Abstract
Papaya production is seriously limited by Papaya ringspot virus (PRSV) worldwide and Papaya leaf-distortion mosaic virus (PLDMV) in Eastern Asia. An efficient transformation method for developing papaya lines with transgenic resistance to these viruses and commercially desirable traits, such as hermaphroditism, is crucial to shorten the breeding program for this fruit crop. In this investigation, an untranslatable chimeric construct pYP08 containing truncated PRSV coat protein (CP) and PLDMV CP genes coupled with the 3′ untranslational region of PLDMV, was generated. Root segments from different portions of adventitious roots of in vitro multiple shoots of hermaphroditic plants of papaya cultivars ‘Tainung No. 2’, ‘Sunrise’, and ‘Thailand’ were cultured on induction medium for regeneration into somatic embryos. The highest frequency of somatic embryogenesis was from the root-tip segments of adventitious roots developed 2–4 weeks after rooting in perlite medium. After proliferation, embryogenic tissues derived from somatic embryos were wounded in liquid-phase by carborundum and transformed by Agrobacterium carrying pYP08. Similarly, another construct pBG-PLDMVstop containing untranslatable CP gene of PLDMV was also transferred to ‘Sunrise’ and ‘Thailand’, the parental cultivars of ‘Tainung No. 2’. Among 107 transgenic lines regenerated from 349 root-tip segments, nine lines of Tainung No. 2 carrying YP08 were highly resistant to PRSV and PLDMV, and 9 lines (8 ‘Sunrise’ and 1 ‘Thailand’) carrying PLDMV CP highly resistant to PLDMV, by a mechanism of post-transcriptional gene silencing. The hermaphroditic characteristics of the transgenic lines were confirmed by PCR with sex-linked primers and phenotypes of flower and fruit. Our approach has generated transgenic resistance to both PRSV and PLDMV with commercially desirable characters and can significantly shorten the time-consuming breeding programs for the generation of elite cultivars of papaya hybrids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Effective gene transfer systems require reliable and efficient protocols for plant regeneration from transformed cells. Although plant regeneration from various explants of papaya (Carica papaya L.), such as cotyledon (Litz et al. 1983), root (Chen et al. 1987; Mondal et al. 1994), protoplast (Chen and Chen 1992), anther (Tsay and Su 1985) and ovule (Litz and Conover 1982) have been attempted, regeneration of transformed cells from these explants into transgenic plants has not been accomplished.
Through the effective somatic embryogenesis from immature zygotic embryos of papaya (Fitch and Manshardt 1990), the coat protein (CP) gene of Papaya ringspot virus (PRSV) HA 5-1 can be incorporated into papaya via microprojectile bombardment to generate transgenic plants resistant to PRSV (Fitch et al. 1990) under glasshouse (Fitch et al. 1992) and field conditions (Lius et al. 1997). The subsequently developed papaya varieties Rainbow and SunUp represent the first commercialized transgenic fruit crop (Tennant et al. 2001; Tripathi et al. 2007). PRSV-resistant transgenic papaya have also been generated via microprojectile bombardment using embryogenic tissues derived from immature zygotic embryos, in Australia (Lines et al. 2002), Jamaica and Brazil (Gonsalves and Fermin 2004). Moreover, the same approach has been used to produce Phytophthora palmivora-resistant transgenic papaya (Zhu et al. 2004).
Transformation of papaya by Agrobacterium was first performed using explants of embryogenic calli and somatic embryos derived from hypocotyl sections (Fitch 1993) or immature zygotic embryos, with long regeneration process (13 months) and low transformation rates (Fitch et al. 1993). Although the regeneration from petiole explants via an intervening callus stage is time-consuming (Yang and Ye 1992), transgenic plantlets can be regenerated after co-cultivation of petioles with Agrobacterium (Yang et al. 1996). However, the long regeneration period (10–11 months) and the high rates of abnormal morphology limit the application of this method. Using an efficient transformation mediated by Agrobacterium following the liquid-phase wounding treatment of embryogenic tissues derived from premature zygotic embryos (Cheng et al. 1996), transgenic lines carrying PRSV YK (a Taiwan strain) CP gene conferring broad-spectrum resistance to PRSV strains of different geographic regions were generated (Bau et al. 2003). By Agrobacterium-mediated transformation, transgenic papaya lines resistant to PRSV have also been generated using immature zygotic embryos (Fermin et al. 2004; Davis and Ying 2004) and somatic embryos derived from hypocotyl or root of seedlings with unknown sex (Chen et al. 2001).
Embryogenic tissues derived from premature zygotic embryos are considered the most effective explants for both biolistic delivery and Agrobacterium transformation. However, immature zygotic embryos are difficult to obtain and often affected by seasonal factors. Because of the polygamous nature of papaya (i.e., male, female and hermaphrodite), the sex types and other horticultural traits of transgenic papaya lines can be determined only after flowering and fruit production. In most world markets, fruits from hermaphroditic plants are commercially desirable, for they contain less seeds and thicker flesh (Yeh et al. 2007). The breeding program required for incorporating the desired sex and other useful traits into a commercial hybrid cultivar of papaya is complicated and lengthy. Transformation using somatic embryos derived from hypocotyl or root of seedlings also faces the same problem.
Papaya production has been seriously limited by PRSV (Purcifull et al. 1984) worldwide and by Papaya leaf-distortion mosaic virus (PLDMV) in Eastern Asia (Bau et al. 2008; Maoka et al. 1996; Maoka and Hataya 2005). An isolate of potyvirus designated PLDMV P-TW-WF was isolated from an infected YK-CP transgenic plant in central Taiwan, and was identified as a strain of a different potyvirus PLDMV, which is serologically distinct from PRSV (Bau et al. 2008). PLDMV was first recorded in the northern part of Okinawa Island, Japan, in 1954 (Yonaha et al. 1976), and spread throughout the island during 1960s (Kawano and Yonaha 1992). The disease caused by PLDMV has been a major constraint to papaya production in Okinawa, Japan (Maoka et al. 1996). The CP genes of the P-TW-WF isolate from Taiwan and the P56 isolate of Okinawa (Maoka et al. 1996) share 95.1% nucleotide and 94.9% amino acid identities. Susceptibility of all PRSV-resistant transgenic papaya lines to PLDMV indicates that the virus is an emerging threat for the application of PRSV-resistant transgenic papaya in Taiwan and elsewhere (Bau et al. 2008).
