Abstract
Transgenic founder rabbits carrying a gene construct consisting of a 2.5 kb murine whey acidic protein promoter (mWAP), 7.2 kb of the human clotting factor VIII (hFVIII) cDNA and 4.6 kb of 3′ flanking sequences of mWAP gene were crossed for three generations. All transgenic animals showed stable transgene transmission. Transgenic females showed high level of recombinant hFVIII (rhFVIII) mRNA expression in biopsed mammary gland tissues, while marginal expression of rhFVIII mRNA was observed in the spleen, lung and brain. No adverse effects of ectopic expression on the physiology of the rabbits were observed. Expression was not detected in the liver, kidney, heart and skeletal muscle. In transgenic females derived from three generations, rhFVIII protein was secreted from the mammary gland of lactating females, as shown by Western blotting. Biological activity of rhFVIII protein, as revealed in clotting assays was ranged from 0.012 to 0.599 IU/ml corresponding to 1.2% and 59.9% of the hFVIII level in normal human plasma. No apparent effect of secreted rhFVIII on the milk performance of rabbits was observed. Our results confirm the possibility of producing a significant amount of a biologically active rhFVIII in the mammary gland of established transgenic rabbit lines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An abnormality or deficiency of coagulation factor VIII in human blood causes a common bleeding hemophilia A disorder occurring in one of 10,000 males. Treatment of this X-linked genetic disease involves replacement therapy with FVIII concentrates prepared from pooled plasma or with hFVIII produced by recombinant technology (rhFVIII). Recombinant human factor VIII is currently produced commercially in cell culture systems. Unfortunately, the available amounts do not meet the worldwide demands, especially for prophylaxis, and the obtained rhFVIII shows considerable variability in post-translational modifications (Garber 2000).
In recent years it has been shown that established lines of transgenic animals can be used as a source of biologically active proteins produced in their mammary gland (Lubon et al. 1996) and such technologies have progressed to the point of advanced stages of clinical trials (Dove 2000). The first recombinant protein (Atryn® = recombinant human antithrombin III) has been approved as regular drug by the European Medicine Agency (EMEA).
Transgenic rabbits offer an attractive alternative to large dairy animals because of their short reproduction cycle, easy milking and the relatively high yield from lactating females of milk with high protein content (e.g. 2.5 times higher compared to sheep milk). These facts, together with the feasibility of generating transgenic rabbits are reflected in more than 30 reports found in the literature of bioreactive protein production in rabbit milk (Fan and Watanabe 2003).
To express hFVIII protein, different mammary gland specific hFVIII gene constructs have previously been used in different animals. Transgenic pigs, sheep, rabbits and mice have been generated and variable levels of rhFVIII expression were obtained depending on the regulatory sequences employed. Usage of a construct consisting of the hFVIII cDNA directed by mouse WAP promotor resulted in transgenic pigs expressing rhFVIII (Paleyanda et al. 1997), a gene construct consisting of ovine β-lactoglobulin (B-LG)-hFVIII cDNA led to low level expression in transgenic sheep (Niemann et al. 1999), whereas a bovine α-lactalbumin (A-LA)-hFVIII-bGHp(A) gene construct expressed rhFVIII at higher levels in transgenic mice (Chen et al. 2002). Our transgenic rabbit founders derived using the mouse WAP-hFVIII cDNA construct also expressed higher levels of rhFVIII (Chrenek et al. 2005), than transgenic pigs or transgenic rabbits with the mWAP-hFVIII-MT1 gene construct (Hiripi et al. 2003).
Here we report the expression of human clotting factor VIII in the mammary gland on three generations of transgenic rabbits used as a model.
