2221
Carvalho EB, Maga EA, Quetz JS, Lima IF, Magalhães HY, Rodrigues FA, Silva AV, Prata MM, Cavalcante PA, Havt A, Bertolini M, Bertolini LR, Lima AA (2012) Goat milk with and without increased concentrations of lysozyme improves repair of intestinal cell damage induced by enteroaggregative Escherichia coli. BMC Gastroenterol 12:106
Chandan RC, Parry RM, Shahani KM (1968) Lysozyme, lipase, and ribonuclease in milk of various species. J Dairy Sci 51:606–607
Cheng JB, Wang JQ, Bu DP, Liu GL, Zhang CG, Wei HY, Zhou LY, Wang JZ (2008) Factors affecting the lactoferrin concentration in bovine milk. J Dairy Sci 91:970–976
Clark M, Murray JD, Maga EA (2014) Assessing unintended effects of a mammary-specific transgene at the whole animal level in host and non-target animals. Transgenic Res 23:245–256
Commare CE, Tappenden KA (2007) Development of the infant intestine: implications for nutrition support. Nutr Clin Pract 22:159–173
Cooper CA, Brundige DR, Reh WA, Maga EA, Murray JD (2011) Lysozyme transgenic goats’ milk positively impacts intestinal cytokine expression and morphology. Transgenic Res 20:1235–1243
Cooper CA, Nelson KM, Maga EA, Murray JD (2012) Consumption of transgenic cows’ milk containing human lactoferrin results in beneficial changes in the gastrointestinal tract and systemic health of young pigs. Transgenic Res 22:571–578
Cooper CA, Garas Klobas L, Maga EA, Murray JD (2013) Consuming transgenic goats’ milk containing the antimicrobial protein lysozyme helps resolve diarrhea in young pigs. PLoS ONE 8:e58409
Cooper CA, Maga EA, Murray JD (2014) Consumption of transgenic milk containing the antimicrobials lactoferrin and lysozyme separately and in conjunction by 6 week old pigs improves intestinal and systemic health. J Dairy Res 81:30–37
Davidson LA, Lönnerdal B (1987) Persistence of human milk proteins in the breast-fed infant. Acta Paediatr Scand 76:733–740
Du SJ, Gong ZY, Fletcher GL, Shears MA, King MJ, Idler DR, Hew CL (1992) Growth enhancement in transgenic Atlantic salmon by the use of an “all fish” chimeric growth hormone gene construct. Biotechnology (NY) 10:176–181
Ellison RT 3rd, Giehl TJ (1991) Killing of gram-negative bacteria by lactoferrin and lysozyme. J Clin Invest 88:1080–1091
Eschenburg G, Heine W, Peters E (1990) Fecal sIgS and lysozyme excretion in breast feeding and formula feeding. Kinderaerztl Prax 58:255–260
Ginsburg I (2002) Role of lipoteichoic acid in infection and inflammation. Lancet Infect Dis 2:171–179
Goldman AS, Thorpe LW, Goldblum RM, Hanson LA (1986) Anti-inflammatory properties of human milk. Acta Paediatr Scand 75:689–695
Goldman IL, Georgieva SG, Gurskiy YG, Krasnov AN, Deykin AV, Popov AN, Ermolkevich TG, Budzevich AI, Chernousov AD, Sadchikova ER (2012) Production of human lactoferrin in animal milk. Biochem Cell Biol 90:513–519
Golovan SP, Meidinger RG, Ajakaiye A, Cottrill M, Wiederkehr MZ, Barney DJ, Plante C, Pollard JW, Fan MZ, Hayes MA, Laursen J, Hjorth JP, Hacker RR, Phillips JP, Forsberg CW (2001) Pigs expressing salivary phytase produce low-phosphorus manure. Nat Biotechnol 19:741–745
Gordon LI, Douglas SD, Kay NE, Yamada O, Osserman EF, Jacob HS (1979) Modulation of neutrophil function by lysozyme. Potential negative feedback system of inflammation. J Clin Invest 64:226–232
Han ZS, Li QW, Zhang ZY, Yu YS, Xiao B, Wu SY, Jiang ZL, Zhao HW, Zhao R, Li J (2008) Adenoviral vector mediates high expression levels of human lactoferrin in the milk of rabbits. J Microbiol Biotechnol 18:153–159
Hettinga K, van Valenberg H, de Vries S, Boeren S, van Hooijdonk T, van Arendonk J, Vervoort J (2011) The host defense proteome of human and bovine milk. PLoS ONE 6:e19433
Hu W, Zhao J, Wang J, Yu T, Wang J, Li N (2012) Transgenic milk containing recombinant human lactoferrin modulates the intestinal flora in piglets. Biochem Cell Biol 90:485–496
Ibrahim HR, Iwamori E, Sugimoto Y, Aoki T (1998) Identification of a distinct antibacterial domain within the N-lobe of ovotransferrin. Biochim Biophys Acta 1401:289–303