For the effective control of PRSV and PLDMV, an untranslatable chimeric construct containing truncated PRSV YK CP and PLDMV P-TW-WF CP genes has been transferred into papaya (Carica papaya cv. ‘Thailand’) by Agrobacterium-mediated transformation via embryogenic tissues derived from immature zygotic embryos of papaya (Kung et al. 2009). However, this attempt ended up in the production of resistant lines (R0) displaying female sex, on attaining flowering stage, after being cultivated under greenhouse conditions for 6 months. Similar results were observed for previously obtained PRSV-YK CP transgenic papaya lines (Bau et al. 2003) and line 55-1 (Fitch et al. 1992). This may be due to the long exposure to the regulator 2,4-dichlorophenoxyacetic acid (2,4-D) during regeneration (Bau et al. 2003). For breeding purpose, these lines have to be crossed with hermaphrodite plants and then selfed to fix the resistance. Since papaya is a fruit crop, these processes take years. Somatic embryos can be induced from adventitious roots of in vitro shoots derived from selective fruit-bearing hermaphroditic papaya plants of cv. ‘Tainung No. 2’ (Lin and Yang 2001). In order to shorten the time-consuming breeding program for the development of transgenic papaya lines with virus resistance and commercially desirable traits, a novel regeneration process from desired hermaphrodite plants coupled with an efficient Agrobacterium-mediated transformation protocol become crucial.
In this investigation, the somatic embryos derived from the adventitious roots of in vitro shoots of selected hermaphroditic ‘Tainung No. 2’ individuals, the most popular hybrid cultivar in Taiwan, were used as explants for transformation. Using our protocol, commercially valuable hermaphrodite lines of an elite papaya variety with double-virus resistance to PRSV and PLDMV were obtained. For hybrid-breeding purpose, we also successfully delivered an untranslatable PLDMV CP construct to ‘Tainung No. 2’ hermaphrodite parental cultivars ‘Thailand’ and ‘Sunrise’ to engineer resistance to PLDMV. Our protocol can avoid time-consuming and labor-intensive breeding programs for producing elite transgenic papaya hybrid cultivars with resistance to both PRSV and PLDMV.
Materials and methods
Plant materials of papaya
After field cultivation for 1 year, shoot tips of fruit-bearing hermaphrodite plants of papaya (Carica papaya L.) cultivars (cv. ‘Tainung No. 2’, ‘Sunrise’, and ‘Thailand’) with good horticultural properties were collected. After surface sterilization, the shoot tips were used to generate multiple shoots, following the method described by Yang and Ye (1992). After proliferation, individual shoots were excised and transferred to MS medium (Murashige and Skoog 1962) containing 0.5 mg/l indole-3-butyric acid (IBA) for 1 week under dark condition for the initiation of roots, and subsequently transferred to perlite supplemented with 1/2 volume of MS basal medium for the development of adventitious roots (50 ml 1/2 volume of MS medium + 100 ml perlite per 350 ml flask). The developed roots were used as explants for the induction of somatic embryos.
Somatic embryogenesis from different root portions and rooting times
The adventitious roots generated after transfer to perlite medium for 1–6 weeks were selected for induction. Three portions of roots [primary root (I), secondary root (II) and root tip (III)] were cut into 0.5–1.0 cm segments and cultured on the MSDB callus/somatic-embryo induction medium [MS medium containing 1 mg/l 2,4-dichlorophenoxyacetic acid (2,4-D) and 0.1 mg/l 6-benzylaminopurine (BAP)], for somatic embryo production in a dark growth chamber at 28 ± 1°C (Lin and Yang 2001). Explants were transferred onto fresh medium at monthly intervals. After 3 months, the numbers of the somatic embryos produced from root segments of cv. ‘Tainung No. 2’, ‘Sunrise’, and ‘Thailand’ were calculated and the condition for highest frequency of somatic embryogenesis determined.
Construction of an untranslatable chimeric transgene
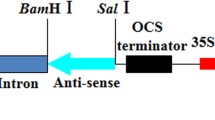
The construct of pPLDMVstop containing frameshift mutation in the CP sequence and the 3′ non-coding region of P-TW-WF strain (Bau et al. 2008) of Papaya leaf-distortion mosaic virus (PLDMV) was previously developed in our laboratory (Kung et al. 2009). The SmaI/SacI fragment of pPLDMVstop was ligated to SwaI/SacI-digested pBGCP that contained the CP coding region and the 3′ UTR of PRSV (Cheng et al. 1996) to form pYP08 through blunt-end ligation. The primers 926stop [5′-CCATGGAGTCCTAATAATGAAG-3′ containing two ochre stop codons (in bold) and NcoI site (underlined)] and dT18SacI [5′-TTTTTTTTTTTTTTTTTTGAGCTCT-3′ containing SacI site (underlined)] were used to amplify the chimeric YP08 fragment from pYP08 by PCR and cloned into TOPO PCR-II vector (Invitrogene, Carlsbad, CA, USA). After NcoI/SacI digestion, the chimeric YP08 fragment was cloned back to pYP08 to generate pYP08stop that contained an untranslatable chimeric construct with a segment of PRSV CP gene and a segment of PLDMV CP gene coupled with the complete 3′ UTR of PLDMV genome, controlled by a CaMV 35S promoter and a nos terminator (Supplementary Fig. 1A).
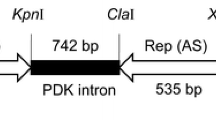
In addition, the NcoI/SacI-digested fragment of pPLDMVstop (Kung et al. 2009) was ligated to NcoI/SacI-digested pBGCP (Cheng et al. 1996) to form pBG-PLDMVstop containing the frame-shifted CP gene of PLDMV with two stop codons, controlled by a CaMV 35S promoter and a nos terminator (Supplementary Fig. 1B).
Plasmids pYP08stop and pBG-PLDMVstop were separately mobilized into disarmed Agrobacterium tumefaciens LBA4404 by triparental mating (Rogers et al. 1986). Cells of A. tumerfaciens cultured in the LB medium containing 50 mg/l kanamycin and 100 mg/l streptomycin at 28°C for 36 h were used for plant transformation.
Generation of transgenic papaya lines
The somatic embryos derived from the root-tip portion (III) of adventitious roots (Supplementary Fig. 2A) were further proliferated on the embryo-induction medium (Fitch et al. 1990) containing 4 mg/l 2,4-D (induction medium 4, IM4) and subcultured monthly 2–3 times to generate sufficient quantities of embryogenic tissue for transformation.
For transformation, embryogenic tissues (3–4 g) were wounded by vortexing with carborundum (600 mesh, 0.5 g) in 30 ml distilled water on a Vortex Genie-2 (Scientific Industries, Inc., Bochemia, NY) at speed 7 for 1 min and 200 μl of A. tumefaciens culture (OD600 = 0.5) was added and allowed to strand for 5 min. After blotting away the excess bacteria, the treated tissues were transferred to the IM4 embryo-induction medium and co-cultivated for 2 days. After co-cultivation, the tissues were subcultured for 20 days on IM4 medium containing 500 mg/l carbenicillin to suppress bacterial growth and then transferred to the same medium with 50 mg/l kanamycin for 30 days and 100 mg/l kanamycin for 60 days. The selected tissues were then nursed on IM4 medium without kanamycin to enhance mature embryo development, with monthly subtransfers. The mature somatic embryos were cultured on MSNB shoot medium (MS medium with 0.02 mg/l α-naphthaleneacetic acid (NAA) and 0.2 mg/l BAP) containing 50 mg/l kanamycin for shoot development.
