Abstract
Gene transferability from transgenic rapeseed to various subspecies and varieties of Brassica rapa was assessed in this study. Artificial crossability was studied in 118 cultivars of 7 B. rapa subspecies and varieties with the transgenic rapeseed GT73 (Brassica napus) as the pollen donor. On average 5.7 seeds were obtained per pollination, with a range from 0.05 to 19.4. The heading type of B. rapa L. showed significantly higher crossability than non-heading types of B. rapa. The spontaneous outcrossing rate between B. rapa (female) and the transgenic rapeseed Ms8 × Rf3 (B. napus) (male) ranged from 0.039 to 0.406%, with an average of 0.19%. The fertilization process and the development of the hybrid seeds as shown by fluorescent staining techniques indicated that the number of adhered pollens on the stigma was reduced by 80%, the number of pollen tubes in the style was reduced by 2/3 and the fertilization time was delayed by over 20 h when pollinated with the transgenic rapeseed Ms8 × Rf3 in comparison with the bud self-pollination of B. rapa as control. About 10–70% of the interspecific hybrid embryos were aborted in the course of development. Some seeds looked cracked in mature pods, which showed germination abilities lower than 10%. The spontaneous outcrossing rates were much lower than the artificial crossability, and their survival fitness of the interspecific hybrid was very low, indicating that it should be possible to keep the adventitious presence of the off-plants under the allowed threshold, if proper measures are taken.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brassica rapa L. (AA, 2n = 20) is a collection of Brassica species consisting of the A genome. Due to natural evolution and artificial selection, B. rapa comprises many subspecies and cultivar groups, including those for swollen edible roots (B. rapa L. ssp. rapifera), stems (B. rapa L. ssp. chinensis var. utilis, B. rapa L. ssp. chinensis var. parachinensis, B. rapa L. ssp. chinensis var. multiceps), leaves (B. rapa L. ssp. pekinensis, B. rapa L. ssp. chinensis, B. rapa L. ssp. chinensis var. rosularis), flower buds (B. rapa L. ssp. chinensis var. purpurea) and oil-rich seeds (B. rapa L. ssp. chinensis var. oleifera). The B. rapa variety derived for its leaves is the most diversified, consisting of various heading and non-heading groups. As one of the biodiversity centers of B. rapa in the world, China has abundant resources of B. rapa all over the country. B. rapa have been cultivated there for thousands of years as very important vegetables and feeds and as an oil source.
With the commercialization of transgenic rapeseed in the world, the problems concerning the transgene dispersal from B. napus to B. rapa are brought much into focus. It has long been known that natural interspecific crossing can and does occur among the oilseed Brassica species (i.e., B. napus, B. rapa and B. juncea) (Bing et al. 1996; Jorgensen and Andersen 1994; Frello et al. 1995) and in some countries a weedy form of B. rapa occurs. Both pre- and postzygotic barriers, e.g., temporal divergence, gametic and zygotic incompatibility, hybrid inviability and sterility have the potential to reduce gene flow between species (Levin 1978). If the barriers are incomplete, first and later generation hybrids may be formed and function as a bridge for gene transfer. Different alleles may flow from one species into another at different rates, depending on rates of dispersal, the strength of selection, breeding system, and linkage to selected loci (Christiansen et al. 1995).
The use of transgenic rapeseed in production requires a careful consideration of the possible risks related to the unintended spread of transgenes into new habitats. Management measures for reducing the gene flow from transgenic populations are needed in order to prevent possible unwanted effects of transgenes on ecosystems (Ellstrand and Hoffman 1990; Raybould and Gray 1993, 1994; Snow and Palma 1997). Consequently, it is important to quantify the gene flow and to try to establish strategies to control or minimize it, while accounting for the possible ecological effect of the newly introduced genes, whether advantageous or disadvantageous.
Although many studies have investigated crossability between B. napus and B. rapa, many of them have been done with wild B. rapa (Jorgensen and Andersen 1994; Halfhill et al. 2001, 2002; Hansen et al. 2001; Snow et al. 1999) or oilseed rape (Li et al. 2006; Metz et al. 1997; Zhao et al. 2005), and very few studies have been done on the gene flow from transgenic rapeseed to various subspecies and varieties of B. rapa. The aim of this study was to quantify the gene transferability from B. napus to various subspecies and varieties of B. rapa and to estimate the germination ability of the interspecific hybrids.