Materials and methods
Animals
Transgenic rabbit founders (female no. 10 produced by single microinjection—SM and female no. 36 produced by double microinjection—DM) carrying mammary gland specific construct consisting of a 2.5 kb murine whey acidic protein promoter (mWAP), 7.2 kb cDNA of the human clotting factor VIII (hFVIII), and 4.6 kb of 3′ flanking sequences of mWAP gene were used (Chrenek et al 2005). The founder rabbits were mated with non-transgenic rabbits of the same breed to obtain F1 hemizygous generation. Transgenic rabbits from F1 generation were crossed to obtain F2 homozygous animals. To obtain heterozygous F3 generation, transgenic females from F2 generation were bred with non-transgenic males. The lines derived from female founder include:
Line SM: female 10 | female 10-1 | female 1-3 | female 1-3-5 |
|---|---|---|---|
female 1-9 | female 1-9-7 | ||
(F0) | (F1) | (F2) | (F3) |
Line DM: female 36 | female 36-2 | female 36-2-1 |
|---|---|---|
(F0) | (F1) | (F2) |
PCR analysis
Total DNA was isolated from ear tissues of newborn rabbits. As a negative control, DNA isolated from non-transgenic rabbits was used. DNA was subjected to 30 cycles of amplification (30 s at 94°C, 30 s at 64°C, and 30 s at 72°C) using hFVIII gene-specific primers: 5′-GTA GAC AGC TGT CCA GAG GAA-3′ and 5′-GAT CTG ATT TAG TTG GCC CAT C-3′ that result in a PCR product of 578 bp (Paleyanda et al. 1997).
Milk yield
Rabbit pups are known to nurse only for approximately 3 min, once every 24 h. Milk consumption or yield during the first lactation was assessed using the weight-suckle-weight method on day 10, 21 and 30 postpartum. The body weight of pups removed from their mothers for a period of 24 h was monitored immediately before and after suckling, and the difference in body weight corresponded to the daily milk consumption.
Milk sample collection
Milk samples were taken from lactating transgenic and non-transgenic rabbit females on the day 10, 21 and 30 of first lactation. In order to stimulate milk down, intramuscular injection of 5IU of oxytocin (Veyx Pharma, Germany) was applied 10 min before milk collection. Thereafter, obtained milk was immediately centrifuged at 10,000 g for 10 min and the upper lipid layer was removed. The samples were either subjected to further analysis or stored at −80°C until used. Milk samples were diluted in PBS for Western blots, in imidazole for clotting assay and in manufacturer buffer for ELISA, as described below.
Content of rabbit milk protein and fat, was investigated using an infrared absorption instrument (Milko-Scan FT 120, Foss Electric, Denmark) according to the manufacturer´s manual.
Purification of rhFVIII
Milk rhFVIII was purified over an immune affinity column. Briefly, immunobeads were prepared by immobilizing the antihuman FVIII Mab 9041 on Sepharose-CL2B using cyanogen bromide. One milligram of Mab was covalently linked to 1 ml of the support, which then bound 110 μg of FVIII antigen. The milk fat was skimmed by centrifugation at 2,000 g, 4°C for 15 min, and the skim milk was dialyzed against 0.05 M imidazole, 0.8 M NaCl, 0.05 CaCl2, pH 7.4. Triton X-100 was added to a concentration of 0.2% and the milk was recentrifuged. Two hundred microliters of a 50% suspension of the immunobeads were added to the supernatant and rotated at 4°C overnight. The supernatant was removed and the immunobeads were washed with 0.1% Triton X-100 in buffer. The bound proteins were then eluted with 40% ethylene glycol in 0.05 M imidazole, 0.04 M CaCl2, pH 6.4.
Western blot detection of rhFVIII
Defatted milk samples were diluted 1:40 in PBS and heated at 95°C for 5 min. Proteins were resolved on 4–15% SDS-PAGE gels (Phast Gels) in the presence of β-mercaptoethanol as a reducing agent and transferred onto a nitrocellulose membrane (Nitrocellulose 0.2 μM, Pierce, USA) by diffusion blotting. Human plasma was used as a positive control. It was diluted 1:30 in PBS buffer (pool of >100 normal plasma donors = coagulation control N, Technoclone, Austria *5020040). Blots were blocked for 1 h in TBS-T Buffer/10% BSA and probed with goat anti-human Factor VIII polyclonal antibody (dilution 1:300, TBS-T/1% BSA, ICN Biomed, USA). Detection of positive bands was carried out using secondary rabbit anti-goat IgG—conjugated with alkaline phosphatase (dilution 1:5,000, Sigma, USA). Visualization of positive signals was achieved using NBT/BCIP substrate.
ELISA
Secretion of rhFVIII into the transgenic milk was determined quantitatively using an ELISA-kit (Asserachrom FVIII:Ag, Diagnostica Stago, France) according to the manufacturer´s manual.