Regenerated individual shoots were then micropropagated on the MSNB shoot medium to form multiple shoots, which were excised and transferred to MS medium containing 0.5 mg/l IBA for 1 week and subsequently transferred to vermiculite supplemented with 1/2 volume of MS basal medium for rooting (Cheng et al. 1996). The putative transformed plantlets micropropagated from a single original shoot, which was considered as an individual R0 regenerant, were acclimatized in a growth chamber and then established in vermiculite soil in temperature-controlled (25 ± 3°C) greenhouse (Cheng et al. 1996). The micropropagated plants of individual R0 lines were used for further evaluation.
DNA extraction and polymerase chain reaction
Total DNA was extracted from leaves of putative transgenic papaya lines or non-transformed papaya plants by the CTAB method (Fulton et al. 1995). One microgram of RNase A-treated total DNA was used as template for PCR (Saiki et al. 1988). The forward primer MO926 (5′-TCTAAAAATGAAGCTGTGGA-3′) and the reverse primer 1,169 (5′-ATAATATCGAACGCCGGTGAATGC-3′) were used for YP08 amplification. The forward primer PLDMVstop (5′-CCATGGAGTCCGCTCTTTGATGATGG-3′) and the reverse primer 1,169 (5′-ATAATATCGAACGCCGGTGAATGC-3′) were used for PLDMV CP amplification. The plasmids pYP08stop and pBG-PLDMVstop were separately used as positive controls. PCR was performed with periods of 1 min at 94°C, 1 min at 55°C, and 1 min at 72°C for 30 cycles. Additionally, nptII-specific primers (Kung et al. 2009) were used in PCR to check the presence of the selection marker gene. PCR products were analyzed by electrophoresis on 0.8% agarose gels.
Inoculation of transgenic lines with PRSV YK or PLDMV P-TW-WF
The resistance of transgenic lines was evaluated by mechanical inoculation with PRSV YK isolate (Wang and Yeh 1997) or PLDMV P-TW-WF isolate (Bau et al. 2008) in a temperature-controlled (25 ± 3°C) greenhouse. Eighty-nine YP08 transgenic lines of ‘Tainung No. 2’ were tested for inoculation with YK or P-TW-WF, with ten plants of each line for each inoculation. In addition, 20 PLDMV-CP transgenic lines, including 18 ‘Sunrise’ lines and 2 ‘Thailand’ lines, were inoculated with P-TW-WF (five plants per each line). Micropropagated R0 transgenic papaya plantlets were grown to a height of 8–10 cm in a temperature-controlled (25 ± 3°C) greenhouse. The first two fully expanded leaves were dusted with 600-mesh carborundum and rubbed with 200 μl of inoculum prepared from papaya leaf tissue 3 weeks after inoculation with PRSV YK or PLDMV P-TW-WF (1:10 w/v, in 0.01 M potassium phosphate buffer, pH 7.0). Non-transformed papaya plants were used as controls. Inoculated plants were kept in temperature-controlled greenhouse and symptom development was monitored for 7 weeks.
Indirect enzyme-linked immunosorbent assays (ELISA)
Indirect ELISA was performed as described previously (Kung et al. 2009). Systemic leaves were ground and diluted (1:100) in 50 mM sodium carbonate buffer (pH 9.6) with 0.01% sodium azide and assayed using 2,000-fold dilution of P-TW-WF CP antiserum (Bau et al. 2008) or PRSV CP antiserum (Yeh et al. 1984). Alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin (KPL, Inc., Gaithersburg, MD) was used at 5,000-fold dilution as the secondary antibody. The absorbance at 405 nm was measured with a Rainbow microplate reader (SLT Lab Instruments, Salzburg, Austria), 30 min after the addition of enzyme substrate. The samples that recorded an average absorbance reading ≧ three times that of the negative control readings (non-transformed papaya) were regarded as positive.
Southern blotting analysis
DNeasy Plant Mini kit (Qiagen, Valencia, CA, USA) was used to extract genomic DNA of non-transformed papaya and transgenic papaya plants, including 11 lines carrying the chimeric construct YP08 and 10 lines carrying PLDMV CP, under greenhouse conditions. Thirty micrograms of genomic DNA was digested with HindIII (a unique HindIII site is located in the T-DNA between the nptII gene and 35S promoter) and separated by electrophoresis at 100 V on a 0.8% agarose gel. The Primer-It II random primer labeling kit (Stratagene, LaJolla, Ca, USA) was used to prepare the [α-P32]dATP labeled probe from the DNA fragment (635 bp, 25 ng) amplified from pYP08stop and the DNA fragment (879 bp) amplified from pBG-PLDMVstop using primer pairs MO926/1169 and PLDMVstop/1169, respectively, according to manufacturer’s instructions. Southern hybridization was performed as described previously (Kung et al. 2009). The blotted Gene Screen Plus nylon membrane (Dupont Co., Boston, MA) was prehybridized at 60°C for 1 h, followed by hybridization of the probe at 60°C for 16 h. After washing, autoradiography was carried out at −80°C for 48 h on an X-ray film (Hyperfilm MP, Amersham Phamacia Biotech, UK) with KODAK™ Biomax® MS intensifying screens (Eastman Kodak Co., Rochester, NY, USA).
Northern blotting analysis for detection of transgene transcript and siRNA
Total RNA was extracted from leaves using the Trizol reagent according to manufacturer’s instruction (Invitrogen, Carlsbad, CA, USA). Detection of the transgene transcript was performed with 25 μg total RNA that was separated on a 1.2% agarose gel with formaldehyde, transferred to a Hybond-N + membrane (Amersham Phamacia Biotech, UK), and hybridized with the α32P-labeled YP08 probe or PLDMV CP probe, as described above at 60°C for 16 h. Autoradiography was conducted as described for Southern blotting.
For the detection of siRNA derived from the transgene constructs, 30 μg total RNA was resolved on a 15% polyacrylamide/1 × TBE (8.9 mM Tris, 8.9 mM boric acid, 20 mM EDTA)/8 M urea gel and transblotted to a Hybond-N + membrane (Amersham Phamacia Biotech, UK). The α32P-labeled YP08 probe or PLDMV CP probe, prepared as described above, was used for hybridization using the ULTRAHyb-Oligo solution according to manufacturer’s instructions (Ambion Inc., Austin, TX), and signals were monitored by autoradiography similar to Southern blotting. RNA Decade™ Marker system (Ambion Inc., Austin, TX) was used as markers in this experiment. The Decade Markers were derived from the cleavage of a single 150 nt gel-purified Decade Marker RNA transcript, which was first 5′ end-labeled by kinase reaction with [γ-P32] ATP, according to the manufacture’s instructions, to generate a marker set in the range of 10–150 nts.
