Abstract
In order to gain further insight into the partly-characterized carotenoid biosynthetic pathway in corn (Zea mays L.), we cloned cDNAs encoding the enzymes carotenoid isomerase (CRTISO) and β-carotene hydroxylase (BCH) using endosperm mRNA isolated from inbred line B73. For both enzymes, two distinct cDNAs were identified mapping to different chromosomes. The two crtiso cDNAs (Zmcrtiso1 and Zmcrtiso2) mapped to unlinked genes each containing 12 introns, a feature conserved among all crtiso genes studied thus far. ZmCRTISO1 was able to convert tetra-cis prolycopene to all-trans lycopene but could not isomerize the 15-cis double bond of 9,15,9′-tri-cis-ζ-carotene. ZmCRTISO2 is inactivated by a premature termination codon in B73 corn, but importantly the mutation is absent in other corn cultivars and the active enzyme showed the same activity as ZmCRTISO1. The two bch cDNAs (Zmbch1 and Zmbch2) mapped to unlinked genes each coding sequences containing five introns. ZmBCH1 was able to convert β-carotene into β-cryptoxanthin and zeaxanthin, but ZmBCH2 was able to form β-cryptoxanthin alone and had a lower overall activity than ZmBCH1. All four genes were expressed during endosperm development, with mRNA levels rising in line with carotenoid accumulation (especially zeaxanthin and lutein) until 25 DAP. Thereafter, expression declined for three of the genes, with only Zmcrtiso2 mRNA levels maintained by 30 DAP. We discuss the impact of paralogs with different expression profiles and functions on the regulation of carotenoid synthesis in corn.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carotenoids are fat-soluble pigments synthesized by all plants and many microorganisms. In plants they are found on photosynthetic membranes where they participate in the light-harvesting reaction and protect the photosynthetic apparatus from photo-oxidation (reviewed by Bramley 2002). However, they are also precursors for the synthesis of abscisic acid (Creelman and Zeevart 1984) and strigolactones (Gomez-Roldan et al. 2008; Umehara et al. 2008), and they are the source of yellow, orange and red pigmentation in some flowers and fruits (Tanaka et al. 2008). In animals, carotenoids provide multiple health benefits (reviewed in Fraser and Bramley 2004), prompting scientists to explore ways to improve carotenoid content and composition in staple crops (reviewed in Sandmann et al. 2006; Howitt and Pogson 2006; Zhu et al. 2007; Giuliano et al. 2008; Fraser et al. 2009; Zhu et al. 2009).
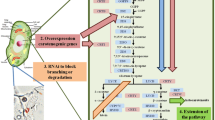
Plant carotenoid synthesis begins with the conversion of geranylgeranyl pyrophosphate into 15-cis phytoene by the enzyme phytoene synthase (PSY; Misawa et al. 1994; Fig. 1). A series of four desaturation reactions carried out by phytoene desaturase (PDS) and ζ-carotene desaturase (ZDS) then generates the carotenoid chromophore. The product of the first desaturase is 9,15,9′-tri-cis-ζ-carotene, which is isomerized by light (and perhaps an unknown enzyme; Li et al. 2007) to yield 9,9′-di-cis-ζ-carotene, the substrate of ZDS (Breitenbach and Sandmann 2005). The end product of the desaturation reactions is converted to all-trans lycopene by a carotenoid isomerase (CRTISO) in non-green tissue, and by light and chlorophyll (acting as a sensitizer) in green tissue (Isaacson et al. 2004; Breitenbach and Sandmann 2005). All-trans lycopene is then cyclized by lycopene ε-cyclase (LCYE) and lycopene β-cyclase (LCYB) to introduce ε- and β-ionone end groups and produce α- and β-carotene, respectively. The introduction of hydroxyl moieties into the cyclic end groups by β-carotene hydroxylase (BCH) and carotene ε-hydroxylase results in the formation of zeaxanthin from β-carotene and lutein from α-carotene (Sun et al. 1996; Bouvier et al. 1998; Tian et al. 2003). In some plant tissues, zeaxanthin can be epoxidized to violaxanthin by zeaxanthin epoxidase (ZEP) (reviewed by Cunningham and Gantt 1998).
The limited data concerning endogenous regulation of carotenogenic genes has made the precise engineering of crop plants to enhance carotenoid content and composition difficult (reviewed in Sandmann et al. 2006; Zhu et al. 2007; Fraser et al. 2009) despite recent progress in cereal crops, particularly corn (Harjes et al. 2008; Zhu et al. 2008; Aluru et al. 2008; Naqvi et al. 2009). The corn genome contains three paralogous psy genes (Li et al. 2008a, b) and PSY1 is the key rate-limiting enzyme in endosperm carotenoid biosynthesis (Buckner et al. 1996; Palaisa et al. 2003; Li et al. 2008a). The endosperm-specific expression of a corn psy1 transgene in white corn, which lacks endogenous PSY1 activity, increased the total endosperm carotenoid content >50-fold (Zhu et al. 2008). Six different corn paralogs encoding β-carotene hydroxylase (BCH) were recently identified through bioinformatics analysis in corn (Vallabhaneni et al. 2009). Two bch genes were reported to encode β-carotene hydroxylase (Vallabhaneni et al. 2009); two paralogs were pseudogenes, while the remaining two paralog functions remain unknown (Vallabhaneni et al. 2009). In contrast, PDS, ZDS, LCYB and LCYE are encoded by single-copy genes in corn (Li et al. 1996; Matthews et al. 2003; Singh et al. 2003; Harjes et al. 2008), and pds and zds at least do not appear to regulate endosperm carotenoid accumulation since the corresponding transcript levels remain constant during endosperm development (Li et al. 1996; Matthews et al. 2003). Here we report the isolation and characterization of corn crtiso and bch cDNAs, their developmental expression profiles and their functional characterization by complementation analysis in bacteria. The presence of small gene families for several carotenogenic genes in corn suggests that diverse regulatory strategies may be used to control the accumulation of carotenoids in endosperm tissue.
Materials and methods
Plant materials
Corn plants (Zea mays L.) representing lines B73, A632, EP42 (yellow corn) and M37W (white corn) were grown in the greenhouse and growth chamber at 28/20°C day/night temperature with a 10-h photoperiod and 60–90% relative humidity for the first 50 days, followed by maintenance at 21/18°C day/night temperature with a 16-h photoperiod thereafter. Plants were self-pollinated to obtain seeds. Mature leaf and endosperm tissue were frozen rapidly in liquid nitrogen and stored at −80°C. Endosperm tissues were dissected at five developmental stages [10, 15, 20, 25 and 30 days after pollination (DAP)].
Nucleic acid isolation and cDNA synthesis
Genomic DNA was extracted from 5 g of leaf tissue as described by Sambrook et al. (1989). Total RNA was isolated using the RNeasy® Plant Mini Kit (QIAGEN, Valencia, CA, USA) and DNA was removed with DNase I (RNase-Free DNase Set, QIAGEN, Valencia, CA, USA). Total RNA was quantified using a NANODROP 1000 spectrophotometer (Thermo Scientific, Vernon Hills, Illinois, USA), and 2 μg total RNA was used as template for first strand cDNA synthesis with Ominiscript Reverse Transcriptase in a 20 μl total reaction volume following the manufacturer’s recommendations (QIAGEN, Valencia, CA, USA).