Biological activity of rhFVIII in rabbit milk
The functional activity of rhFVIII in rabbit milk samples was determined by assaying its ability to restore clotting activity using FVIII-depleted human plasma together with a partial prothrombin time reagent (DAPTTIN, Technoclone).
Chromogenic assay
The ability of rhFVIII to generate Factor Xa was assessed using Technochrom F VIII:C reagent kit (Technoclone, Austria) according to the manufacturer’s manual. In brief, activity of FXa is measured by cleavage of a chromogenic substrate. The reagents which contain FIXa and Factor X are designed in such a way that the amount of FXa generated is only dependent on the amount of FVIII present in the sample.
Real time –PCR and sequencing
Tissue samples from the mammary gland, liver, spleen, kidney, lung, skeletal muscle, heart and brain were collected from transgenic and non-transgenic rabbits on day 35 of first lactation and stored immediately in RNAlater ® (Ambion, USA). Total RNA was isolated from tissues using TRIzol® Reagent (Invitrogen Life Technologies, USA) according to the manufacturer’s instructions. The obtained RNA was subsequently treated with Deoxyribonuclease I (MBI Fermentas, Lithuania) to remove any genomic DNA contamination. About 900 ng of total RNA was reverse-transcribed with MuLV Reverse Transcriptase using the GeneAmp® RNA PCR Kit (Applied Biosystems, USA) and Oligo d(T)16 primers.
hFVIII specific sense 5′-TGC CTG ACC CGC TAT TAC TC-3′ and antisense 5′-TGA GGT ACC AGC TTC GGT TC-3′ primers, and hypoxanthine phosphoribosyltransferase housekeeping gene (HPRT) specific sense 5′-CTT TGC TGA CCT GCT GGA TT-3′ and antisense 5′-GCT TGA CCA AGG AAA GCA AG-3′ primers were designed using the PRIMER3 software (Whitehead Institute for Biomedical Research, Cambridge, MA, USA). These primers were used for quantitative real-time PCR (qPCR) performed using LightCycler-FastStart DNA Master SYBR Green I Kit (Roche Diagnostics, Switzerland) at following conditions: 10 s denaturing; 55 amplification cycles consisting of 5 s at 95°C, 15 s at 65°C and 15 s at 72°C. Tissue-specific expression of the rhFVIII was normalized to the expression of the endogenous HPRT gene using a mathematical model by Pfaffl (2001). All obtained PCR products were subsequently analyzed by agarose gel electrophoresis and sequenced by ABI PRISM® BigDye™ Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems, USA) on the ABI PRISM™ 310 Genetic Analyzer (Perkin Elmer, USA).
Statistical analysis
The t-test was used to compare milk yield between transgenic and non-transgenic rabbit females.
Results
Transgenic rabbits
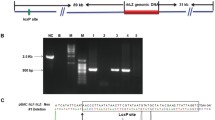
Transgenic rabbit founders were crossed with non-transgenic rabbits to derive the F1 hemizygous generation (females no. 10-1 and no. 36-2). Breeding of transgenic animals from F1 generation resulted in F2 homozygous generation (females no.1-3, 1-9 and no. 36-2-1). To obtain the F3 heterozygous generation (females no. 1-3-5, and no. 1-9-7), transgenic females from F2 generation were bred with non-transgenic males. Stability of transgene transmission via transgenic females was confirmed in all three generations of transgenics females at 43, 100 and 75% for F1, F2 and F3 generation, respectively (Table 1). PCR analysis was employed to detect the integration of the hFVIII transgene construct into the genome of rabbits and hFVIII-specific PCR product of 578 bp was obtained (Fig. 1).
Representative figure of hFVIII transgene integration into genome of rabbits by PCR amplification. Lane 1—no DNA template (negative control), lanes 2, 5, 6 and 7—transgenic rabbits (female 10-1, 1-9, 1-3 and 1-9-7), lanes 3 and 4—non-transgenic rabbits, lane M—DNA marker (GeneRuler 100 bp DNA Ladder Plus, MBI Fermentas). Size of hFVIII-specific PCR product is 578 bp
Milk yield of transgenic females
There were no significant differences between transgenic and non-transgenic females in milk quantity (milk production) and quality (protein and fat content) investigated during the first lactation on day 10, 20 and 30 (Table 1.). This indirectly indicates that the rabbits grew normal and were of normal health. No mortality of pups, except for one pup of female 1-9-7 which was trampled during the birth, was seen.