Confirmation of hermaphroditic characteristics of transgenic lines
For determination of papaya sex, the sex-linked primers pairs SDP-1 (5′-GCACGATTTAGATTAGATGT-3′) and SDP-2 (5′-GGATAGCTTGCCCAGGTCAC-3′) (Urasaki et al. 2002) were used for detection of hermaphroditism of transgenic lines. In addition to non-transformed controls, two female transgenic papaya lines, 18-2-4 and 10-4, derived in previous studies (Bau et al. 2003; Kung et al. 2009) were also used as controls. PCR and gel analysis were performed as described above.
Moreover, the selected transgenic lines were planted in an isolated greenhouse to observe the flower types, fruit shape and other horticultural properties as compared to those of the non-transformed controls.
Results
Construction of an untranslatable chimeric construct
Construction of an untranslatable chimeric construct containing the truncated PRSV and PLDMV CP coding regions coupled with 3′ UTR of PLDMV is summarized in Supplementary Fig. 1. The construct of pYP08stop contained a segment of PRSV CP gene (nts 1–263) and a segment of PLDMV CP gene (nts 719–879) and the entire 3′ UTR (211 nts) of PLDMV with a total length of 635 nucleotides. The final untranslatable chimeric construct YP08 containing two upstream stop codons is under the control of a CaMV 35S promoter and a nos terminator (Supplementary Fig. 1A).
The other untranslatable construct pBG-PLDMVstop containing a frame-shifted full-length (879 nts) PLDMV CP reading frame coupled with two upstream stop codons was also generated, and put under the control by a CaMV 35S promoter and a nos terminator (Supplementary Fig. 1B).
Frequency of somatic embryogenesis
A total of 5,728 root segments, which were cumulated from three root portions, primary-root (I), secondary-root (II) and root-tip (III) (Supplementary Fig. 2A-a), of the adventitious roots of 36 ‘Tainung. No. 2’ plantlets developed 1–6 weeks after rooting in the perlite medium (six plants per week at weekly intervals), were cultured on the MSDB medium for 3 months for induction of somatic embryos (Supplementary Fig. 2A-b, c). An average of 12.2% somatic embryogenesis occurred (Supplementary Fig. 2A-c), with the highest frequency of 22.1% at 2 weeks after rooting (Supplementary Table 1). When these 5,728 root segments were analyzed according to the origin of three portions, the highest average frequency of 21.7% somatic embryogenesis was from the root-tip segments (III), superior to those from the primary-root (I) (9.8%), and secondary-root (II) portions (7.7%) (Supplementary Table 1). With 28.2–34.3% somatic embryogenesis frequencies, the root-tip segments (III) from adventitious root 2–4 weeks after rooting were selected as best sources of explants for somatic embryogenesis of ‘Tainung No. 2’.
When the efficiency of somatic embryogenesis of 3,465 total root segments of ‘Sunrise’ and 2,058 total root segments of ‘Thailand’ were determined, an average of 9.9 and 17.8% of somatic embryogenesis occurred, respectively. Highest frequencies of 16.8 and 36.4% for ‘Sunrise’ and ‘Thailand’, respectively, were noticed 2 weeks after rooting (Supplementary Table 1). When efficiencies of somatic embryogenesis from the three root portions were analyzed, higher frequencies (22.2% for ‘Sunrise’ and 35.0% for ‘Thailand’) were from the root-tip segments (III), compared to those from the primary-root (I) (6.5 and 19.1%), and secondary-root (II) portions (5.4 and 7.0%). The root-tip segments (III) from adventitious roots 2–4 weeks after rooting were chosen as the best explants for somatic embryogenesis for ‘Sunrise’ and ‘Thailand’, for given somatic embryogenesis frequencies of 29.8–33.3% and 43.8–55.4%, respectively.
Furthermore, our results indicated that the highest efficiency of root development occurred with ‘Tainung No. 2’ (a total 5,728 root segments obtained from 36 plants/6 weeks at weekly intervals), compared to those from ‘Sunrise’ (3,465 root segments) and ‘Thailand’ (2,058 root segments) (Supplementary Table 1).
Establishment of transgenic lines
The somatic embryos regenerated from root-tip segments (III) were proliferated on IM4 medium for 2–3 subcultures and then used for transformation (Supplementary Fig. 2A-c). The somatic embryogenic tissues were wounded by carborundum in liquid-phase and then co-cultivated with Agrobacterium carrying pYP08stop or pBG-PLDMVstop on the IM4 medium for 2 days (Supplementary Fig. 2B-a). After culturing on IM4 medium with kanamycin selection for 90 days, the selected tissues were further nursed on IM4 medium without kanamycin for 30–60 days for embryo development (Supplementary Fig. 2B-b). Two to four weeks after germination, putative transformed somatic embryos grew rapidly into multiple shoots on the MSNB shoot medium containing 50 mg/l kanamycin (Supplementary Fig. 2B-c, d). Non-transgenic embryos were not able to germinate and gradually declined on the same medium. Putative transgenic, kanamycin resistant green shoots were established on MSNB medium containing 100 mg/l kanamycin within 3 weeks (Supplementary Fig. 2B-e left), while non-transformed shoots derived from somatic embryos of the control turned pale and gradually declined (Supplementary Fig. 2B-e right). Multiple shoots (1.0–1.5 cm) micropropagated from individual R0 regenerates were transferred to MS medium containing 0.5 mg/l IBA for 1 week and subsequently transferred to vermiculite supplemented with 1/2 volume of MS basal medium for rooting (Supplementary Fig. 2B-f) and five to 20 clonal plantlets of each R0 line were established and used for further assay.
Plantlets of a total of 89 putative transgenic R0 lines of ‘Tainung No. 2’ regenerated from the somatic embryos derived from 237 root segments were established and analyzed further. When PCR was used to analyze the presence of the chimeric construct in putative transgenic R0 lines, an expected DNA fragment of 635 bp was noticed in all transgenic lines, but not in non-transformed plants. Moreover, when the nptII—specific primers were used for PCR, an amplified DNA fragment of 1.0 kb was obtained.
A total of 20 putative transgenic lines (18 lines of ‘Sunrise’ and 2 lines of ‘Thailand’) carrying the untranslatable PLDMV CP construct were obtained, as checked by PCR using transgene-specific and nptII-specific primers described above.
A total of 109 transgenic lines obtained from 349 treated root-tip segments (III), thus transformation frequencies ranging from 3.7 to 37.6%, with an average of 31.2% (Supplementary Table 2). Among the three papaya cultivars tested, ‘Tainung No. 2’ had the best transformation efficiency (37.6%), while ‘Thailand’ had the lowest transformation efficiency (3.7%) (Supplementary Table 2).