Cloning and sequencing the corn crtiso and bch cDNAs
A partial corn crtiso cDNA was isolated by PCR using 1 μl cDNA prepared as above from 25 DAP corn endosperm tissue. The 50-μl reaction was carried out using the GoTaq® DNA Polymerase Kit (Promega, Madison, WI, USA) and synthetic degenerate oligonucleotide primers matching two conserved regions of the homologous Arabidopsis and tomato carotenoid isomerase (CRTISO) enzymes: CWKIFNS (forward primer 5′-TGY TGG AAR ATH TTY AAY IS-3′) and TYGPMPR (reverse primer 5′-CKI GGC ATI GGI CCR TAI GT-3′). The samples were heated to 95°C for 3 min, followed by 30 cycles at 94°C for 45 s, 55°C for 45 s and 72°C for 90 s. After the last amplification cycle, samples were incubated at 72°C for 10 min. The crtiso product was purified from a 1.0% w/v agarose gel using the Geneclean® II Kit (BIO® 101 Systems, Solon, OH, USA) and cloned in the PCR® II TOPO® vector (TA Cloning Kit, Invitrogen, Carlsbad, CA, USA) for sequencing using the Big Dye Terminator v3.1 Cycle Sequencing Kit on a 3130x1 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). The sequences were used to query EST databases and matches were used to design primers for full-length cDNA cloning. The EST sequences (DR812825 and EE176013) from B73 were found with high identities (100 and 98.7% in the overlapping part, respectively) to the 5′ and 3′-end of the partial Zmcrtiso sequence. Zmcrtiso was isolated using forward primer 5′-CAT GCC GCC GCT CGC CGC GCG CCT C-3′ based on 5′ EST sequence (accession number DR812825) and reverse primer 5′-CAG AAA GTT GAA GGG TAT CTC AA-3′ based on 3′ EST sequence (accession number EE176013). Utilizing the published maize genomic DNA, bacterial artificial chromosome (BAC) and expressed sequence tags (EST) sequences (MaizeGDB, http://www.maizegdb.org/), we identified a second and distinct crtiso gene (designated Zmcrtiso2) which has higher identity (86.1%) at the nucleotide level with the crtiso gene we report in this manuscript (designated Zmcrtiso1). The primer combination designed to amplify the full CRTISO protein coding Zmcrtiso2 mRNA sequence was designed based on the EST sequences in the MaizeGDB database. The primers were: forward, 5′-CTC CCG AGT CCC AAT CCA AAC GGC TTC ACT C-3′ based on EST sequence with GenBank accession number EE045563; reverse, 5′-AAT TAC ACT GTT TGG CAT ACC ATG TAA CTT GT-3′ based on EST sequence with GenBank accession number FL449103. PCR, cloning and sequencing was carried out using the components described above with initial heating to 95°C for 3 min followed by 35 cycles of 94°C for 45 s, 60°C for 45 s and 72°C for 3 min, followed by a final incubation at 72°C for 10 min.
Nested PCR was used to amplify the corn β-carotene hydroxylase 1 (Zmbch1) cDNA from endosperm tissue, based on the putative Zmbch1 cDNA sequences already deposited in GenBank from an unknown corn cultivar (accession number AY844956). The PCR was carried out in a 50-μl reaction volume using the components described above, but substituting forward primer 5′-CAT GGC CGC CGG TCT GTC CGG CGC CGC GAT-3′ (BCH1F1) and reverse primer 5′-TGA GCT GGT GGT TCA TAA CAT GTC TCT AC-3′ (BCH1R1) in the first reaction and forward primer 5′-AGA ATT CCA TGG CCG CCG GTC TGT CCG-3′ (BCH1F2) (the terminal EcoRI restriction site and start codon are underlined) and 5′-AGG ATC CGG ACG AAT CCA TCA GAT GGT C-3′ (BCH1R2) (the terminal BamHI restriction site is underlined) in the second reaction. The first reaction mix included 5% (v/v) dimethyl sulfoxide (DMSO), and 5 μl of the product was used to initiate the second reaction. Cloning and sequencing were carried out as above.
A similar nested PCR strategy was used to isolate the full-length Zmbch2 cDNA using primer combinations based on the corresponding GenBank sequence (accession number AY844956). For this cDNA, the forward primers were 5′-GGA GAC TCG AGG CCA CTC TGC CTT-3′ (BCH2F1) and 5′-GAA TTC CAT GGC CGC CGC GAT GAC CAG-3′ (BCH2F2) (terminal EcoRI restriction site and start codon are underlined) and the reverse primers were 5′-GCT AGA ACT CAT TTG GCA CAC TCT G-3′ (BCH2R1) and 5′-GGA TCC TAG AAC TCA TTT GGC ACA CTC-3′ (BCH2R2) (terminal BamHI restriction site is underlined). The PCR was carried out as above without DMSO in the reaction mix, and the products were cloned and sequenced as described.
Bioinformatic analysis
The Maize Genetics and Genomic Database (MaizeGDB, http://www.maizegdb.org/), the GRAMENE database (http://www.gramene.org/) and GenBank (http://blast.ncbi.nlm.nih.gov/Blast.cgi) were searched for homologous sequences using BLAST, and multiple sequence alignments were performed using ClustalW2 (http://www.ebi.ac.uk/clustalw/). Protein sequences were screened for chloroplast signal peptides using the ChloroP 1.1 Server at http://www.cbs.dtu.dk/services/ChloroP/ (Emanuelsson et al. 1999).
Construction of CRTISO and BCH expression vectors
Gene-specific primers, with terminal EcoRI and BamHI restriction sites (forward primer 5′-CGA ATT CCA TGC CGC CGC TCG CCG CGC GCC TC-3′ and reverse primer 5′-GGA TCC CTA TGC AAG TGT TCT CAA CCA TCT GAG TAG-3′) were used to amplify the full-length Zmcrtiso1 coding sequence, which was then inserted into the same sites in vector pUC8 to create pUC8-Zmcrtiso1. A similar strategy was used to create pUC8-Zmcrtiso2, with the primers in this case designed to incorporate terminal EcoRI and HindIII restriction sites (forward primer 5′-GAA TTC CAT GTT CGG CTT CTC CGA CAA G-3′ and reverse primer 5′-AAG CTT CTA TGC AAG TGT TCT CAG CCA T-3′). The stop codon in Zmcrtiso2 was changed to serine using a recombinant PCR strategy (Higuchi 1990) with forward P1 primer 5′-GAA TTC CAT GCC GCC GCT CGC CGC GCG CCT CT-3′ (initiation codon underlined) incorporating an EcoRI site, reverse P2 primer 5′-CGC GAC CGC CAC CGC CGC CGC CTT CTC CGA-3′ (modified stop codon underlined), forward P3 primer 5′-GGC GGG TTC AGG AGA GGC GCG CTG GCA TCG-3′ (modified stop codon underlined) and reverse P4 primer 5′-AAG CTT CTA TGC AAG TGT TCT CAG CCA TCT GAG-3′ incorporating a HindIII site (stop codon underlined). The P1/P2 and P3/P4 reactions were performed separately by heating to 95°C for 3 min followed by 30 cycles of 94°C for 45 s, 60°C for 45 s and 72°C for 90 s, and a 10-min incubation at 72°C. The products were recovered and used as templates for a subsequent PCR with primers P1 and P4, wherein the samples were heated to 95°C for 3 min, followed by one cycle of 94°C for 45 s, 60°C for 45, 72°C for 10 min and 94°C for 3 min, then 30 cycles of 94°C for 45 s, 60°C for 45 s and 72°C for 2 min, followed by a final 10-min incubation at 72°C. The mutagenized Zmcrtiso2 cDNA was cloned and sequenced as above to generate pUC8-Zmcrtiso2C.
pUC8-Zmbch1 and pUC8-Zmbch2 plasmids were constructed by digesting pCR-Zmbch1 and pCR-Zmbch2 with EcoRI and BamHI simultaneously, and subcloned as in frame fusion into a pUC8 vector also digested with the same restriction enzymes. They were then utilized for functional analysis of the corresponding genes.