Ibrahim HR, Imazato K, Ono H (2011) Human lysozyme possesses novel antimicrobial peptides within its N-terminal domain that target bacterial respiration. J Agric Food Chem 59:10336–11045
Irwin DM, Gong Z (2003) Molecular evolution of vertebrate goose-type lysozyme genes. J Mol Evol 56:234–242
Jackson KA, Berg JM, Murray JD, Maga EA (2010) Evaluating the fitness of human lysozyme transgenic dairy goats: growth and reproductive traits. Transgenic Res 19:977–986
Jespers L, Sonveaux E, Fastrez J (1992) Is the bacteriophage lambda lysozyme an evolutionary link or a hybrid between the C and V-type lysozymes? Homology analysis and detection of the catalytic amino acid residues. J Mol Biol 228:529–538
Jia HP, Starner T, Ackermann M, Kirby P, Tack BF, McCray PB Jr (2001) Abundant human beta-defensin-1 expression in milk and mammary gland epithelium. J Pediatr 138:109–112
Jimenez-Flores R, Richardson T (1988) Genetic engineering of the caseins to modify the behavior of milk during processing: a review. J Dairy Sci 71:2640–2654
Kanyshkova TG, Buneva VN, Nevinsky GA (2001) Lactoferrin and its biological functions. Biochemistry 66:1–7
Kawakami H, Lönnerdal B (1991) Isolation and function of a receptor for human lactoferrin in human fetal intestinal brush-border membranes. Am J Physiol 261:G841–G846
Krimpenfort P, Rademakers A, Eyestone W, van der Schans A, van den Broek S, Kooiman P, Kootwijk E, Platenburg G, Pieper F, Strijker R (1991) Generation of transgenic dairy cattle using ‘in vitro’ embryo production. Biotechnology (NY) 9:844–847
Król J, Litwińczuk Z, Brodziak A, Barłowska J (2010) Lactoferrin, lysozyme and immunoglobulin G content in milk of four breeds of cows managed under intensive production system. Pol J Vet Sci 13:357–361
Kurasawa T, Takada K, Ohno N, Yadomae T (1996) Effects of murine lysozyme on lipopolysaccharide-induced biological activities. FEMS Immunol Med Microbiol 13:293–301
Lee M, Kovacs-Nolan J, Yang C, Archibold T, Fan MZ, Mine Y (2009) Hen egg lysozyme attenuates inflammation and modulates local gene expression in a porcine model of dextran sodium sulfate (DSS)-induced colitus. J Agric Food Chem 57:2233–2240
Leitch EC, Willcox MD (1999) Lactoferrin increases the susceptibility of S. epidermidis biofilms to lysozyme and vancomycin. Curr Eye Res 19:12–19
León-Sicairos N, López-Soto F, Reyes-López M, Godínez-Vargas D, Ordaz-Pichardo C, de la Garza M (2006) Amoebicidal activity of milk, apo-lactoferrin, sIgA and lysozyme. Clin Med Res 4:106–113
Liao Y, Lopez V, Shafizadeh TB, Halsted CH, Lönnerdal B (2007) Cloning of a pig homologue of the human lactoferrin receptor: expression and localization during intestinal maturation in piglets. Comp Biochem Physiol A: Mol Integr Physiol 148:584–590
Liao Y, Jiang R, Lönnerdal B (2012) Biochemical and molecular impacts of lactoferrin on small intestinal growth and development during early life. Biochem Cell Biol 90:476–484
Liu J, Luo Y, Liu Q, Zheng L, Yang Z, Wang Y, Su J, Quan F, Zhang Y (2013) Production of cloned embryos from caprine mammary epithelial cells expressing recombinant human β-defensin-3. Theriogenology 79:660–666
Ma QQ, Lv YF, Gu Y, Dong N, Li DS, Shan AS (2013) Rational design of cationic antimicrobial peptides by the tandem of leucine-rich repeat. Amino Acids 44:1215–1224
Maga EA, Murray JD (1995) Mammary gland expression of transgenes and the potential for altering the properties of milk. Nat Biotech 13:1452–1457
Maga EA, Anderson GB, Murray JD (1995) The effect of mammary gland expression of human lysozyme on the properties of milk from transgenic mice. J Dairy Sci 78:2645–2652
Maga EA, Cullor JS, Smith W, Anderson GB, Murray JD (2006a) Human lysozyme expressed in the mammary gland of transgenic dairy goats can inhibit the growth of bacteria that cause mastitis and the cold-spoilage of milk. Foodborne Pathog Dis 3:384–392