Western blot analysis
In milk samples obtained at day 10, 21 and 30 from lactating females, a band corresponding to the light chain of rhFVIII-specific (54 kDa) was detected by Western blot analysis. This signal was not detected in non-transgenic milk, but was observed in human plasma (Fig. 2, Table 2).
Representative Western blot analysis of rhFVIII expression in the milk of transgenic rabbits (Day 21). Lane 1—non-transgenic female, lane 2—transgenic female no. 10-1 (F1), lane 3—transgenic female no. 1-3-5 (F3), lane 4—transgenic female no. 1-9-7 (F3) and lane 5—human plasma. Detection was carried out with a goat anti-human Factor VIII polyclonal antibody, AP-linked secondary antibody and NBT/BCIP substrate
Concentration of rhFVIII in rabbit milk
Using ELISA, the concentrations of rhFVIII detected in the milk of transgenic rabbit females ranged within 5–161 μg/ml, depending on generation and lactation day, but also on individual transgenic female (Table 2). No measurable rhFVIII concentrations in non-transgenic rabbit milk were detected.
Functional activity of rhFVIII in rabbit milk
The functional activity of rhFVIII was examined using clotting and chromogenic assays (Table 2). Up to 25 mU/ml of rhFVIII procoagulant activity was detected in milk from female no. 10-1 from F1 generation.
The coagulation clotting activity of rhFVIII in milk samples determined by reduction in the activated partial thromboplastin time (APTT test) varied between animals and generations. The highest activity of rhFVIII was detected in female no. 1-3 from F2 generation on second lactation (599 mU/ml), which corresponds to 59.9% of the level of normal human plasma. On the other hand, the lowest activity of rhFVIII was found in female no. 1-3-5 from F3 generation (12 mU/ml).
Real time—PCR analysis
To evaluate the tissue specific expression pattern of rhFVIII mRNA as driven by the mouse WAP promoter in the tissues of transgenic rabbits, mRNA was prepared from eight different organs and analyzed by Real time—PCR. As expected, high level hFVIII mRNA expression was almost exclusively found in the mammary gland of the transgenic rabbits (Table 3), where 100% homology between all Real time-PCR products and hFVIII cDNA sequence was confirmed by sequencing. Slight hFVIII mRNA expression was detected in the spleen, in the lung and in the brain, while it was absent in the liver, in the kidney, in the skeletal muscle and in the heart of analysed females.
Discussion
Stable transgene transmission and consistent production of recombinant protein in different generations is a prerequisite to establishing transgenic lines for protein production purposes (Van Cott et al. 1997; Zinovieva et al. 1996; Chrenek et al. 2002). Integration of foreign DNA into the embryonic genome with respect to the chromosomal locus is generally a random event. Transgene often inserts into functional genomic sequences. Stability of transgene transmission has been reported in many transgenic animals including mice, rabbits and pigs (Chen et al. 2002; Zinovieva et al. 1996; Chrenek et al. 2002; Van Cott et al. 1997). Our PCR results confirmed the mWAP-hFVIII transmission in all three generations of transgenic females. Present results concerning receiving three generations of transgenic rabbits with integration, expression and transgene transmission of the hFVIII gene correspond to our earlier studies (Chrenek et al. 2006a), where transgene transmission through transgenic males at similar percentage was also obtained in several generations. Efficiency of transgene transmission (100%) allowed us to select homozygous animal in F2 generation.
The presence of the mWAP-hFVIII transgene in rabbit genome and secretion of rhFVIII into milk of transgenic females did not have any adverse phenotypic effect on the offspring. All transgenic rabbit females of F1, F2 and F3 generation had normal reproductive behaviour, producing litters ranging from 7 to 9 offspring, which is in accordance with our previous results (Chrenek et al. 2005). The transgenic animals did not exhibit pathological signs such as infertility, respiratory distress or impairment of mammary gland development and lactation, compared to non-transgenic rabbits, as it was previously found (Suvegova et al. 2004; Dragin et al. 2006). In this study we showed that there were no significant differences between transgenic and non-transgenic females in milk yield investigated on day 10, 21 and 30 at the first lactation. These results confirmed our previous report (Chrenek et al. 2006b) that mammary gland specific transgenic over-expression of hFVIII can be obtained without any strong influence on rabbit milk yield in different lactations, indicating that this technology can be applied to “pharmaceutical farming”.