Materials and methods
Plant materials
Glyphosate-tolerant transgenic Brassica napus c.v. GT73 was provided by Monsanto Company. Phosphinothricin-tolerant transgenic B. napus c.v. Ms8 × Rf3 was provided by Byer Crop Science Company. One hundred eighteen cultivars of B. rapa were maintained by Kerun Vegetable Research Institute of Tianjin, including 60 cultivars of B. rapa L. ssp. pekinensis Olsson, 33 cultivars of B. rapa L. ssp. chinensis var. chinensis Kitam, 4 cultivars of B. rapa L. ssp. chinensis var. purpurea Mao, 10 cultivars of B. rapa L. ssp. chinensis var. parachinensis Tsen et Lee, 6 cultivars of B. rapa L. ssp. chinensis var. rosularis Tsen et Lee, 3 cultivars of B. rapa L. ssp. chinensis var. oleifera and 2 cultivars of B. rapa L. ssp. raifera Matzg (Table 1). The representative morphologies of the various subspecies and varieties are shown in Fig. 1.
Representative morphologies of various subspecies and varieties of B. rapa. a Brassica rapa L. ssp. pekinensis Olsson; b Brassica rapa L. ssp. chinensis var. chinensis Kitam; c B. rapa L. ssp. chinensis var. purpurea Mao; d B. rapa L. ssp. chinensis var. parachinensis Tsen et Lee; e B. rapa L. ssp. chinensis var. rosularis Tsen et Lee; f B. rapa L. ssp. raifera Matzg; g B. rapa L. ssp. chinensis var. oleifera
GT73 and Ms8 × Rf3 are two representative transgenic rapeseed events, the cultivars from which have occupied over 90% of the cultivation area of the total transgenic rapeseed commercialized so far. One hundred eighteen cultivars varieties of B. rapa represent almost all of the types of the cultivated B. rapa in China.
Interspecific crossability analysis
Experiments were conducted in the greenhouse. The interspecific crossability analysis was made with B. napus c.v. GT73 as the pollen donor and 118 cultivars of B. rapa as the seed parents, respectively by means of artificial emasculation and crossing (Table 1). For each cross combination, about 100–200 flowers from 10 separate plants were crossed. The pod number and seed number per pod were investigated after harvest. The crossability index was calculated using the formula:
Crossability index = Number of full seeds obtained/Number of flowers pollinated
Seed germination test
The germination rate of the F 1 hybrid seeds from 34 cross-combinations was measured with two replications with B. napus c.v. Zhongyou 821 as a control to provide the normal germination rate estimate. Germination was conducted in a 90 mm Petri dish padded with a moistened filter paper disc, on which 100 seeds were placed evenly, in a growth chamber at a temperature of 23°C, humidity of 90% and day length of 12 h (3000–6000 lux). Germinated seeds were counted after 7 days to estimate the germination rate as described in Lu et al. (2001).
Seed morphology observation
The F 1 hybrid seeds of transgenic rapeseed and the parents were observed and photographed under Olympus stereromicroscope SZX12.
Spontaneous outcrossing rate analysis
Experiments were conducted at the Hanchuan Experimental Station of the Inspection, Detection and Testing Center of Transgenic Crops Environmental Biosafety of the Ministry of Agriculture (Wuhan). The spontaneous outcrossing rate was assessed using B. rapa c.v. Wutacai, c.v. Wuyuemanqingcai and c.v. Shiyuehong as the seed parents and transgenic B. napus c.v. Ms8 × Rf3 as the pollen donor. B. napus c.v. Zhongyou 821(CK) was used as the control of the seed parents. The experiments were carried out in a randomized block design with 3 replicates. Each plot contained two rows of transgenic rapeseed and two rows of a seed parent. The space between rows and plants was 35 × 25 cm and each row was 6 m long. To synchronize the flowering time, the seed parents were sown at two times, each at an interval of one row every 15 days. All plants were pollinated naturally. After maturation, the seeds were harvested and then sown again to estimate the outcrossing rate.
Rate of herbicide resistance plants
The outcrossing rate was estimated by the survival rate of the plants after treatment with the herbicide BASTA. The experiments were carried out with a randomized block design with 4 genotypes and 3 replications (Table 3). Each plot was 10 m2 in size with an established plant density of about 200–300/m2. Seedlings at the 4–5 leaf stage were sprayed with 1:200 diluted 13.5% BASTA herbicide. The second spray was conducted 4 days later. Both dead and survived seedlings were counted 4–7 days later, after the second spraying. The spontaneous outcrossing rate was calculated using the formula:
Outcrossing rate = 100 × Number of herbicide-tolerant plants/total number of plants treated.