Evaluation of transgenic resistance to PRSV YK or PLDMV P-TW-WF
At least ten plants (8–10 cm) for each of the 89 transgenic ‘Tainung No. 2’ lines carrying the chimeric construct YP08 were mechanically inoculated with PRSV YK or PLDMV P-TW-WF. Symptom development in transgenic lines was compared with that of non-transformed (NT) control. During the period of 49 days after inoculation, the inoculated plants exhibited various levels of resistance, as shown by different percentages of inoculated plants that displayed symptoms. Based on the results, the transgenic lines were classified into four categories: susceptible (S), weakly resistant (WR), moderately resistant (MR), and highly resistant (HR) types (Table 1). Fourteen days after inoculation, 47 susceptible lines (S) showed symptoms of mosaic and distortion on leaves, and water-soaked streaks on petioles and stems, similar to those of the non-transformed control (Fig. 1A-a, b). Fifteen lines were considered as weakly resistant (WR) type, with 1–2 week delay in symptom development and up to 80–95% of test plants infected 4 weeks after inoculation. Eighteen lines were classified as moderately resistant (MR) type, with a 3–5 week delay in development of attenuated symptoms with mild mottling or chlorotic spots and only 20–30% of test plants were infected 4 weeks after inoculation. Similar degrees of resistance were noticed in the same MR and WR lines after inoculation with PRSV or PLDMV. Infection with PRSV or PLDMV was confirmed in ELISA assays with samples of symptomatic WR and MR plants. Nine lines, which showed no symptoms during a test period of 2 months and ELISA negative when analyzed with PRSV or PLDMV antiserum, were regarded as highly resistant (HR) type (Fig. 1A-c, d; Table 1). When these nine HR lines were re-inoculated with another virus, either PLDMV or PRSV, they also displayed high degrees of resistance to the second virus challenge.
Transgenic resistance to Papaya ringspot virus (PRSV) YK or Papaya leaf-distortion mosaic virus (PLDMV) P-TW-WF of transgenic papaya lines. A reactions of the YP08 transgenic plants photographed 4 weeks after mechanical inoculation with PRSV or PLDMV. Non-transformed plants developed typical severe mosaic and leaf-distortion symptoms after inoculation with PRSV YK (a) and PLDMV P-TW-WF (b), respectively. No symptom was evidenced on plants of transgenic line H2-1 after inoculation with PRSV YK (c) or PLDMV P-TW-WF (d). B reactions of the PLDMV CP transgenic plants photographed 4 weeks after mechanical inoculation with PLDMV. Non-transformed plants developed typical severe mosaic and leaf-distortion symptoms after inoculation with PLDMV P-TW-WF (a). No symptom was evidenced on plants of transgenic line 2-13-1 after inoculation with PLDMV P-TW-WF (b)
Five plants (8–10 cm) for each of the 20 transgenic papaya lines (18 lines of ‘Sunrise’ and two lines of ‘Thailand’) carrying untranslatable PLDMV CP gene were inoculated with PLDMV P-TW-WF. The transgenic lines were classified into three categories: including susceptible (S), weakly resistant (WR) and highly resistant (HR) types, based on the level of resistance to P-TW-WF (Table 2). Of the 18 ‘Sunrise’ lines, eight susceptible (S) lines showed symptoms of mosaic and distortion on leaves, and water-soaked streaks on petioles and stems, similar to those of the NT control (Fig. 1B-a). Two lines were considered as weakly resistant (WR) type, with 1–2 week delay in symptom development after inoculation. The symptomatic plants of all the S and WR lines were ELISA positive, when tested against PLDMV antiserum. Eight lines, which showed no symptoms during the observation period of 2 months (Fig. 1B-b) and tested negative in ELISA for PLDMV, were regarded as highly resistant (HR) type.
Of the two ‘Thailand’ lines, one line was susceptible (S), showing symptoms and ELISA positive similar to those of NT. Another line, which showed no symptoms 2 months after inoculation and ELISA negative, was regarded as highly resistant (HR) type.
Southern blotting analysis of R0 transgenic lines
The copy numbers of the chimeric construct inserts in the 3 S and 8 HR lines carrying YP08 construct were determined using the YP08-specific probe and in the 3 S and 7 HR lines carrying PLDMV CP using PLDMV CP-specific probe by Southern hybridization analyses. The chimeric construct was randomly inserted into the host genome, as reflected by the different hybridization patterns of samples from different lines. The copy numbers of different lines ranged from 1 to 5, as shown in Supplementary Fig. 3A and Fig. 2A. Among these lines, the HR lines were associated with two or multiple copies of the chimeric insert. Furthermore, all the tested susceptible lines carried single copy insert with the chimeric YP08 (Supplementary Fig. 3A) or untranslatable PLDMV CP (Fig. 2A). These results indicated that higher levels of transgenic resistance in papaya lines have a trend to be correlated with high transgene copy numbers of the inserted transgene.
Southern analysis for the integration of the untranslatable PLDMV CP construct in transgenic papaya lines and analysis of the accumulations of the transgene transcript and siRNA by northern blotting. A Analysis of the copy numbers of the chimeric construct in transgenic papaya lines, as detected by Southern hybridization using PLDMV CP-specific probe. The copy numbers (Copy No.) are indicated at the bottom of each lane. B Expression levels of transgene transcript of susceptible (S) and highly resistant (HR) lines detected by northern hybridization. Non-transformed papaya (NT) was used as a negative control. The arrow indicates where the expected transcript migrated. The quantities of the total RNA (25 μg/lane) loaded were monitored by ethidium bromide staining. C siRNA accumulations derived from the transgene in susceptible (S) and highly resistant (HR) lines were analyzed by PLDMV CP specific probe. Loading control of total RNA (30 μg/lane) was monitored by ethidium bromide staining. RNA Decade™ Marker system (Ambion Inc., Austin, TX) was used as markers
The expression levels of chimeric CP transgene transcript and siRNA
Twenty-one lines, including 11 lines carrying the chimeric construct YP08 and 10 lines carrying untranslatable PLDMV CP, in which the presence of the transgene was confirmed by PCR and Southern blot analysis, were selected and their expression levels of transgene transcript and siRNA were analyzed by northern blotting.
The results revealed that two S lines accumulated higher level of the chimeric YP08 transcript, but no transcript was detected from S line D3-1 (Supplementary Fig. 3B). In contrast, lower levels of the chimeric YP08 transcript were detected from all the HR lines (Supplementary Fig. 3B). Similarly, high levels of transcript were noticed in all S lines of PLDMV CP transgenic lines, while no transcript was detected from all the HR lines tested (Fig. 2B).
When siRNA of these 21 lines was analyzed by YP08-specific probe or PLDMV CP-specific probe, the results revealed that high levels of siRNA related to the chimeric YP08 construct or PLDMV CP construct were detected from all HR lines tested, but not from all S lines tested (Supplementary Fig. 3C and Fig. 2C).