Functional characterization of crtiso and bch cDNAs
Different carotenoid backgrounds were established in Escherichia coli for functional complementation experiments with the corn crtiso and bch cDNAs using a selection of plasmids containing carotenogenic genes. Plasmid pACCRT-EBP contained genes for geranylgeranyl pyrophosphate synthase (CRTE), phytoene synthase (CRTB) and a pds type phytoene desaturase from Synechococcus, allowing the formation of ζ-carotene (Linden et al. 1993). Plasmid pBBR1MCS2-zds contained a ζ-carotene desaturase gene (Breitenbach et al. 2001a). Plasmid pRKcrtY contained a lycopene cyclase gene (Schnurr et al. 1996). Plasmid pACCAR16ΔcrtX contained all the genes required to synthesize β-carotene (Misawa et al. 1990).
DNA and RNA blots
Leaf genomic DNA (20 μg) was digested separately with BamHI, EcoRI, EcoRV, HindIII and XbaI. The resulting fragments were separated by electrophoresis on a 0.8% (w/v) agarose gel and blotted onto a positively-charged nylon membrane (Roche, Mannheim, Germany) according to the manufacturer’s instructions. Nucleic acids were fixed by UV crosslinking. The transferred DNA fragments were hybridized with appropriate digoxigenin-labeled probes at 42°C overnight using DIG Easy Hyb buffer (Roche Diagnostics GmbH, Mannheim, Germany). The membrane was washed twice for 5 min in 2× SSC, 0.1% SDS at room temperature, twice for 20 min in 0.2× SSC, 0.1% SDS at 68°C, and then twice for 10 min in 0.1× SSC, 0.1% SDS at 68°C. After immunological detection with anti-DIG-AP (Fab-Fragments Diagnostics GmbH, Germany) chemiluminescence generated by disodium 3-(4-methoxyspiro {1,2-dioxetane-3,2′-(5′-chloro)tricyclo[3.3.1.13,7] decan}-4-yl) phenyl phosphate (CSPD) (Roche, Mannheim, Germany) was detected on Kodak BioMax light film (Sigma–Aldrich, St. Louis, USA) according to the manufacturer’s instructions. The primer combinations used to generate Zmcrtiso probe (394 bp) were: 5′-CAT GCC GCC GCT CGC CGC GCG CCT C-3′ and 5′-CTT GTC GGA GAA GCC GAA CAT GAC A-3′ using Zmcrtiso1 cDNA as a template. This Zmcrtiso1 DNA probe had 90.1% identity with Zmcrtiso2. The primer combinations for 3′ UTR specific probes were: 5′-GGA ATA CGA ATG GGT GTA CAG GTT-3′ and 5′-TGA AGG GTA TCT CAA AAC AGA ACT-3′ for Zmcrtiso1 (212 bp), 5′-GAG GCT GGG CTA GCA GCA TGC GGT-3′ and 5′-AAT TAC ACT GTT TGG CAT ACC ATG-3′ for Zmcrtiso2 (216 bp), 5′-TGG AAA AGG AGC TCG CGC GAA TCG-3′ and 5′-TGA GCT GGT GGT TCA TAA CAT GTC T-3′ for Zmbch1 (377 bp), and 5′-GCT TGT TAG CAG TCC GGT GAG TGA A-3′ and 5′-GAA AGG AAG ATG GCG ATA GAT GTA-3′ for Zmbch2 (251 bp).
Total RNA (30 μg) was fractionated on a denaturing 1.2% (w/v) agarose gel containing formaldehyde prior to blotting. The membrane was probed with digoxigenin-labeled partial cDNAs prepared as above using the PCR-DIG Probe Synthesis Kit (Roche, Mannheim, Germany), with hybridization carried out at 50°C overnight using DIG Easy Hyb. Washing and immunological detection and CSPD chemiluminescence were carried out as described above.
Carotenoid extraction and quantification
Carotenoids from freeze-dried E. coli co-transformants were extracted in darkness with acetone at 60°C for 20 min, partitioned into 10% ether in petrol (bp. 40–60°C) and analyzed by HPLC on a non-endcapped polymeric 3 μm C30 column (YMC Wilmington NC, USA) according to Sander et al. (1994). The mobile phase was methanol/methyl-tert-butyl ether/water (56:40:4, v/v/v) for 30 min followed by a change to 26:70:4 (v/v/v) at a flow rate of 1 ml/min (Breitenbach and Sandmann 2005). Spectra were recorded on-line with a Kontron 440 diode array detector. The sources of reference carotene isomers are described in previous publications (Breitenbach and Sandmann 2005; Breitenbach et al. 2001b). Carotenoids from freeze dried ground endosperm tissue were extracted in a similar manner, although the acetone was replaced with tetrahydrofuran/methanol (50:50, v/v) followed by acetone re-extraction. The HPLC system used for the corn carotenoids was a C18 Vydac 218TP54 column with 1% water in methanol as the mobile phase.
Quantitative real time PCR
Real-time PCR was performed on a BIO-RAD CFX96™ system using a 25-μl mixture containing 10 ng of synthesized cDNA, 1× iQ SYBR green supermix (BIO-RAD) and 0.2 mM forward and reverse primers for the target genes and the internal glyceraldehyde-3-phosphate dehydrogenase control (Zmgapdh) (Iskandar et al. 2004) as listed in Table 1. To calculate relative expression levels, serial dilutions (0.2–125 ng) were used to produce standard curves for each gene. PCRs were performed in triplicate using 96-well optical reaction plates, comprising a heating step for 3 min at 95°C, followed by 40 cycles of 95°C for 15 s, 58°C for 1 min and 72°C for 20 s. Amplification specificity was confirmed by melt curve analysis on the final PCR products in the temperature range 50–90°C with fluorescence acquired after each 0.5°C increment. The fluorescence threshold value and gene expression data were calculated using the CFX96™ system software. Values represent the mean of three real time PCR replicates ±SD.