Hybridity confirmation by PCR
The plants that survived herbicide treatment were analyzed by PCR in order to prevent the false positive results. About 1.0 g of leaves from each plant was selected for DNA extraction using the improved 1% sodium dodecyl sulfate (SDS) method. Primers of the bar gene were designed according to the coding region of the sequence of GenBank (Accession NO. AY582737) as BarF: ATCGGATCCATGAGCCCAGAACGACGCCC, and BarR: ATCAAGCTTCAGATTTCGGTGACGGGCA, producing a 544 bp fragment after amplification. The 20 μl PCR mixture contained 0.2 mmol/l dNTPs, 2 mmol/l MgCl2, 1× Buffer, 0.15 mmol/l primers, 30 ng template DNA, and 1 unit of Taq polymerase. The PCR amplification was performed in PE9700 with the program as follows: 4 min at 95°C, 35 cycles of 30 s at 94°C, 30 s at 60°C, and 1 min at 72°C, and a final extension step of 7 min at 72°C. The PCR products were analyzed by electrophoresis using a 1% agarose gel.
Pollen germination and fertilization
The pollen germination and fertilization processes of B. napus on B. rapa stigma were observed with the self-pollinated B. rapa as a control by aniline blue fluorescence (ABF) staining under a microscope according to the protocol of Hu (1994). B. rapa ssp. rosularis Tsen et Lee(Wutacai), B. rapa ssp. chinensis Kitam (Wuyuemanqingcai) and B. rapa ssp. oleifera (0069) were crossed separately as female parents with the phosphinothricin-tolerant B. napus c.v. Ms8 × Rf3. The flowers sampled at 1, 3, 5, 8, 27, 31 and 48 h after pollination were rinsed, fixed and stored in 70% alcohol after 1 h fixation in Carnoy’s Fluid to assess the pollen germination and fertilization.
Results
Crossability test
In total 20,225 flowers of 118 B. rapa varieties were emasculated and pollinated with GT73. On average, 171.4 flower buds were pollinated for each variety and 5.7 seeds were obtained from one pollination. The crossability varied largely from 0.05 to 19.4 seeds per pollination, depending on different seed parents, with 5 seed parents bearing over 15 seeds, accounting for 4.2% of the total cross combinations, 22 parents bearing more than 10 seeds, for 18.6%, and 20 parents bearing fewer than 3 seeds, for 17% (Table 1). Degao 8, a heading type of B. rapa, showed the highest crossability (19.4 seeds/flower) and Zhongmaowawacai, a non-heading type, exhibited the lowest crossability (0.05 seeds/flower). Luxing 80, 87 Chun 34, Yangchun, Chunqiuyu and Qiangshi also showed relatively high crossability (>14 seeds/pod), whereas the varieties Wenzhoupancai, Aijiaosuzhouqing, Japanese Zaoshengxiaobaicai, Qiulu 55, Chihua 4 and Xiawangaiqiqing produced fewer than 2 seeds per pollination (Table 1). On average, B. rapa L. ssp. pekinensis showed the highest crossability (7.86), followed by B. rapa L. ssp. chinensis var. rosularis (7.04), B. rapa L. ssp. chinensis var. purpurea (6.77), B. rapa L. ssp. chinensis var. parachinensis (5.32), B. rapa L. ssp. chinensis var. chinensis (5.06), B. rapa L. ssp. chinensis var. oleifera (4.91) and B. rapa L. ssp. raifera (3.18) (Table 2). A groupwise t-test showed that the difference between the heading and the non-heading types of B. rapa L. was highly significant (P = 0.00022), and no significant differences were found among the non-heading types of B. rapa.
Spontaneous outcrossing rate
The spontaneous outcrossing rate was measured with three B. rapa varieties. The highest outcrossing rate was 0.406%, found in Wutacai (B. rapa L. ssp. chinensis var. rosularis Tsen et Lee), followed by 0.144% in Wuyuemanqingcai (B. rapa L. ssp. chinensis var. chinensis Kitam) and 0.039% in Shiyuehong (B. rapa L. ssp. chinensis var. purpurea Mao) (Table 3). A groupwise t-test showed that the difference between Wutacai and Zhongyou 821 (CK) was not significant (P = 0.468) but the differences between Wutacai and Wuyuemanqingcai or Shiyuehong were significant (P = 0.045 and P = 0.002). However, differences between Wuyuemanqingcai and Shiyuehong were not significant (P = 0.231). This was consistent with the results of the artificial crossability, suggesting that the stronger the crossability, the higher was the spontaneous outcrossing rate. The frequency of gene flow to Wutacai was higher than that of Wuyuemanqingcai and Shiyuehong.