Sex determination and horticultural properties of transgenic lines
A 450 bp marker fragment, named PSDM (Papaya Sex Determination Marker), exists in all male and hermaphrodite plants, but not in the female (Urasaki et al. 2002). Two primers, SDP-1 and SDP-2, were designed from sequences within the PSDM sequence (Urasaki et al. 2002). PCR performed with these primers amplified a 225 bp fragment in the hermaphrodite non-transformed papaya and all the transgenic papaya lines obtained in this study, but not in the female papaya controls (Fig. 3a).
PCR analysis and hermaphroditic phenotypes of transgenic lines. A PCR analysis with the hermaphrodite-linked primers, SDP-1 and SDP-2 (Urasaki et al. 2002). Non-transgenic hermaphrodite and female plants of non-transformed papaya (NT) cv. Sunrise (SR), Tainung No. 2 (TN) and Thailand (TL) and two female transgenic papaya lines, 18-2-4 and 10-4, generated in previous studies (Bau et al. 2003; Kung et al. 2009) were used as controls. A specific fragment of 225 bp, similar to that of the hermaphrodite controls, was amplified from all the transgenic papaya lines, including 11 lines of Tainung No. 2 (TN lines), three lines of Sunrise (SR lines) and one line of Thailand (TL line). B Hermaphroditic phenotypes of transgenic lines. After planting in greenhouse for 1 year, plants of selected transgenic papaya lines SR 2-13-1, TN B1-4, and TL 3-5-1 showed hermaphroditic flowers (upper panel, gray arrow = anther and white arrow = pistil) and fruits (lower panel) similar to those of the original NT plants of Tainung No. 2, Sunrise, and Thailand
After 1 year culturing under greenhouse conditions, plants of 15 selected transgenic papaya lines, including 11 lines of cv. ‘Tainung No. 2’, three lines of cv. ‘Sunrise’ and one line of cv. ‘Thailand’, showed hermaphrodite characteristics at the flowering stage (Fig. 3b, upper panel), and normal horticultural properties such as leaf size, height, fruit-setting, fruit size and shape (Fig. 3b, lower panel), and sweetness indistinguishable from those of the original non-transformed papaya plants of cv. ‘Tainung No. 2’, ‘Sunrise’, and ‘Thailand’.
Discussion
The lengthy breeding procedure (about 5–7 years) is usually required for incorporating the desired sex and other useful horticultural properties like shape, size, color, sweetness and flavor of fruits into a commercially valuable hybrid cultivar of papaya. For example, because all YK-CP transgenic papaya lines were with female sex (Bau et al. 2003), we have spent 5–7 years to fix the transgenic resistance through backcross with parental lines to obtain hermaphrodite sex with desirable horticultural traits and fix the homozygosity of the transgene, and the selective progenies were then used for generation of a commercial hybrid cultivar. In this study, in order to shorten the time for the generation of bisexual papaya sex types, the somatic embryos derived from the roots of in vitro multishoots of selected plants of C. papaya cv. ‘Tainung No. 2’, ‘Sunrise’, and ‘Thailand’, with desired bisexual type and horticultural properties, were used as explants to replace the zygotic embryos from premature seeds with unknown sex and horticultural properties. A commercially valuable ‘Tainung No. 2’ papaya hybrid variety with double-virus (PRSV and PLDMV) resistance was generated within 7 months after Agrobacterium-mediated transformation. An efficient micropropagation protocol (Yu et al. 2000) has been widely used for production of large numbers of hermaphrodite plants of ‘Tainung No. 2’ hybrid in Taiwan (Yeh et al. 2007). Thus, the transgenic lines derived from ‘Tainung No. 2’ generated in this study can be directly used for commercial purposes without further breeding. Compared with recently generated double-virus resistant papaya lines with female sex regenerated from immature zygotic embryos (Kung et al. 2009), which need several years to generate hermaphroditic papaya with desired properties via breeding, our new approach significantly shortens the breeding program.
The sequence content of the presently used PRSV-PLDMV CP chimeric construct pYP08 [(635 bp, containing 5′-end segment (263 bp) of PRSV CP and 3′-end segment (161 bp) of PLDMV CP with 3′ UTR (211 bp) of PLDMV] is completely different from that of the previously reported construct pPY16 (1,526 bp, containing 5′-end segment of PLDMV CP (718 bp) and 3′-end segment (601 bp) of PRSV CP with 3′ UTR (206 bp) of PRSV] (Kung et al. 2009). The construct of pYP08 is much shorter and contains CP segments and 3′ UTR different from those of pPY16 (Kung et al. 2009). Despite these differences, we showed that the chimeric construct YP08 was also able to provide high degrees of transgenic against PRSV and PLDMV. Our results suggested lack of significant influence of different segments, arrangement and length of PRSV-PLDMV CP sequence components in the PRSV-PLDMV CP chimeric construct in its ability to provide resistance against PRSV and PLDMV. However, the shorter length of the chimeric construct used in this study may minimize the possibility of recombination between the genome of invading virus and the transgene.
Our new approach was also successfully applied to other papaya cultivars of ‘Thailand’ and ‘Sunrise’, in which PLDMV resistant hermaphrodite papaya lines carrying the PLDMV untranslatable CP sequence were also obtained. Since cultivars of both ‘Thailand’ and ‘Sunrise’ are the parental lines of the hybrid cultivar ‘Tainung No. 2’, their hermaphrodite individuals with desired horticultural traits can greatly shorten the breeding time to generate a new ‘Tainung No. 2’ hybrid with resistance to PLDMV. Moreover, these PLDMV HR lines can also be crossed with previously developed homozygous PRSV-YK CP transgenic papaya lines (Bau et al. 2003) to generate a double-virus resistant papaya hybrid via breeding in future to control the two viruses in Eastern Asia and elsewhere.
In this investigation, transformation frequencies using somatic embryos derived from root-tip segments (III) ranged from 3.7–37.6%, with an average of 31.2%. The transformation frequency of ‘Thailand’ was significantly lower than those of ‘Sunrise’ and ‘Tainung No. 2’ (Supplementary Table 2). The transformation frequency is apparently variety-dependent, and it may be due to lower efficiency of rooting, as reflected in the lower amounts of adventitious roots developed from ‘Thailand’, as compared to those of ‘Tainung No. 2’ and ‘Sunrise’ (Supplementary Table 1). In general, the average transformation frequency of our new approach is higher than the transformation frequency of 0.42% of bombarded embryos (Fitch et al. 1990), 0.15% of embryogenic callus by Agrobacterium-mediated transformation (Fitch et al. 1993), and 16.9% of immature embryos treated with Agrobacterium following liquid-phase wounding with caborundum (Cheng et al. 1996). Moreover, our results indicated that the new transformation protocol can be used for different papaya varieties including the parental lines of hybrid variety such as cultivars ‘Thailand’ and ‘Sunrise’.