Results
Cloning and characterization of the corn crtiso genes
Two different cDNAs (Zmcrtiso1 and Zmcrtiso2), encoding full-length carotenoid isomerase (CRTISO) enzymes, were amplified from 25-DAP corn endosperm mRNA (line B73) by RT-PCR. The sequences were deposited in GenBank with accession numbers FJ603466 and FJ765413, respectively. The full-length Zmcrtiso1 cDNA encoded a 587-amino-acid protein with a molecular weight of 63.7 kDa, a pI of 8.24, and a putative 43-residue transit peptide for chloroplast targeting (Fig. 2). The ZmCRTISO1 amino acid sequences showed 80.7% similarity and 74.6% identity to Arabidopsis CRTISO (Park et al. 2002), 78.7% similarity and 71.5% identity to tomato CRTISO (Isaacson et al. 2002), and 73.2% similarity and 58.8% identity to a cyanobacterial CRTISO (Breitenbach et al. 2001a; Masamoto et al. 2001; Fig. 2).
a Alignment of deduced amino acid sequences of the N- and C-terminal region encoded by the crtiso genes. In B73 ZmCRTISO2, the 48th amino acid is interrupted by a stop codon as marked with the asterisk. Consequently, the predicted protein of Zmcrtiso2 starts with the second methionine (M) marked in black as indicated by the arrow; it is truncated by 127 amino acid residues as compared to its M37W, A632 and EP42 paralogs. The putative ZmCRTISO1 protein possesses a 43-residue transit peptide for chloroplast targeting as marked by the arrow. ZmCRTISO1 (Zea mays L., accession no. FJ603466); ZmCRTISO2 (Zea mays L., accession no. FJ765413); AtCRTISO (Arabidopsis thaliana L., accession no. AC011001); LeCRTISO (Lycopersicon esculentum cv. M82, accession no. AF416727) and SyneCRTISO (Synechocystis sp. PCC 6803, gene sll0033). b Comparison of the nucleotide sequence regions around position 143 (the putative translation start codon of the Zmcrtiso2 cDNA is referred to as +1) of Zmcrtiso2 in different corn cultivars
The B73 Zmcrtiso2 cDNA contained a C-to-A transversion at position +143 (the putative translation start codon of the Zmcrtiso2 cDNA is referred to as +1) which was not present in homologous EST and cDNA sequences from other cultivars (accession nos. FL133727 and EU957482). The resulting nonsense mutation prematurely terminates protein synthesis and yields a truncated 127-amino-acid protein (Fig. 2). The presence of the mutation specifically in B73 corn genomic DNA was confirmed by PCR using the Zmcrtiso2 gene-specific primers 5′-CAC CGT CCT CCG CCC TCC ACT GCA ACT AGC-3′ and 5′-CAG CCG CAG AGC AGA GTA AAC TGT AGA GTA-3′, based on the sequence of a Zmcrtiso2 BAC clone (accession no. AC183901). Eight different clones were used to confirm the presence of the mutation specifically in B73 genomic DNA. Importantly, the mutation was not present in genomic DNA from M37W, A632 and EP42 corn (GenBank accession nos. GQ366381, GQ366382 and GQ366383, respectively; Fig. 2b). The truncated ZmCRTISO2 amino acid sequence showed 96.3% identity and 98.1% similarity to the corresponding region of ZmCRTISO1 (Fig. 2a).
The cDNA sequences were used to screen on-line corn genomic resources in order to identify the corresponding genes. Zmcrtiso1 was localized to chromosome 4 (accession no. AC205563) and Zmcrtiso2 to chromosome 2 (accession no. AC183901). Eleven introns were found in the available Zmcrtiso1 gene sequence with the coding region located 383–3,826 bp downstream from the transcriptional start site. Since the BAC clone containing Zmcrtiso1 did not overlap the first 392 bp of the cDNA, a pair of primers matching this sequence (5′-CAT GCC GCC GCT CGC CGC GCG CCT CCA-3′ and 5′-CAA CTG CTT CCA ATG CTT GTG TGA TCA-3′) was used to amplify the missing genomic DNA fragment. The 857-bp product was cloned and sequenced (accession no. FJ838766). The comparison of the amplified genomic DNA sequence, Zmcrtiso1 cDNA and the BAC clone (accession no. AC205563) showed that the Zmcrtiso1 gene contains 12 introns. The Zmcrtiso2 BAC clone (accession no. AC183901) spanned the entire gene, which also contained 12 introns.
Cloning and characterization of the corn bch genes
The sequence of the Zmbch1 cDNA from B73 endosperm predicted a 309-amino-acid protein with a molecular weight of 33.6 kDa, a pI of 10.92 and a 68-amino-acid transit peptide, suggesting the 241-amino-acid mature protein has a molecular weight of 26.5 kDa and a pI of 9.10 (accession no. GQ131287). The cloned Zmbch1 cDNA sequence has 98.9% identity at DNA level with β-carotene hydroxylase 4 cDNA (Vallabhaneni et al. 2009; accession number AY844956). The deduced amino acid sequence of ZmBCH1 shared 99.4% identity with hydroxylase 4 as reported by Vallabhaneni et al. 2009. These authors do not report the specific corn cultivar used to identify hydroxylase 4 (Vallabhaneni et al. 2009) consequently one cannot determine if our cloned gene (Zmbch1) is different from hydroxylase 4 because of a difference in cultivar used to clone the genes or different isozymes in B73. The sequence of the cloned Zmbch2 cDNA is the same as β-carotene hydroxylase 3 (Vallabhaneni et al. 2009; accession number AY844958). Zmbch2 encodes a predicted 319-amino-acid protein with a molecular weight of 34.6 kDa, a pI of 8.82 and a 69-residue transit peptide, yielding a mature 250-amino-acid protein with a molecular weight of 27.5 kDa and a pI of 6.67. The predicted amino acid sequences of ZmBCH1 and ZmBCH2 are aligned in Fig. 3. The N-terminal sequences are highly conserved, despite the presence of four small gaps in ZmBCH2, whereas the C-terminal sequences differ considerably, not least because of the presence of 29 additional residues in the ZmBCH2 sequence (Fig. 3). The screening of corn genomic resources allowed us to localize Zmbch1 to chromosome 2 (accession nos. AC196442) and Zmbch2 to chromosome 10 (accession no. AC194430). A comparison of the cDNAs and corresponding BAC clones showed that both genes were present in their entirety in the BAC clones and both coding sequences contained five introns. The full-length amino acid sequences are 76.6% identical, both containing four predicted transmembrane helices and one highly conserved region described by Sun et al. (1996) as “Motif 1”. The most striking features were the conserved histidine motifs, often found in iron-containing monooxygenases and fatty acid desaturases including β-carotene hydroxylases (Bouvier et al. 1998), which may act as iron-binding ligands (Shanklin et al. 1994). The four regions HX4H, HX2HH, HX4H and HX2HH are underlined in Fig. 3.