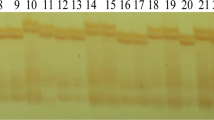
All hybrid plants were confirmed by PCR assay. The results showed that a target DNA band with over 500 bps was detected from all tolerant plants and the positive control Ms8 × Rf3 but not from the sensitive plants after PCR amplification (Fig. 2), indicating that herbicide-tolerant plants contain the bar gene from the spontaneous outcrossing.
Identification of resistant plants by PCR. M DNA Marker; 1 transgenic rapeseed Ms8Rf3; 2 no template control; 3 negative control; 4–6 resistant plants of Wutacai; 7–9 sensitive plants of Wutacai; 10–12 resistant plants of Wuyuemanqingcai; 13–15 sensitive plants of Wuyuemanqingcai; 16–18 resistant plants of Shiyuehong; 19–21 sensitive plants of Shiyuehong
Pollen germination and fertilization
When B. rapa L. ssp. chinensis var. rosularis (Wutacai) was pollinated with transgenic rapeseed, about 8–15 adhesive pollens were observed on the stigma 1 h after pollination (HAP; Fig. 3b). The number increased to 30–50 at 3 HAP, with a few pollen tubes found in stigma. At 8 HAP, the beam of pollen tubes penetrated into the style, and the pollen tubes reached approximately the upper 1/3 of the style (Fig. 3d). At 27 HAP, the pollen tubes penetrated into the lower part of the style, with 10–20 pollen tubes in each style (Fig. 3f). At 48 HAP, some pollen tubes penetrated into the ovule via the micropyle and they began to fertilize, whereas bright yellow-green pollen tubes were seen on the micropyle (Fig. 3h). In the self-pollinated control, 50–100 adhesive pollens were observed on the stigma for an hour after self-pollination (Fig. 3c), Three hours later, the number of adhesive pollens increased to 200–300 and many germinated and penetrated into papilla cells. Eight hours later, more than 50 beams of pollen tubes penetrated into half the style (Fig. 3e). Twenty-seven hours after pollination, the pollen tubes had penetrated into the ovule via the micropyle and began to fertilize (Fig. 3g). Forty-eight hours later, it was clear that the bundled pollen tubes had penetrated into the ovule and fertilized completely (Fig. 3i).
a No pollination of B. rapa; b B. rapa pollinated with pollens of Ms8 × Rf3 at 1 HAP; c Self-pollination of B. rapa at 1 HAP; d B. rapa pollinated with pollens of Ms8 × Rf3 at 8 HAP; e Self-pollination of B. rapa at 8 HAP; f B. rapa pollinated with pollens of Ms8 × Rf3 at 27 HAP; g Self-pollination of B. rapa at 27 HAP; h B. rapa pollinated with pollens of Ms8 × Rf3 at 48 HAP; i Self-pollination of B. rapa at 48 HAP
Similar results were also found in B. rapa L. ssp. chinensis var. chinensis Kitam (Wuyuemanqingcai) and B. rapa L. ssp. chinensis var. oleifera (0069), which were pollinated with transgenic B. napus. There were 8–15 adhesive pollens on the stigma of B. rapa for 1 HAP and 30–50 adhesive pollens 3–8 HAP with a few pollens germinating. A large amount of callose was observed on the surface of the papillary cells that touched the pollen tubes. At 27 HAP, a few pollen tubes were seen penetrating into the bottom of the styles, whereas numerous pollen tubes penetrated into the ovary and began to fertilize the eggs in the control. Up to 48 HAP, a few pollen tubes were still seen in ovaries and were begining to fertilize the eggs.
The above observation indicated that the number of pollens on the stigma was reduced by 80%, the number of pollen tubes in the style were reduced by 2/3, and the fertilization time was delayed by over 20 h when pollinated with the transgenic B. napus in comparison with the bud self-pollination of B. rapa as a control.
Seed morphology
The seeds of B. napus c.v. GT73 were large and black, whereas the seeds of B. rapa observed in this experiment were generally small and red (Fig. 4). When B. rapa was pollinated with GT73, the F 1 hybrids tended to have an intermediate size, red seed coat and high frequency of cracked seeds (Fig. 4b, e–g), which was not found in the F1 hybrids when the resynthesized B. napus was used as the male parent (data not cited). The rate of the cracked seeds varied with cross-combinations from 15 to 70%, generally about 30%.