The endogenous WAP transgene driven by WAP promotor in mice was tissue-specific, expressed predominantly in the mammary tissue and, to a lesser extent, in the salivary glands (Van Cott et al. 1997). When this mouse protein was expressed in transgenic sheep, this transgene contributed to health problems and high morbidity (Wall et al. 1996). In our transgenic rabbits we confirmed high levels tissue-specific expression of hFVIII in the mammary glands. The expression levels of rhFVIII in the lung, spleen and brain were below 1%, while no expression in the liver, kidney, skeletal muscles and heart of these females were found by Real—time PCR. This hFVIII ectopic expression in transgenic rabbits did not lead to any histological changes or health problems (Suvegova et al. 2004; Jurcik et al. unpublished data).
As in previously established transgenic pigs and mice (Paleyanda et al 1997; Chen et al. 2002) we also observed differences in the level of rhFVIII, when different assays for quantification were used. The ELISA measured values from 3 to 161 μg/ml, while activity assays resulted in determination of 12–599 mU/ml of active recombinant protein corresponding to 1.2–59.9% of the level of normal plasma. In spite of that, totally higher rhFVIII concentration in our transgenic rabbits was detected as compared to previously reported rhFVIII concentration in mammary gland of transgenic pigs (Paleyanda et al. 1997), sheep (Niemann et al. 1999), mice (Chen et al. 2002) and rabbits (Hiripi et al. 2003). The majority of our transgenic rabbits derived from three generations produced more rhFVIII than the transgenic founders described earlier (Chrenek et al. 2005). There are, however, many factors which may negatively influence the level of rhFVIII activity, such as incomplete glycosylation. N-linked oligosacharide in 25 potential sites within hFVIII polypeptide sequences is necessary to build the complex—type of tertiary structure (Dorner et al. 1987). The second reason might be unsuccessful processing of factors that are required for post-translational modifications (Chen et al. 2002). Low clotting activity of rhFVIII could be also due to the instability of the rhFVIII in the rabbit milk lacking von Willebrand factor (vWF) as a carrier protein. A FVIII-vWF complex formation might prevent premature binding of factor VIII to components of the factor X activating complex. It is also possible that rhFVIII, produced in rabbit mammary gland interacting with rabbit milk components, such as casein micelles or lipids are reorganized by the antibodies in the ELISA assay as explained previously by Paleyanda et al. (1997) in transgenic pigs. The genetic and in vitro stability of rhFVIII may also influence final quality during purification, dilution and analyses (Parti et al. 2000). Western-blots revealed that single rhFVIII chain content was dependent on the expression level and varied between transgenic rabbit females. This may suggest, as reported in transgenic pigs (Van Cott et al. 1997), that rabbit genetics may play a role in selection of production lines with optimal post-translational proteolytic processing capability.
Although rabbits are not conventional dairy livestock, it is agreed that the short generation time, multiple offspring per litter, stable patternal transmission of the transgene and milk yield offer advantages over conventional dairy livestock for the establishment of a line producing a therapeutic recombinant protein in sufficient concentration and biological activity. Since a high variability between generations and individual transgenic rabbits in rhFVIII concentration and biological activity is observed, an individual evaluation and selection of transgenic animals is required for commercial use.