Alignment of deduced amino acid sequences of ZmBCH1 (Zea mays L., accession no. GQ131287) and ZmBCH2 (Zea mays L., accession no. AY844957). Four predicted transmembrance (TM) helices, one highly conserved region described by Sun et al. (1996) as “Motif 1” and the four regions HX4H, HX2HH, HX4H and HX2HH are underlined
Crtiso and bch gene copy numbers
Most plants studied thus far appear to contain a single gene encoding CRTISO (Park et al. 2002; Isaacson et al. 2002) so the presence of a small gene family in corn warranted further investigation. Southern blot analysis was carried out by digesting the corn genomic DNA with five different restriction enzymes, followed by hybridization under high stringency with a 394-bp Zmcrtiso1 probe lacking any of the enzyme sites. This Zmcrtiso1 DNA probe had 90.1% identity with Zmcrtiso2. At least three hybridizing bands were present in each lane under high stringency conditions (Fig. 4a), suggesting that a further crtiso gene was present in addition to the two we had already cloned. Based on Zmcrtiso1 and Zmcrtiso2 cDNA and genomic DNA sequences, two gene specific probes without intron DNA fragments were prepared as described in Materials and Methods. These two probes have just 43.3% identity at the nucleotide levels. There are two adjacent XbaI sites (791 bp) which cover Zmcrtiso1 probe DNA fragment in Zmcrtiso1 genomic DNA (accession number: AC205563). Thus, one 791 bp hybridizing band was detected in the XbaI digested genomic DNA for Zmcrtiso1 gene specific DNA hybridization (Fig. 4b). There are two adjacent EcoRV sites (5,330 bp), two adjacent HindIII sites (6,202 bp) and two adjacent XbaI sites (867 bp), which cover Zmcrtiso2 probe DNA fragment in Zmcrtiso2 genomic DNA (accession number: AC183901). However, at least two hybridization bands were detected for EcoRV, HindIII and XbaI digested genomic DNA for Zmcrtiso2 gene specific DNA hybridization (Fig. 4c). Thus we demonstrated that Zmcrtiso1 probably exists as a single copy whereas there are two copies of Zmcrtiso2 (Fig. 4b, c). A similar experiment using gene specific Zmbch1 and Zmbch2 probes detected at least two bands per lane for Zmbch1 and at least three for Zmbch2 (Fig. 4d, e), indicating there are two or more copies of Zmbch1 and three or more copies of Zmbch2 in the corn genome bringing the total of Zmbch genes in corn to 5 or more. The Zmbch1 and Zmbch2 probe nucleotide sequences were used to query Maize Genetics and Genomic Database (MaizeGDB, http://www.maizegdb.org/), respectively. Three different BAC clones (AC196442 located on chromosome 2, AC215681 on chromosome 4, and AC217349 on chromosome 6) in B73 matched the Zmbch1 probe nucleotide sequence (96–100% identities), while four distinct BAC clones (AC194430 on chromosome 10, AC205313 on chromosome 1, AC197362 on chromosome 8, and AC201889 on chromosome 7) in B73 matched the Zmbch2 probe nucleotide sequence (87–100% identities). Thus our data is consistent with the maize genome sequence.
DNA blot analysis of Zmcrtiso and Zmbch gene family in corn. Genomic DNA (20 μg) from mature leaves was separately digested with BamHI (B), EcoRI (RI), EcoRV (RV), HindIII (H) and XbaI (X). Five different blots were hybridized with the Zmcrtiso probe (a), Zmcrtiso1 (b), Zmcrtiso2 (c), Zmbch1 (d) and Zmbch2 (e) specific probes described in “Materials and methods”
Functional analysis of corn crtiso and bch
The activity of our cloned cDNAs was investigated by genetic complementation analysis in E. coli. For this purpose, Zmcrtiso1, Zmcrtiso2 and Zmcrtiso2C cDNAs, the latter with the premature stop codon corrected, were expressed in E. coli strains cotransformed with plasmids pACCRT-EBP and pBBR1MCS2-zds, allowing the synthesis of prolycopene as the substrate for CRTISO (Fig. 5A). The composition of ζ-carotene isomers is shown in trace A′. When functional CRTISO1 is present, all-trans lycopene and 5-cis lycopene are produced at the expense of prolycopene (Fig. 5B). The cis ζ-carotene composition remains unchanged following the introduction of Zmcrtiso1 (Fig. 5B′). Lycopene cyclase is unable to utilize prolycopene (Fig. 5C). However, in the presence of Zmcrtiso1, the correct all-trans lycopene isomer is formed, allowing cyclization (Fig. 5D). By complementation, we demonstrated that the B73 Zmcrtiso2 is non-functional (data not shown). However, when the nonsense mutation is corrected, the functional enzyme was able to convert prolycopene into all-trans lycopene (Fig. 5E). This indicates that Zmcrtiso2 encodes a functional isomerase in other corn cultivars. Figure 5F shows different lycopene isomers produced by CrtI phytoene desaturase.
Functional analyses of Zmcrtiso cDNAs. Formation of carotenes in E. coli transformed with different combinations of carotenogenic genes: A with plasmids pACCRT-EBP + pBBR1MCS2-zds establishing a cis-lycopene and a ζ-carotene (trace A′) background; B conditions as in A but with the B73 carotenoid isomerase cDNA crtiso1 in pUC8-Zmcrtiso1 (iso1); C cis-lycopene background as in A with an additional lycopene cylase gene in pRK-crtY; D conditions as in C plus pUC8-Zmcrtiso1 (iso1); E conditions as in A but with the corn carotenoid isomerase gene crtiso2C in pUC8-Zmcrtiso2C (iso2c); Trace F shows several lycopene isomers as standards produced by plasmid pACCRT-EBI with the crtI-type phytoene desaturase. Abbreviations: proL, prolycopene; N, neurosporene; L, lycopene; ζ, ζ-carotene; βZc, β-zeacarotene; βC, β-carotene; c, cis; t, trans
The Zmbch1 and Zmbch2 cDNAs were tested by complementation in E. coli strains accumulating β-carotene (Fig. 6A). In strains cotransformed with Zmbch1 cDNA, more than half of the β-carotene was converted into the monohydroxyl derivative β-cryptoxanthin (about 80% of the product) and the dihydroxyl derivative zeaxanthin (about 20% of the product; Fig. 6B). However, in strains cotransformed with Zmbch2 cDNA, only 4.3% of β-carotene was converted to β-cryptoxanthin and no zeaxanthin was formed (Fig. 6C).
Functional analyses of Zmbch1 and Zmbch2. (A) E. coli expressing pACCAR16ΔcrtX. (B) E. coli expressing pACCAR16ΔcrtX with additional plasmid pUC8-Zmbch1 (Bch1). C E. coli expressing pACCAR16ΔcrtX with additional plasmid pUC8-Zmbch2 (Bch2). Abbreviations: bCar, β-carotene; Zeax, zeaxanthin; bCry, β-cryptoxanthin; bCEp, β-carotene epoxide
Carotenoid accumulation and gene expression during endosperm development
The profile of carotenoid accumulation in developing B73 corn endosperm was assessed up to 30 DAP (Table 2). Yellow corn endosperm contains β-carotene, its monohydroxylated product β-cryptoxanthin and its dihydroxylated product zeaxanthin. Although α-carotene could not be detected, its hydroxylated products α-cryptoxanthin and lutein were present at detectable levels. A steady increase in total carotenoid content was observed during kernel maturation, although the levels of lutein, zeaxanthin and α-cryptoxanthin increased, β-carotene and β-cryptoxanthin levels decreased from 25 to 30 DAP.