Maga EA, Shoemaker CF, Rowe JD, Bondurant RH, Anderson GB, Murray JD (2006b) Production and processing of milk from transgenic goats expressing human lysozyme in the mammary gland. J Dairy Sci 89:518–524
Maga EA, Walker RL, Anderson GB, Murray JD (2006c) Consumption of milk from transgenic goats expressing human lysozyme in the mammary gland results in the modulation of intestinal microflora. Transgenic Res 15:515–519
Maga EA, Desai PT, Weimer BC, Dao N, Kültz D, Murray JD (2012) Consumption of lysozyme-rich milk can alter microbial fecal populations. Appl Environ Microbiol 78:6153–6160
Mao R, Teng D, Wang X, Xi D, Zhang Y, Hu X, Yang Y, Wang J (2013) Design, expression, and characterization of a novel targeted plectasin against methicillin-resistant Staphylococcus aureus. Appl Microbiol Biotechnol 97:3991–4002
Mcinnis EA, Kalanetra KM, Mills DA, Maga EA (2015) Analysis of raw goat milk microbiota: impact of stage of lactation and lysozyme on microbial diversity. Food Microbiol 46:121–131
Montagne P, Cuillière ML, Molé C, Béné MC, Faure G (2001) Changes in lactoferrin and lysozyme levels in human milk during the first twelve weeks of lactation. Adv Exp Med Biol 501:241–247
Mountzouris KC, McCartney AL, Gibson GR (2002) Intestinal microflora of human infants and current trends for its nutritional modulation. Br J Nutr 87:405–420
Murray JD, Maga EA (2010) Is there a risk from not using GE animals? Transgenic Res 19:357–361
Newburg DS, Walker WA (2007) Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr Res 61:2–8
Nicholas K, Loughnan M, Messer M, Munks S, Griffiths M, Shaw D (1989) Isolation, partial sequence and asynchronous appearance during lactation of lysozyme and alpha-lactalbumin in the milk of a marsupial, the common ringtail possum (Pseudocheirus peregrinus). Comp Biochem Physiol B 94:775–778
Nielsen SM, Hansen GH, Danielsen EM (2010) Lactoferrin targets T cells in the small intestine. J Gastroenterol 45:1121–1128
Ohno N, Morrison DC (1989a) Lipopolysaccharide interaction with lysozyme binding of lipopolysaccharide to lysozyme and inhibition of lysozyme enzymatic activity. J Biol Chem 264:4434–4441
Ohno N, Morrison DC (1989b) Lipopolysaccharide interactions with lysozyme differentially affect lipopolysaccharide immunostimulatory activity. Eur J Biochem 186:629–636
Palmiter RD, Brinster RL, Hammer RE, Trumbauer ME, Rosenfeld MG, Birnberg NC, Evans RM (1982) Dramatic growth of mice that develop from eggs microinjected with metallothionein–growth hormone fusion genes. Nature 300:611–615
Piotte CP, Marshall CJ, Hubbard MJ, Collet C, Grigor MR (1997) Lysozyme and alpha-lactalbumin from the milk of a marsupial, the common brush-tailed possum (Trichosurus vulpecula). Biochim Biophys Acta 1336:235–242
Polcyn P, Jurczak M, Rajnisz A, Solecka J, Urbanczyk-Lipkowska Z (2009) Design of antimicrobially active small amphiphilic peptide dendrimers. Molecules 14:3881–3905
Schanler RJ, Goldblum RM, Garza C, Goldman AS (1986) Enhanced fecal excretion of selected immune factors in very low birth weight infants fed fortified human milk. Pediatr Res 20:711–715
Schenkels LC, Veerman EC, Nieuw Amerongen AV (1995) Biochemical composition of human saliva in relation to other mucosal fluids. Crit Rev Oral Biol Med 6:161–175
Short ML, Nickel J, Schmitz A, Renkawitz R (1996) Lysozyme gene expression and regulation. EXS 75:243–257
Simojoki H, Hyvönen P, Orro T, Pyörälä S (2010) High concentration of human lactoferrin in milk of rhLf-transgenic cows relieves signs of bovine experimental Staphylococcus chromogenes intramammary infection. Vet Immunol Immunopathol 136:265–271
Takada K, Ohno N, Yadomae T (1994a) Binding of lysozyme to lipopolysaccharide suppresses tumor necrosis factor production in vivo. Infect Immun 62:1171–1175
Takada K, Ohno N, Yadomae T (1994b) Detoxification of lipopolysaccharide (LPS) by egg white lysozyme. FEMS Immunol Med Microbiol 9:255–263
Thomassen EA, van Veen HA, van Berkel PH, Nuijens JH, Abrahams JP (2005) The protein structure of recombinant human lactoferrin produced in the milk of transgenic cows closely matches the structure of human milk-derived lactoferrin. Transgenic Res 14:397–405