Seed germination ability
The seeds collected from the B. rapa plants pollinated with transgenic B. napus germinated poorly (Fig. 5; Table 4). The germination rate of the seeds without discarding the cracked seeds averaged 19.7%, with a range from 1 to 70%. B. rapa L. ssp. pekinensis Olsson showed the highest germination ability, with an average rate of 41.7% and B. rapa L. ssp. raifera Matzg exhibited the lowest, with an average rate of 3%. B. rapa L. ssp. chinensis var. rosularis Tsen et Lee, B. rapa L. ssp. chinensis var. oleifera, B. rapa L. ssp. chinensis var. parachinensis Tsen et Lee, B. rapa L. ssp. chinensis var. purpurea Mao and B. rapa L. ssp. chinensis var. chinensis Kitam showed a medium germination rate ranging from 5 to 17.7%. Once discarding the cracked seeds, the germination rate of the hybrids increased conspicuously to an average of 72.9%, ranging from 36 to 98%, which did not differ statistically from the rate of control B. napus c.v. Zhongyou 821 (P = 0.587). The highest germination rate (98%) was found in the Zaoshubaicaitai hybrid and the lowest (36%) in the Gaohuaqinggengbaicai hybrid. The results indicated that the cracked seeds germinate poorly. On the other hand, the germinated interspecific hybrids appeared to grow faster and showed significant heterosis compared with the control Zhongyou 821 (Fig. 5). Different repetitions of the same genotype were consistent in the germination rate, indicating that the germination rate was highly inherited.
Discussion
Numerous studies have investigated the gene flow of transgenic rapeseed to wild weed B. rapa and oilseed rape B. rapa (Paul et al. 1995; Baranger et al. 1995; Mikkelsen et al. 1996; Rieger et al. 1999; Messeguer 2003; Brown 1995), but few studies have been conducted on various subspecies or varieties of B. rapa. In this study, we investigated the interspecific crossability of 118 cultivars from 7 B. rapa subspecies and varieties, and showed that all of the B. rapa varieties could bear seeds by artificial pollination with transgenic B. napus. Different varieties can exhibit different levels of crossability and outcrossing under the same experimental and environmental conditions (Tables 2, 3). The average crossability of B. rapa by the transgenic B. napus was 5.7 seeds per flower, with a range from 0.05 to 19.4, which was about 1/3 of the self-pollination. On the whole, the heading types of B. rapa showed a higher crossability than the non-heading types of B. rapa.
The variation of crossability among the different genotypes of B. rapa may derive from different factors: (1) Adhesion of pollens to the stigma; (2) Penetration of pollen tubes into the style; and (3) Abortion of fertilized embryos during development. Compared with the flower-bud self-pollination of B. rapa, the amount of pollen adhesion on the stigma and the number of pollen tubes in the style were significantly reduced. Furthermore, the amount of callose deposited in papillose cells increased with the pollination of B. napus on B. rapa (Fig. 3), indicative of reproductive barriers before fertilization, which was in agreement with the reports of Meng (1990). Many studies also reported that there were similar fertilization barriers with self-incompatibility during the self-pollination of B. rapa. Although most varieties of B. rapa used in this experiment were self-incompatible, they did not show such heavy self-incompatibility because we made the crosses with the flower-buds and the self-incompatibility had not yet developed.
Under natural pollination, the outcrossing rate between B. rapa and the transgenic B. napus ranged from 0.039 to 0.406%, with an average of 0.19%, which was about 1/3 of the rate of the control (Table 3). Scott and Wilkinson (1998) found that the spontaneous outcrossing rate of B. napus and B. rapa ranged from 0.4 to 1.5%, which was closer to our results in this experiment. However, other researchers reported that the spontaneous outcrossing rate of B. napus and B. rapa was as high as 9–93%, with the condition that a B. rapa plant was surrounded by a large number of B. napus plants (Jorgensen et al. 1996). In addition to the genotypic difference of crossability, the spontaneous outcrossing rate is also affected by flowering synchrony, the height of parental plants, the topography and weather factors (Scheffler et al. 1995; Staniland et al. 2001). In addition, in the case of homozygous glyphosate and glufosinate resistant plant lines, all pollen carries the herbicide resistance gene. By contrast, in studies of cross-fertilization from glufosinate-resistant hybrids, the amount of transgenic pollen was lower, resulting in only about 5/8 of the outcrossing frequencies of homozygous herbicide-resistant lines.