In order to understand how carotenoid accumulation is regulated during corn endosperm development, northern blots containing B73 endosperm mRNA from different developmental stages were hybridized with Zmcrtiso1, Zmcrtiso2, Zmbch1 and Zmbch2 gene-specific DNA probes. The signals for Zmcrtiso1, Zmcrtiso2 and Zmbch1 were below the detection threshold regardless of the probe used (data not shown). The steady-state levels of Zmbch2 mRNA increased in the endosperm between 10 and 15 DAP then remained constant until 25 DAP, then declined at 30 DAP, but no transcripts could be detected in leaves (Fig. 7a). To increase sensitivity, expression profiles were monitored by quantitative real time PCR (Fig. 7b) which showed that the transcripts for all four genes increased throughout endosperm development to 25 DAP. Thereafter, the level of Zmcrtiso2 mRNA remained constant until 30 DAP whereas the levels decreased for the other three genes.
a mRNA blot analysis of Zmbch2 transcript in leaves and corn endosperms. Each lane was loaded with 30 μg of total RNA. rRNA stained with ethidium bromide is shown as a control for loading of equal amounts of RNA. b Transcript levels for Zmcrtiso1, Zmcrtiso2, Zmbch1 and Zmbch2, from greenhouse grown B73 corn harvested at 10, 15, 20, 25 and 30 DAP (days after pollination). Values are a mean of three quantitative real time PCR replicates with ±SD. Abbreviations: Zm, Zea mays; crtiso1, carotenoid isomerase 1 gene; crtiso2, carotenoid isomerase 2 gene; bch1, β-carotene hydroxylase 1 gene; bch2, β-carotene hydroxylase 2 gene
Discussion
Carotenoids are nutritionally valuable compounds that provide a range of health benefits including protection against cancer and other chronic diseases (review by Fraser and Bramley 2004). Humans cannot synthesize carotenoids and must obtain them from their diet, notably from fresh fruit and vegetables and seafood. However, since many people, particularly those in developing countries, subsist on a monotonous diet of staple cereal grains, there has been much interest in the development of strategies to improve carotenoid levels and composition in staple crops (Zhu et al. 2007; 2009). One significant hurdle to the improvement of carotenoid levels in cereal grains is the limited understanding of how carotenoid synthesis is normally regulated, particularly in the endosperm, which is the most nutritious component of the grain. A number of recent studies have shown the potential for carotenoid enhancement in corn endosperm, either through conventional breeding or transgenic strategies (Harjes et al. 2008; Zhu et al. 2008; Aluru et al. 2008; Naqvi et al. 2009). Despite this progress, much remains to be learned about the carotenoid synthesis pathway in corn endosperm and the regulatory mechanisms that control the accumulation of specific carotenoid molecules.
The first step towards understanding how carotenoids are synthesized is to identify the enzymes involved and isolate the corresponding genes. Several corn cDNAs encoding carotenogenic enzymes have already been cloned and identified including psy1, psy2 and psy3 (phytoene synthase), pds (phytoene desaturase), zds (ζ-carotene desaturase), lcyb (lycopene β-cyclase) and lcye (lycopene ε-cyclase) (Buckner et al. 1996; Gallagher et al. 2004; Li et al. 2008a; Li et al. 1996; Matthews et al. 2003; Singh et al. 2003; Bai et al. 2009). We have cloned and characterized four additional cDNAs, two representing the enzyme carotenoid isomerase (CRTISO) and two representing non-heme di-iron monooxygenase β-carotene hydroxylase (BCH).
The two crtiso cDNAs (Zmcrtiso1 and Zmcrtiso2) mapped to genes on different chromosomes and the predicted amino acid sequences showed >96% identity to each other, as well as >70% identity to the single CRTISO enzymes in Arabidopsis and tomato (Park et al. 2002; Isaacson et al. 2002). The corn genes, like the Arabidopsis and tomato genes, contained 12 introns, and a candidate crtiso gene with 12 introns was also identified in the rice genome (accession no. AC108871). Similarly, the two bch cDNAs (Zmbch1 and Zmbch2) mapped to different chromosomes but encoded very similar proteins (76.6% identity, with highly conserved motifs typical for an iron-containing monooxygenase). However, in this case, the presence of two distinct genes reflected the situation in other plants such as Arabidopsis (Sun et al. 1996; Tian and DellaPenna 2001), pepper (Bouvier et al. 1998), citrus (Kim et al. 2001), saffron (Castillo et al. 2005) and tomato (Galpaz et al. 2006), where two bch genes are also present.
The presence of multiple isoenzymes in plant metabolic pathways is a common phenomenon which often reflects the requirement for the same catalytic activity in different subcellular compartments, often the plastids and cytosol (Gottlieb 1982). However, both the crtiso and bch cDNAs appear to encode proteins with the transit peptide sequences, suggesting the isoenzymes are destined for the plastids. Another source of isoenzymes in diploid plants such as corn is the random duplication of chromosome segments followed by the functional diversification of the duplicated genes. We therefore sought to determine whether the two cDNAs encoding each enzyme were differentially expressed or whether the enzymes themselves were functionally distinct.
We looked at the expression of all four cDNAs during endosperm development using a combination of mRNA blot and quantitative real-time RT–PCR. The latter was necessary because only Zmbch2 was expressed at a high enough level to be detected by mRNA blot. Real-time PCR showed that the two crtiso mRNAs were differentially expressed. Zmcrtiso1 increased during endosperm development to 25 DAP before dropping off, whereas Zmcrtiso2 followed the same profile until 25 DAP but thereafter remained at a constant level. Although this suggests a distinct role for Zmcrtiso2 in later endosperm development, the two enzymes appear to have identical activities when in their functional forms, both isomerizing prolycopene into all-trans lycopene and providing the correct isomer for the subsequent cyclization step forming either a β- or ε-ionone ring. Since the composition of ζ-carotene isomers is almost unchanged (Fig. 5A′, B′) when the CRTISO enzymes are expressed, additional ζ-carotene isomerase activity can be excluded, although this may be the function of the corn y9 allele (Li et al. 2007). Interestingly, the Zmcrtiso2 cDNA from inbred corn line B73 contained a nonsense mutation in the first exon converting it into a mutated gene which is not present in M37W (accession no. GQ366381), A632 (accession no. GQ366382), EP42 (GQ366383) and other unknown corn lines (accession nos. FL133727, EU957482). Translation can initiate from a subsequent methionine codon resulting in a truncated, non-functional protein. It also lacks a transit sequence for plastid targeting. Therefore, ZmCRTISO2 does not contribute to carotenogenesis in the inbred line B37, and from this we can conclude that CRTISO is not a limiting step in carotenogenesis otherwise the pathway would be restricted at the level of prolycopene isomerization once the expression of ZmCRTISO1 dwindles during late endosperm development.
The two bch mRNAs were also differentially expressed, with Zmbch2 alone detectable by mRNA blots. This showed that the mRNA was restricted to endosperm and below the limit of detection in leaves (Fig. 7a), unlike other carotenogenic genes in corn which are thought to be expressed constitutively (Vallabhaneni and Wurtzel 2009). The steady state Zmbch2 mRNA levels in endosperm were significantly higher than in leaves (Fig. 7a), a finding which is in disagreement with the report by Vallabhaneni et al. 2009 claiming similar mRNA levels in leaves and endosperm at 10 and 30 DAP (Vallabhaneni et al. 2009). The expression of both Zmbch1 and Zmbch2 increased during endosperm development, suggesting that the expression profiles in the endosperm were concordant. The Zmbch2 was preferentially expressed in amyloplast-containing endosperm rather than chloroplast-containing leaves (Fig. 7a), similar to the situation in Arabidopsis where the Atbch2 gene is induced rapidly and strongly during seed development. This suggests that AtBCH2 is preferentially involved in xanthophyll synthesis in seeds (Kim et al. 2009). One of the two bch genes in bell pepper, tomato and saffron is also preferentially expressed in flowers or during fruit development (Bouvier et al. 1998; Castillo et al. 2005; Galpaz et al. 2006). In tomato, a bch2 mutant results in a colorless petal phenotype with no impact on xanthophyll synthesis in leaves (Galpaz et al. 2006), and the massive accumulation of xanthophylls during stigma maturation in saffron correlates with high expression of a single bch gene (Castillo et al. 2005). Although showing concordant expression profiles in endosperm, the activity of the two BCH isoenzymes is distinct. In bacteria producing β-carotene and expressing the Zmbch1 cDNA, more than half of the β-carotene was converted into downstream products, approximately 80% β-cryptoxanthin and 20% zeaxanthin. In contrast, similar bacteria expressing Zmbch2 cDNA were able to convert less than 5% of the available β-carotene and only β-cryptoxanthin was produced. This functional difference might indicate that the two genes are diverging to fulfill slightly different roles in carotenoid biosynthesis.