In previous study, the tissue culture conditions for producing somatic embryos from adventitious roots of in vitro shoots of Carica papaya cv. ‘Tainung No. 2’ were described, and the root-tip part was considered the best explant (Lin and Yang 2001). But, suggestions were not provided regarding the best time for root harvesting and for the root portion of other papaya cultivars. In this investigation, our results indicated that higher frequencies (28.2–34.3%) of somatic embryogenesis for cultivars ‘Tainung No. 2’, ‘Thailand’, and ‘Sunrise’ were from root-tip segments (III portions) of adventitious roots obtained 2–4 weeks after culturing in the rooting medium. Apical meristem is located on the root tip that differentiates into different tissues. This portion of the adventitious roots is considered prone to be inducible by 2,4-D medium for the formation of somatic embryos.
In this study, after kanamycin selection for 90 days, the selected tissues were subcultured on the kanamycin-free IM4 medium for embryo development. This step is important for embryo development from transformed cells. It appears that antibiotic selection retards the regeneration process of transgenic papaya lines (Yu et al. 2003). Several studies have addressed this issue by limiting the period under antibiotic selection and allowing regeneration of plants in the absence of kanamycin, as described by Cai et al. (1999). We also had difficulties in regenerating plantlets from tissues kept on IM4 medium with kanamycin for long periods (more than 3 months).
In northern blotting analysis, lower levels of transgene transcript or no transcript were detected in most of highly resistant lines, as compared with those of susceptible lines. Furthermore, higher levels of siRNA were detected in all the highly resistant lines, suggesting that the resistance was mediated by post-transcriptional gene silencing (PTGS) (Baulcombe 1996). However, a relatively higher level of transgene transcript was detected from the highly resistant A2-2 line that contained five inserts (Supplementary Fig. 3A). The transcript accumulation in this line implied that the siRNA did not completely silence the expression of multiple inserts, but apparently the RNA degradation through siRNA signaling was capable of silencing the invading viral RNA to provide a high level of resistance (Supplementary Fig. 3B, C). No transcript and no siRNA of the transgene were detected in the susceptible line D3-1, suggesting that the susceptibility of this line is due to transcriptional gene silencing (TGS) (Smith et al. 1994). The highly resistant lines were associated with two or multiple copies of the chimeric YP08 construct or PLDMV CP construct. Furthermore, transgenic papaya lines with single copy insertions were susceptible. These results corroborate previous report that more transgenic copies may enhance PTGS (Sijen et al. 1996; Kung et al. 2009).
In this investigation, a tissue culture method was developed for efficient Agrobacterium-mediated transformation via somatic embryos derived from root-tip explants (III) from desired hermaphroditic papaya plants of cultivars ‘Tainung No. 2’, ‘Sunrise’, and ‘Thailand’ to generate transgenic papaya lines. The selected transgenic papaya lines growing under greenhouse conditions were hermaphrodite and had normal horticultural properties similar to those of the original non-transformed papaya plants of cv. ‘Tainung No. 2’, ‘Sunrise’, and ‘Thailand’. Following this procedure, we developed single-virus (PLDMV) resistance and double-virus (PRSV and PLDMV) resistance in transgenic papaya lines of different papaya cultivars with hermaphrodite sex and desired horticultural properties. The commercially valuable ‘Tainung No. 2’ papaya hybrid variety with double-virus resistance to PRSV and PLDMV can be directly micropropagated or used in breeding for a hybrid. Therefore, we consider our new approach is a fast and efficient transformation method for different papaya varieties and it can significantly shorten the time-consuming breeding program.
Abbreviations
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- BAP:
-
6-benzylaminopurine
- MSDB:
-
MS medium containing 0.125 mg/l 2,4-D and 0.4 mg/l BAP
- NAA:
-
α-Naphthaleneacetic acid
- MSNB:
-
MS medium containing 0.02 mg/l NAA and 0.2 mg/l BAP
- IM4:
-
Induction medium containing 4 mg/l 2,4-D
- IBA:
-
Indole-3-butyric acid
References
Bau HJ, Cheng YH, Yu TA, Yang JS, Yeh SD (2003) Broad spectrum resistance to different geographic strains of Papaya ringspot virus in coat protein gene transgenic papaya. Phytopathology 93:112–120
Bau HJ, Kung YJ, Raja JAJ, Chan SC, Chen KC, Chen YK, Wu HW, Yeh SD (2008) Potential threat of a new pathotype of Papaya leaf-distortion mosaic virus infecting transgenic papaya resistant to Papaya ringspot virus. Phytopathology 98:848–858
Baulcombe DC (1996) RNA as a target and an initiator of post-transcriptional gene silencing in transgenic plants. Plant Mol Biol 32:79–88
Cai W, Gonalves C, Tennant P, Fermin G, Souza M, Sarinud N, Jan FJ, Zhu HY, Gonsalves D (1999) A protocol for efficient transformation and regeneration of Carica papaya L. In Vitro Cell Dev Biol-Plant 35:61–69
Chen MH, Chen CC (1992) Plant regeneration from Carica protoplasts. Plant Cell Rep 11:404–407
Chen MH, Wang PJ, Maeda E (1987) Somatic embryogenesis and plant regeneration in Carica papaya L. tissue culture derived from root explants. Plant Cell Rep 6:348–351
Chen G, Ye CM, Huang JC, Yu M, Li BJ (2001) Cloning of the Papaya ringspot virus (PRSV) replicase gene and generation of PRSV-resistant papayas through the introduction of PRSV replicase gene. Plant Cell Rep 20:272–277
Cheng YH, Yang JS, Yeh SD (1996) Efficient transformation of papaya by coat protein gene of Papaya ringspot virus mediated by Agrobacterium following liquid-phase wounding of embryogenic tissues with carborundum. Plant Cell Rep 16:127–132
Davis MJ, Ying Z (2004) Development of papaya breeding lines with transgenic resistance to Papaya ringspot virus. Plant Dis 88:352–358
Fermin G, Inglessis V, Garboza C, Rangle S, Dagert M, Gonsalves D (2004) Engineered resistance against Papaya ringspot virus in Venezuelan transgenic papaya. Plant Dis 88:516–522
Fitch MMM (1993) High frequency somatic embryogenesis and plant regeneration from papaya hypocotyl callus. Plant Cell Tissue Organ Cult 32:205–212