van Berkel PH, Welling MM, Geerts M, van Veen HA, Ravensbergen B, Salaheddine M, Pauwels EK, Pieper F, Nuijens JH, Nibbering PH (2002) Large scale production of recombinant human lactoferrin in the milk of transgenic cows. Nat Biotechnol 20:484–487
van der Linden DS, Short D, Dittmann A, Yu PL (2009) Synergistic effects of ovine-derived cathelicidins and other antimicrobials against Escherichia coli O157:H7 and Staphylococcus aureus 1056 MRSA. Biotechnol Lett 31:1265–1267
Wakabayashi H, Koji Yamauchi K, Takase M (2006) Lactoferrin research, technology and applications. Int Dairy J 16:1241–1251
Walker A (2010) Breast milk as the gold standard for protective nutrients. J Pediatr 156:S3–S7
Wang X, Liu S, Xu H, Yan W (2012) Effects of recombinant human lactoferrin on improving the iron status of IDA rats. Wei Sheng Yan Jiu 41:13–17
Wheeler MB, Bleck GT, Donovan SM (2001) Transgenic alteration of sow milk to improve piglet growth and health. Reprod Suppl 58:313–324
Wiesner J, Vilcinskas A (2010) Antimicrobial peptides: the ancient arm of the human immune system. Virulence 1:440–464
Xie H, Huff GR, Huff WE, Balog JM, Rath NC (2002) Effects of ovotransferrin on chicken macrophages and heterophil-granulocytes. Dev Comp Immunol 26:805–815
Yang P, Wang J, Gong G, Sun X, Zhang R, Du Z, Liu Y, Li R, Ding F, Tang B, Dai Y, Li N (2008) Cattle mammary bioreactor generated by a novel procedure of transgenic cloning for large-scale production of functional human lactoferrin. PLoS ONE 3:e3453
Yang B, Wang J, Tang B, Liu Y, Guo C, Yang P, Yu T, Li R, Zhao J, Zhang L, Dai Y, Li N (2011) Characterization of bioactive recombinant human lysozyme expressed in milk of cloned transgenic cattle. PLoS ONE 6:e17593
Yeh TC, Wilson AC, Irwin DM (1993) Evolution of rodent lysozymes: isolation and sequence of the rat lysozyme genes. Mol Phylogenet Evol 2:65–75
Yen CC, Lin CY, Chong KY, Tsai TC, Shen CJ, Lin MF, Su CY, Chen HL, Chen CM (2009) Lactoferrin as a natural regimen for selective decontamination of the digestive tract: recombinant porcine lactoferrin expressed in the milk of transgenic mice protects neonates from pathogenic challenge in the gastrointestinal tract. J Infect Dis 199:590–598
Yu T, Guo C, Wang J, Hao P, Sui S, Chen X, Zhang R, Wang P, Yu G, Zhang L, Dai Y, Li N (2011) Comprehensive characterization of the site-specific N-glycosylation of wild-type and recombinant human lactoferrin expressed in the milk of transgenic cloned cattle. Glycobiology 21:206–224
Yu H, Chen J, Sun W, Liu S, Zhang A, Xu X, Wang X, He Z, Liu G, Cheng G (2012) The dominant expression of functional human lactoferrin in transgenic cloned goats using a hybrid lactoferrin expression construct. J Biotechnol 161:198–205
Yu H, Gao J, Lu Y, Guang H, Cai S, Zhang S, Wang Y (2013) Molecular cloning, sequence analysis and phylogeny of first caudata g-type lysozyme in axolotl (Ambystoma mexicanum). Zoolog Sci 30:938–943
Zhang P, Sawicki V, Lewis A, Hanson L, Nuijens JH, Neville MC (2001) Human lactoferrin in the milk of transgenic mice increases intestinal growth in ten-day-old suckling neonates. Adv Exp Med Biol 501:107–113
Zhang R, Guo C, Sui S, Yu T, Wang J, Li N (2012) Comprehensive assessment of milk composition in transgenic cloned cattle. PLoS ONE 7:e49697
Zhou C, Wang JW, Huang KL, He X, Chen XP, Sun H, Yu T, Che HL (2011) A 90-day safety study in Sprague-Dawley rats fed milk powder containing recombinant human lactoferrin (rhLF) derived from transgenic cloned cattle. Drug Chem Toxicol 34:359–368
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cooper, C.A., Maga, E.A. & Murray, J.D. Production of human lactoferrin and lysozyme in the milk of transgenic dairy animals: past, present, and future. Transgenic Res 24, 605–614 (2015). https://doi.org/10.1007/s11248-015-9885-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-015-9885-5