Any hypothesis addressing the roles of isoenzymes in carotenoid biosynthesis must look at the expression profiles and enzyme activities in the context of carotenogenesis during seed development. Previous studies have shown that total carotenoid levels increase steadily up to 30 DAP coincident with the upregulation of psy1, while pds and zds transcripts remain at a constant level (Li et al. 1996; Matthews et al. 2003; Li et al. 2008a; Vallabhaneni and Wurtzel 2009). The coordinated upregulation of Zmcrtiso1, Zmcrtiso2, Zmbch1 and Zmbch2 until 25 DAP is consistent with the observed accumulation of carotenoids, although only Zmcrtiso2 remains at high levels for the next 5 days while the carotenoid content continues to increase. The expression of lcye follows a similar profile (Vallabhaneni and Wurtzel 2009). It is apparent that PSY1 is the rate-limiting enzyme of the carotenoid biosynthetic pathway (Li et al. 2008b), but the impact of increasing Zmcrtiso1, Zmcrtiso2, Zmbch1 and Zmbch2 expression over the same timescale is to gradually increase the availability of the β-branch of the pathway. The only remaining carotenogenic gene in corn lacking expression data is carotene ε-ring hydroxylase, catalyzing the 3-hydroxylation of the ε-ionone ring in the synthesis of lutein (Fig. 1). When we compare carotenoid accumulation during seed development (Table 2) with bch1 and bch2 transcript kinetics (Fig. 7b), we can predict that ε-ionone ring carotene hydroxylase will demonstrate similar transcript kinetics. This can be concluded from a proportional increase of zeaxanthin and lutein over the whole period of seed development and the equal participation of β-carotene hydroxylase and the ε-ionone ring carotene hydroxylase during formation of lutein from α-carotene (Fig. 1).
In conclusion, we have cloned and characterized four cDNAs encoding corn carotenogenic enzymes, two encoding CRTISO and two encoding BCH. The enzymes are highly conserved in sequence, expression and activity, but subtle differences in the expression profiles of the CRTISO enzymes and the expression and activities of the BCH enzymes hint at divergent roles in plant carotenoid biosynthesis that may be useful in the development of more refined strategies to engineer carotenoid synthesis and composition in staple crops.
References
Aluru M, Xu Y, Guo R, Wang Z, Li S, White W, Wang K, Rodermel S (2008) Generation of transgenic maize with enhanced provitamin A content. J Exp Bot 59:3551–3562
Bai L, Kim EH, DellaPenna D, Brunell TP (2009) Novel lycopene epsilon cyclase activities in maize revealed through perturbation of carotenoid biosynthesis. Plant J 59:588–599
Bouvier F, Keller Y, d’Harlingue A, Camara B (1998) Xanthophyll biosynthesis: molecular and functional characterization of carotenoid hydroxylases from pepper fruits (Capsicum annuum L.). Biochim Biophys Acta 1391:320–328
Bramley PM (2002) Regulation of carotenoid formation during tomato fruit ripening and development. J Exp Bot 53:2107–2113
Breitenbach J, Sandmann G (2005) ζ-Carotene cis isomers as products and substrates in the plant poly-cis carotenoid biosynthetic pathway to lycopene. Planta 220:785–793
Breitenbach J, Vioque A, Sandmann G (2001a) Gene sll0033 from Synechocystis 6803 encodes a carotene isomerase involved in the biosynthesis of all-E lycopene. Z Naturforsch 56C:915–917
Breitenbach J, Braun G, Steiger S, Sandmann G (2001b) Chromatographic performance on a C30-bonded stationary phase of mono hydroxycarotenoids with variable chain length or degree of desaturation and of lycopene isomers synthesized by different carotene desaturases. J Chromatogr A 936:59–69
Buckner B, Miguel PS, Janick-Buckner D, Bennetzen JL (1996) The y1 gene of maize codes for phytoene synthase. Genetics 143:479–488
Castillo R, Fernandez JA, Gomez-Gomez L (2005) Implications of carotenoid biosynthetic genes in apocarotenoid formation during the stigma development of Crous sativa and its closer relatives. Plant Physiol 139:674–689
Creelman RA, Zeevart JA (1984) Incorporation of oxygen into abscisic acid and phaseic acid from molecular oxygen. Plant Physiol 75:166–169
Cunningham FX, Gantt E (1998) Genes and enzymes of carotenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 49:557–583
Emanuelsson O, Nielsen H, von Heijne G (1999) ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Protein Sci 8:978–984
Fraser PD, Bramley PM (2004) The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res 43:228–265
Fraser PD, Enfiss EMA, Bramley PM (2009) Genetic engineering of carotenoid formation in tomato fruit and the potential application of systems and synthetic biology approaches. Arch Biochem Biophys 483:196–204
Gallagher CE, Matthews PD, Li F, Wurtzel ET (2004) Gene duplication in the carotenoid biosynthetic pathway preceded evolution of the grasses. Plant Physiol 135:1776–1783
Galpaz N, Ronen G, Khalfa Z, Zamir D, Hirschberg J (2006) A chromoplast-specific carotenoid biosynthesis pathway is revealed by cloning of the tomato white-flower locus. Plant Cell 18:1947–1960
Giuliano G, Tavazza R, Diretto G, Beyer P, Taylor MA (2008) Metabolic engineering of carotenoid biosynthesis in plants. Trends Biotechnol 26:139–145
Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, Bouwmeester H, Becard G, Beveridge CA, Rameau C, Rochange SF (2008) Strigolactone inhibition of shoot branching. Nature 455:189–194
Gottlieb LD (1982) Conservation and duplication of isozymes in plants. Science 216:373–380
Harjes CE, Rocheford TR, Bai L, Brutnell TP, Kandianis CB, Sowinski SG, Stapleton AE, Vallabhaneni R, Williams M, Wurtzel ET, Yan J, Buckler ES (2008) Natural genetic variation in lycopene epsilon cyclase tapped for maize biofortification. Science 319:330–333
Higuchi R (1990) Recombinant PCR. In: PCR protocols. Academic Press, New York
Howitt CA, Pogson BJ (2006) Carotenoid accumulation and function in seeds and non-green tissues. Plant Cell Environ 29:435–445
Isaacson T, Ronen G, Zamir D, Hirschberg J (2002) Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of β-carotene and xanthophylls in plants. Plant Cell 14:333–342
Isaacson T, Ohad I, Beyer P, Hirschberg J (2004) Analysis in vitro of the enzyme CRTISO establishes a poly-cis-carotenoid biosynthesis pathway in plants. Plant Physiol 136:4246–4255
Iskandar HM, Simpson RS, Casu RE, Bonneti GD, Maclean DJ, Manners JM (2004) Comparison of reference genes for quantitative real-time polymerase chain reaction analysis of gene expression in sugarcane. Plant Mol Biol Rep 22:325–337
Kim IJ, Ko KC, Kim CS, Chung WI (2001) Isolation and characterization of cDNAs encoding β-carotene hydroxylase in Citrus. Plant Sci 161:1005–1010