Fitch MMM, Manshardt RM (1990) Somatic embryogenesis and plant regeneration from immature zygotic embryos of papaya (Carica papaya L.). Plant Cell Rep 9:320–324
Fitch MMM, Manshardt RM, Gonsalves D, Slightom JL, Sanford JC (1990) Stable transformation of papaya via microprojectile bombardment. Plant Cell Rep 9:189–194
Fitch MMM, Manshardt RM, Gonsalves D, Slightom JL, Sanford JC (1992) Virus resistance papaya plants derived from tissues bombarded with the coat protein gene of Papaya ringspot virus. Bio/Technology 10:1466–1472
Fitch MMM, Manshardt RM, Gonsalves D, Slightom JL (1993) Transgenic papaya plants from Agrobacterium-mediated transformation of somatic embryos. Plant Cell Rep 12:245–249
Fulton TM, Chunwongse J, Tanksley SD (1995) Microprep protocol for extraction of DNA from tomato and other herbaceous plants. Plant Mol Biol Rep 13:207–209
Gonsalves D, Fermin G (2004) The use of transgenic papaya to control papaya ringspot virus in Hawaii and transfer to this technology to other countries. In: Christou P, Klee H (eds) Handbook of plant biotechnology. Wiley, London, pp 1165–1182
Kawano S, Yonaha T (1992) The occurrence of Papaya leaf-distortion mosaic virus in Okinawa. Tech. Bull. of FFTC 132: 13–23, Food and Fertilizer Technology Center for the Asian and Pacific Regions, Taipei
Kung YJ, Bau HJ, Wu YL, Chen TM, Su WC, Yeh SD (2009) Generation of transgenic papaya resistant to Papaya ringspot virus and Papaya leaf-distortion mosaic virus. Phytopathology 99:1312–1320
Lin CM, Yang JS (2001) Papaya somatic embryo induction from fruiting-bearing field plants: effects of root supporting material and position of the rooting explants. Acta Hort 560:489–492
Lines RE, Persley D, Dale JL, Drew R, Bateson MF (2002) Genetically engineered immunity to Papaya ringspot virus in Australian papaya cultivars. Mol Breed 10:119–129
Litz RE, Conover RA (1982) In vitro somatic embryogenesis and plant regeneration from Carica papaya L. ovular callus. Plant Sci Lett 26:153–158
Litz RE, O’Hair SK, Concver RA (1983) In vitro growth of Carica papaya L. cotyledons. Sci Hort 19:287–293
Lius S, Manshardt RM, Fitch MMM, Slightom JL, Sanford JC, Gonsalves D (1997) Pathogen-derived resistance provides papaya with effective protection against Papaya ringspot virus. Mol breed 3:161–168
Maoka T, Hataya T (2005) The complete nucleotide sequence and biotype variability of Papaya leaf-distortion mosaic virus. Phytopathology 95:128–135
Maoka T, Kashiwazaki S, Tsuda S, Usugi T, Hibino H (1996) Nucleotide sequence of the capsid protein gene of Papaya leaf-distortion mosaic potyvirus. Arch Virol 141:197–204
Mondal M, Gupta S, Mukherjee BB (1994) Callus culture and plantlet production in Carica papaya (var. Honey Dew). Plant Cell Rep 13:390–393
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:437–497
Purcifull DE, Edwardson JR, Hiebert E, Gonsalves D (1984) Papaya ringspot virus. CMI/AAB Descriptions of Plant Viruses, No. 84
Rogers SG, Horsh RB, Fraley RT (1986) Gene transfer in plants: production of transformed plants using Ti-plasmid vectors. Methods Enzymol 118:627–640
Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB, Erlich HA (1988) Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239:487–491
Sijen T, Wellink J, Hiriart JB, Kammen AV (1996) RNA-mediated virus resistance: role of repeated transgenes and delineation of targeted regions. Plant Cell 8:2277–2294
Smith HA, Swaney SL, Parks TD, Wernsman EA, Dougherty WG (1994) Transgenic plant virus resistance mediated by untranslatable sense RNAs: expression, regulation, and fate of nonessential RNAs. Plant Cell 6:1441–1453
Tennant P, Fermin G, Fitch MM, Manshardt RM, Slightom JL, Gonsalves D (2001) Papaya ringspot virus resistance of transgenic Rainbow and SunUp is affected by gene dosage, plant development, and coat protein homology. Eur J Plant Pathol 107:645–653
Tripathi S, Suzuki J, Gonsalves D (2007) Development of genetically engineered resistant papaya for Papaya ringspot virus in a timely manner: a comprehensive and successful approach. Methods Mol Biol 354:197–240
Tsay HS, Su CY (1985) Anther culture of papaya (Carica papaya L.). Plant Cell Rep 4:28–30
Urasaki N, Tokumoto M, Tarora K, Ban Y, Kayano T, Tanaka H, Oku H, Chinen I, Terauchi R (2002) A male and hermaphrodite specific RAPD marker for papaya (Carica papaya L.). Theor Appl Genet 104:281–285
Wang CH, Yeh SD (1997) Divergence and conservation of the genomic RNAs of Taiwan and Hawaii strains of Papaya ringspot virus. Arch Virol 142:271–285
Yang JS, Ye CA (1992) Plant regeneration from petioles of in vitro regenerated papaya Carica papaya L. shoots. Bot Bull Acad Sin 33:375–381
Yang JS, Yu TA, Cheng YH, Yeh SD (1996) Transgenic papaya plants from Agrobacterium-mediated transformation of petioles of in vitro propagated multishoots. Plant Cell Rep 15:549–564
Yeh SD, Gonsalves D, Provvidenti R (1984) Comparative studies on host range and serology of Papaya ringspot virus and Water melon mosaic virus1. Phytopathology 74:1081–1085
Yeh SD, Bau HJ, Kung YJ, Yu TA (2007) Papaya Biotechnology. Biotechology in agriculture and forestry. Transgenic crops V, pp 73–96
Yonaha T, Yonemori S, Tamori M (1976) Relation between the flight occurrence of alate aphids and the spread of papaya virus disease in the field. Okinawa Agric 14:7–15
Yu TA, Yeh SD, Cheng YH, Yang JS (2000) Efficient rooting for establishment of papaya plantlets by micropropagation. Plant Cell Tissue Organ Cult 61:29–35
Yu TA, Yeh SD, Yang JS (2003) Comparison of the effects of kanamycin and geneticin on regeneration of papaya from root tissues. Plant Cell Tissue Organ Cult 74:169–178
Zhu YJ, Agbayani R, Jackson MC, Tang CS, Moore PH (2004) Expression of the grapevine stilbene synthase gene VST1 in papaya provides increased resistance against diseases caused by Phytophthora palmivora. Planta 220:241–250
Acknowledgments
We thank the financial supports by grants 94AS-5.2.1-ST-a1(6), 95AS-6.2.1-ST-a1(33), and 96AS-1.2.1-ST-a2 (1) from the Council of Agriculture of Taiwan, R.O.C.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kung, YJ., Yu, TA., Huang, CH. et al. Generation of hermaphrodite transgenic papaya lines with virus resistance via transformation of somatic embryos derived from adventitious roots of in vitro shoots. Transgenic Res 19, 621–635 (2010). https://doi.org/10.1007/s11248-009-9344-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-009-9344-2