If interspecific fertilization does take place, the F 1 hybrid embryos and plants often suffer from malfunction and low survival and fertility upon reproduction (Stebbins 1958). The interspecific embryos were aborted and stopped during their development to seeds at various stages (Fig. 4). Only some of the embryos developed to seeds with normal germination ability (Table 4; Fig. 5). Usually a pistil of B. rapa has about 30 ovules and, on average, 1/6 of the ovules develop into normal seeds after pollination with B. napus c.v. GT73, which means that about 5/6 of the ovules were aborted in various stages of the development.
A high frequency of broken or germinated seeds was found in pods in all 118 interspecific hybrids, whereas few were found in hybrids when the resynthesized B. napus was used as the male parent, indicating that the frequency of broken seeds in pods was highly related to the genotype of the pollen donor. On the other hand, hybrids derived from different female parents showed significant differences in the frequency of broken seeds in the pods. For example, with the same transgenic rapeseed GT73 as the male parent hybrids from Gongguan2, Yuqingbaicai and Luxing80 showed relatively low frequency of broken seeds (<15%), whereas most of the B. rapa varieties as females showed frequencies higher than 40%. Broken seeds observed in this experiment may have significant ecological and agronomical impacts due to the reduced fitness of the seeds of interspecific hybrids and the reduced quality of seeds of B. rapa vegetables.
Broken or germinated seeds were also reported by other researchers in a similar experiment (Hauser and Ostergard 1999) and was called precocious germination in the pod. According to our observation, however, the broken seeds in pods resulted not only from precocious germination but also from the abortion of the fertilized embryos at various developmental stages. According to previously published work (Black 1991), in immature seeds, the high abscisic acid (ABA) levels prevent precocious germination, whereas desiccation does the same in dry seeds. This indicates that ABA and low water potentials control seed germination. The seed-specific immunomodulation resulted in the switch from seed maturation to germination (Phillips et al. 1997). Therefore, the precocious germination found in this experiment may result from the wrong timing of the switch from the seed maturation to a germination. In general, seeds of B. rapa are smaller than seeds of B. napus. The hybrids look like B. rapa female parents in seed size and seed color (Fig. 4). Because the hybrid embryos come from the fertilization of both parents, whereas the pods and the seed coat comprised maternal tissue coming from the B. rapa female parent, the disharmonization of the developing embryos in a limited space of seed coat and pod may be partly responsible for the high frequency of broken seeds found in this experiment.
So far, few reports exist on the germination rate and morphological features of the hybrid seeds between B. rapa and B. napus. We found that the germination rate of the full seeds of the interspecific hybrids ranged from 36% to 98%, with an average of 72.9%, which a little lower than the rate of normal seeds. By contrast, the cracked seeds showed a much lower germination ability.
The interspecific hybrid between B. rapa and B. napus is a sesquidiploid, with an AAC genome composition and a chromosome number of 2n = 29. The F 1 hybrid plants grew well and bore an average of 4 seeds per pod in open pollination (Lu et al. 2002). A mean of 0.1–2.0 seeds per flower were set in the backcrosses of the interspecific hybrids to B. rapa (Lu et al. 2002). Most backcross progenies were aneuploid (Lu et al. 2001), and their survival fitness was about one-tenth of the normal fitness. The backcross progeny was relatively weak in growth and very low in pollen and seed fertility, with only a few exceptions that were close to the normal rates (Lu and Kato 2001; Hauser et al. 1998a, b). If there was no pressure of positive selection, the frequency of the exogenous gene in the population of B. rapa would gradually decline and disappear (Lu et al. 2002). However, if foreign genes immigrated continuously, especially under favorable selection pressure, there should be a greater possibility that the foreign genes will be integrated into B. rapa (Lu et al. 2002). The spread of transgenic plants to conventional populations depends on the traits introduced by genetic modification and on the fitness advantage of the genotypes carrying the transgene. Therefore, careful estimation of the relative fitness of transgenic plants in conventional populations is needed to assess the risk of transgene spread.
Some practical measures suggested to control gene flow are isolation distances and local and aerial restrictions and barriers (Eastham and Sweet 2002). It appears that the use of predominantly self-pollinating, male sterile, or cleistogamous cultivars as a biological containment strategy will also reduce gene flow. Our data indicated that such genotypes of B. rapa as the cultivar Zhongmaowawacai tend to be very low in crossability with B. napus in favor of reduction of gene flow. On the other hand about 4% of the B. rapa cultivars showed high crossiblity with B. napus, being over 15 seeds per pollination. For those B. rapa cultivars more strict measures should be taken in order to prevent the pollen contamination. This is particularly important for vegetable seed production, because the pollen contamination may result in high frequency of cracked seeds.