Kim J, Smith JJ, Tian L, DellaPenna D (2009) The evolution and function of carotenoid hydroxylases in Arabidopsis. Plant Cell Physiol 50:463–479
Li ZH, Matthews PD, Burr B, Wurtzel ET (1996) Cloning and characterization of a maize cDNA encoding phytoene desaturase, an enzyme of the carotenoid biosynthetic pathway. Plant Mol Biol 30:269–279
Li F, Murillo C, Wurtzel ET (2007) Maize Y9 encodes a product essential for 15-cis-ζ-carotene isomerization. Plant Physiol 144:1181–1189
Li F, Vallabhaneni R, Wurtzel ET (2008a) PSY3, a new member of the phytoene synthase gene family conserved in the poaceae and regulator of abiotic stress-induced root carotenogenesis. Plant Physiol 146:1333–1345
Li F, Vallabhaneni R, Rocheford T, Wurtzel ET (2008b) The maize phytoene synthase gene family: overlapping roles for carotenogenesis in endosperm, photomorphogenesis, and thermal stress tolerance. Plant Physiol 147:1334–1346
Linden H, Vioque A, Sandmann G (1993) Isolation of a carotenoid biosynthesis gene coding for a zeta-carotene desaturase from Anabaena PCC7120 by heterologous complementation. FEMS Microbiol Lett 106:99–104
Masamoto K, Wada H, Kaneko T, Takaichi S (2001) Identification of a gene required for cis-to-trans carotene isomerization in carotenogenesis of the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol 42:1398–1402
Matthews PD, Luo R, Wurtzel ET (2003) Maize phytoene desaturase and zetacarotene desaturase catalyze a poly-Z desaturation pathway: implication for genetic engineering of carotenoid content among cereal crops. J Exp Bot 54:2215–2230
Misawa N, Nakagawa M, Kobayashi K, Yamano S, Nakamura K, Harashima K (1990) Elucidation of the Erwinia uredovora carotenoid biosynthetic pathway by functional analysis of gene products expressed in Escherichia coli. J Bacteriol 172:6704–6712
Misawa N, Truesdale MR, Sandmann G, Fraser PD, Bird C, Schuch W, Bramley PM (1994) Expression of a tomato cDNA coding for phytoene synthase in Escherichia coli, phytoene formation in vivo and in vitro, and functional analysis of the various truncated gene products. J Biochem 116:980–985
Naqvi S, Zhu C, Farre G, Ramessar K, Bassie L, Breitenbach J, Perez Conesa D, Ros G, Sandmann G, Capell T, Christou P (2009) Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways. Proc Natl Acad Sci USA 106:7762–7767
Palaisa KA, Morgante M, Williams M, Rafalski A (2003) Contrasting effects of selection on sequence diversity and linkage disequilibrium at two phytoene synthase loci. Plant Cell 15:1795–1806
Park H, Kreunen SS, Cuttriss AJ, DellaPenna D, Pogson BJ (2002) Identification of the carotenoid isomerase provides insight into carotenoid biosynthesis, prolamellar body formation, and photomorphogenesis. Plant Cell 14:321–332
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Plainview
Sander LC, Sharpless KE, Craft NE, Wise SA (1994) Development of engineered stationary phases for the separation of carotenoid isomers. Anal Chem 66:1667–1674
Sandmann G, Romer S, Fraser PD (2006) Understanding carotenoid metabolism as a necessity for genetic engineering of crop plants. Metab Eng 8:291–302
Schnurr G, Misawa N, Sandmann G (1996) Expression, purification and properties of lycopene cyclase from Erwinia uredovora. Biochem J 315:869–874
Shanklin J, Whittle E, Fox BG (1994) Eight histidine residues are catalytically essential in a membrane-associated iron enzyme, stearoyl-CoA desaturase, and are conserved in alkane hydroxylase and xylene monooxygenase. Biochemistry 33:12787–12794
Singh M, Lewis PE, Hardeman K, Bai L, Rose JKC, Mazourek M, Chomet P, Brutnell TP (2003) Activator mutagenesis of the Pink scutellum1/viviparous7 locus of maize. Plant Cell 15:874–884
Sun Z, Gantt E, Cunningham FX (1996) Cloning and functional analysis of the β-carotene hydroxylase of Arabidopsis thaliana. J Biol Chem 271:24349–24352
Tanaka Y, Sasaki N, Ohmiya A (2008) Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. Plant J 54:733–749
Tian L, DellaPenna D (2001) Characterization of a second carotenoid β-hydroxylase gene from Arabidopsis and its relationship to the LUT1 locus. Plant Mol Biol 47:379–388
Tian L, Musetti V, Kim J, Magallanes-Lundback M, DellaPenna D (2003) Functional analysis of β- and ε-ring carotenoid hydroxylases in Arabidopsis. Plant Cell 15:1320–1332
Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, Kyozuka J, Yamaguchi S (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455:195–200
Vallabhaneni R, Wurtzel ET (2009) Timing and biosynthetic potential for provitamin A accumulation in maize. Plant Physiol 150:562–572
Vallabhaneni R, Gallagher CE, Licciardello N, Cuttriss AJ, Quinlan RF, Wurtzel ET (2009) Metabolites sorting of a germplasm collection reveals the Hydroxylases3 locus as a new target for maize provitamin A biofortification. Plant Physiol 151:1635–1645
Zhu C, Naqvi S, Gomez-Galera S, Pelacho AM, Capell T, Christou P (2007) Transgenic strategies for the nutritional enhancement of plants. Trends Plant Sci 12:548–556
Zhu C, Naqvi S, Breitenbach J, Sandmann G, Christou P, Capell T (2008) Combinatorial genetic transformation generates a library of metabolic phenotypes for the carotenoid pathway in maize. Proc Natl Acad Sci USA 105:18232–18237
Zhu C, Naqvi S, Capell T, Christou P (2009) Metabolic engineering of ketocarotenoid biosynthesis in higher plants. Arch Biochem Biophys 483:182–190
Acknowledgments
This work was supported by grants from the Spanish Ministry of Science and Innovation (MICINN) (BFU2007-61413), European Research Council Advanced Grant (BIOFORCE) to PC, Aciones complementarias, BIO2007-30738-E MICINN, Spain, a grant from the National Natural Science Foundation of China (grant no. 30870222) and the Foundation from Science and Technology Agency of Jilin Province (grant no. 20050543). G.F. and S.N. were supported by MICINN PhD fellowships. We would like to thank Dr. Ana Ana Butrón and Amando Ordás in Misión Biológica de Galicia, Consejo Superior de Investigaciones Cientificas, Apartado 28, 36080 Pontevedra, Spain, for supplying corn seeds.
Author information
Authors and Affiliations
Corresponding author
Additional information
Qunrui Li and Gemma Farre contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, Q., Farre, G., Naqvi, S. et al. Cloning and functional characterization of the maize carotenoid isomerase and β-carotene hydroxylase genes and their regulation during endosperm maturation. Transgenic Res 19, 1053–1068 (2010). https://doi.org/10.1007/s11248-010-9381-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-010-9381-x