References
Chen CM, Wang CH, Wu SC, Lin CC, Lin SH, Cheng WTK (2002) Temporal and spatial expression of biologically active human factor VIII in the milk of transgenic mice driven by mammary-specific bovine α-lactalbumin regulation sequences. Transgenic Res 11:257–268
Chrenek P, Vasicek D, Makarevich A, Uhrin P, Petrovicova I, Lubon H, Binder BR, Bulla J (2002) Integration and expression of the WAP-hPC gene in three generations of transgenic rabbits. Czech J Anim Sci 47:45–49
Chrenek P, Vasicek D, Makarevich AV, Jurcik R, Suvegova K, Bauer M, Parkanyi V, Rafay J, Batorova A, Paleyanda RK (2005) Increased transgene integration efficiency upon microinjection of DNA into both pronuclei of rabbit embryos. Transgenic Res 14:417–428
Chrenek P, Trandzik J, Massanyi P, Makarevich AV, Lukac N, Peskovicova D, Paleyanda RK (2006a) Effect of transgenesis on reproductive traits of rabbit males. Anim Reprod Sci (accepted in April 2006a), in press
Chrenek P, Chrastinova L, Kirchnerova K, Makarevich AV and Foltys V (2006b) The yield and composition of milk from transgenic rabbits. AJAS (accepted in June 2006b), in press
Dove A (2000) Milking the genome for profit. Nat Biotechnol 18:1045–1048
Dorner AJ, Bole DG, Kaufman RJ (1987) the relationship of N-linked glycosylation and heavy chain-binding protein association with the secretion of glycoproteins. J Cell Biol 105:2665–2674
Dragin S, Pivko J, Massanyi P, Lukac N, Makarevich A, Paleyanda RK, Chrenek P (2006) Ultrastructural morphometry of mammary gland in transgenic and non-transgenic rabbits. Anat Histol Embryol 35:351–356
Fan J, Watanabe T (2003) Transgenic rabbits as therapeutic protein bioreactors and human disease models. Pharmacol Ther 99:261–282
Garber K (2000) rhFVIII deficit questioned. Nat Biotechnol 18:133–136
Hiripi L, Makovics F, Halter R, Baranyi M, Paul D, Carnwath JW, Bösze Zs, Niemann H (2003) Expression of active human blood clotting Factor VIII in the mammary gland of transgenic rabbits. DNA Cell Biol 22:41–45
Lubon H, Paleyanda RK, Velander WH, Drohan WN (1996) Blood proteins from transgenic animal bioreactors. Transfus Med Rev 2:131–143
Niemann H, Halter R, Carnwath JW, Herrmann D, Lemme E, Paul D (1999) Expression of human blood clotting factor VIII in the mammary gland of transgenic sheep. Transgenic Res 8:137–149
Paleyanda RK, Velander WH, Lee TK, Scandella DH, Gwazdauskas FG, Knight JW, Hoiyer LW, Drohan WN, Lubon H (1997) Transgenic pigs produce functional human factor VIII in milk. Nat Biotechnol 15:971–975
Parti R, Ardosa J, Yang L, Mankarious S (2000) In vitro stability of recombinant human factor VIII (Recombinate). Haemophilia 6:513–522
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:9, E45
Suvegova K, Jurcik R, Chrenek P, Vasicek D, Gazovicova Z, Rafay J and Hanusova E (2004) Comparison of inner organs weight and selected hematological and biochemical blood parameters of transgenic and nontransgenic rabbits. 8th world rabbit Congress, Mexico City, Mexico, September, pp 632–638
Van Cott KE, Lubon H, Russell CG, Butler SP, Gwazdauskas FC, Knight J, Drohan WN, Velander WH (1997) Phenotypic and genotypic stability of multiple lines of transgenic pigs expressing recombinant human protein C. Transgenic Res 6:203–212
Wall RJ, Rexroad CE Jr, Powell A, Shamay A, McKnight R, Hennighausen L (1996) Synthesis and secretion of the mouse whey acidic protein in transgenic sheep. Transgenic Res 5:67–72
Zinovieva N, Lassnig C, Schams D, Besenfelder U, Wolf E, Muller S, Frenyo L, Seregi J, Muller M, Brem G (1996). Stable production of human insulin-like growth factor 1 (IGF-1) in the milk of hemi- and homozygous transgenic rabbits over several generations. Transgenic Res 7:437–447
Acknowledgements
This work was supported by the grant no: 2003 SP51/028 09 00/028 09 03 coordinated by the Slovak Academy of Science, Slovak republic and by the grant of Ministry of Agriculture of Slovak republic.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chrenek, P., Ryban, L., Vetr, H. et al. Expression of recombinant human factor VIII in milk of several generations of transgenic rabbits . Transgenic Res 16, 353–361 (2007). https://doi.org/10.1007/s11248-007-9070-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-007-9070-6