In summary, the gene transferability from B. napus to various B. rapa subspecies (or varieties) were documented. The interspecific crossability varied with heading and non-heading varieties and with different cultivars. The natural outcrossing rates were much lower than the artificial crossability, indicating that it should be possible to keep the adventitious presence of the off-plants under the allowed threshold, if proper measures are taken.
References
Baranger A, Chever AM, Eber F, Renard M (1995) Effect of oilseed rape genotype on the spontaneous hybridization rate with weedy species: an assessment of transgene dispersal. Theor Appl Genet 91(6–7):956–963. doi:10.1007/BF00223906
Bing DJ, Downey RK, Rakow GFW (1996) Hybridization among Brassica napus, B. rapa and B. juncea and their two weedy relatives B. nigra and Sinpis arvensis under open pollinations in the field. Plant Breed 115:470–473. doi:10.1111/j.1439-0523.1996.tb00959.x
Black M (1991) Involvement of ABA in the physiology of developing and mature seeds. In: Daviesa WJ, Jones HG (eds) Abscisic acid: physiology and biochemistry. Bios Scientific, Oxford, pp 99–124
Brown AP (1995) Gene transfer between Canola (B. napus and B. campestris) and related weed species. In: Proceedings of 9th International Rapeseed Congress, Cambridge, UK, 4: 1445–1447
Christiansen FB, Andreasen V, Poulsen ET (1995) Genotypic proportions in hybrid zones. J Mathemat Biol 33:225–249
Eastham K, Sweet J (2002) Genetically modified organisms (GMOs): the significance of gene flow through pollen transfer. Environmental issue report no 28. European environmental agency. http://www.ewindows.eu.org/Agriculture/GMOs/gmospollen
Ellstrand NC, Hoffman CA (1990) Hybridization as an avenue of escape for engineered genes. Bioscience 40:438–442. doi:10.2307/1311390
Frello S, Hansen KR, Jensen J, Jorgensen RB (1995) Inheritance of rapeseed (Brassica napus) specific RAPD markers and a transgene in the cross B. juncea × (B. juncea × B. napus). Theor Appl Genet 91:236–241. doi:10.1007/BF00220883
Halfhill MD, Richards HA, Mabon SA, Stewart CNJ (2001) Expression of GFP and Bt transgenes in Brassica napus and hybridization and introgression with Brassica rapa. Theor Appl Genet 103:362–368. doi:10.1007/s001220100613
Halfhill MD, Millwood RJ, Raymer PL, Stewart CNJ (2002) Bt transgenic oilseed rape hybridization with its weedy relative, Brassica rapa. Environ Biosafety Res 1:19–28. doi:10.1051/ebr:2002002
Hansen LB, Siegismund HR, Jorgenson RB (2001) Introgression between oilseed rape (Brassica napus L.) and its weedy relative B. rapa L. in a natural population. Genet Resour Crop Evol 48:621–627. doi:10.1023/A:1013825816443
Hauser TP, Ostergard H (1999) Precocious germination of Brassica rapa × B. napus seeds within pods. Hereditas 130:89–93. doi:10.1111/j.1601-5223.1999.00089.x
Hauser TP, Shaw RG, Ostergard H (1998a) Fitness of F 1 hybrids between weedy Brassica rapa and oilseed rape (B. napus). Heredity 81:429–435. doi:10.1046/j.1365-2540.1998.00424.x
Hauser TP, Shaw RG, Ostergard H (1998b) Fitness of backcross and F2 hybrids between weedy Brassica rapa and oilseed rape (B. napus). Heredity 81:436–443. doi:10.1046/j.1365-2540.1998.00425.x
Hu SY (1994) Method of preparation of slides used to examine the pollen germination on the stigma and pollen tube growth in the style. Chin Bulletion Bot 11:58–60
Jorgensen RB, Andersen B (1994) Spontaneous hybridization between oilseed rape (Brassica napus) and weedy B. Campestris: a risk of growing genetically modified oilseed rape. Am J Bot 81(12):1620–1626. doi:10.2307/2445340
Jorgensen RB, Anderson B, landbe L, Mikkelsen TR (1996) Spontaneous hybridization between oilseed rape (Brassica napus) and weedy relatives. Acta Hortic 407(1–2):193–200
Levin DA (1978) The origin of isolating mechanisms in flowering plants. Evol Biol 11:185–317
Li J, Guan CY, Li X, Chen SY, Zheng Z, Chen PP (2006) Barstargene transfer from transgenic rapeseed to relatives. J Hunan Agric Univ 32(6):591–595
Lu CM, Kato M (2001) Fertilization fitness and relation to chromosome number in interspecific progeny between Brassica napus and B. rapa: a comparative study using natural and resynthesized B. napus. Breed Sci 51:73–81. doi:10.1270/jsbbs.51.73
Lu CM, Shen FS, Hu K (2001) Interspecific heterosis in hybrids between Brassica napus and B. rapa Sabrai. J Genet Breed 30:73–86
Lu CM, Kato M, Kakihara F (2002) Destiny of a transgene escape from Brassica napus to B. rapa. Theor Appl Genet 105:78–84. doi:10.1007/s00122-001-0856-2
Meng JL (1990) Studies on pollen-pistil interaction between Brassica napus and its relative species and genus. Acta Agronomica Sin 16(1):19–25
Messeguer J (2003) Gene flow assessment in transgenic plants. Plant Cell 73:201–212
Metz PLJ, Jacobsen E, Nap JP, Pereira A, Stiekema WJ (1997) The impact on biosafety of the phosphinothricin-tolerance transgene in inter-specific B. rapa × B. napus hybrids and their successive backcrosses. Theor Appl Genet 95:442–450. doi:10.1007/s001220050581
Mikkelsen TR, Andersen B, Jorgensen RB (1996) The risk of crop transgene spread. Nature 380:31. doi:10.1038/380031a0
Paul EM, Thompson C, Dunwell JM (1995) Gene dispersal from genetically modified oil seed rape in the field. Euphytica 81:283–289. doi:10.1007/BF00025619
Phillips J, Artsaenko O, Fiedler U, Horstmann C, Mock H, Müntz K, Conrad U (1997) Seed-specific immunomodulation of abscisic acid activity induces a developmental switch. EMBO J 16:4489–4496. doi:10.1093/emboj/16.15.4489
Raybould AF, Gray AJ (1993) Genetically modified crops and hybridization with wild relatives: a UK perspective. J Appl Ecol 30:199–219. doi:10.2307/2404623
Raybould AF, Gray AJ (1994) Will hybrids of genetically modified crops invade natural communities? Trends Ecol Evol 9:85–89. doi:10.1016/0169-5347(94)90201-1
Rieger MA, Preston C, Powles SB (1999) Risks of gene flow from transgenic herbicide-resistant oilseed rape (Brassica napus) to weedy relatives in southern Australian cropping systems. Aust J Agric Res 50:115–128. doi:10.1071/A97138
Scheffler JA, Parkinson R, Dale PJ (1995) Evaluating the effectiveness of isolation distances for field plots of oilseed rape (Brassica napus) using a herbicide-resistance transgene as a selectable marker. Plant Breed 114:317–321. doi:10.1111/j.1439-0523.1995.tb01241.x
Scott SE, Wilkinson MJ (1998) Transgene risk is low. Nature 393:320. doi:10.1038/30642
Snow AA, Palma PM (1997) Commercialization of transgenic plants: potential ecological risks. Bioscience 47:86–96. doi:10.2307/1313019
Snow AA, Andersen B, Jorgensen RB (1999) Costs of transgenic herbicide resistance introgressed from Brassica napus into weedy B. rapa. Mol Ecol 8:605–615. doi:10.1046/j.1365-294x.1999.00596.x
Staniland BK, McVetty PBE, Friesen LF, Yarrow S, Freyssinet G, Freyssinet M (2001) Effectiveness of border areas in confining the spread of transgenic Brassica napus pollen. Can J Plant Sci 80:521–526
Stebbins GL (1958) The inviability, weakness, and sterility of interspecific hybrids. Adv Genet 9:147–215. doi:10.1016/S0065-2660(08)60162-5
Zhao XX, Lu WP, Qi CK, Pu HM, Xia QX, Lu DL, Liu GS, Wang YP (2005) Assessment on alien herbicide-resistant gene flow among crucifers by sexual compatibility. Chin Sci Bull 50(15):1604–1611. doi:10.1360/982005-621
Acknowledgments
This study was supported by the Major Special Project for Development of Transgenic Organisms (2008–2010), Ministry of Agriculture, China and the Social Welfare Project on Environmental Protection, Ministry of Environmental Protection, China (2009–2011).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xiao, L., Lu, C., Zhang, B. et al. Gene transferability from transgenic Brassica napus L. to various subspecies and varieties of Brassica rapa . Transgenic Res 18, 733–746 (2009). https://doi.org/10.1007/s11248-009-9261-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-009-9261-4