Abstract
Au–Pd catalysts supported on SBA-16, SBA-16-CeO2, and CeO2 had been studied for partial oxidation of methanol to produce H2. The physicochemical characteristics of the catalysts prepared by deposition–precipitation using urea hydrolysis were examined by inductively coupled plasma atomic emission spectroscopy (ICP-AES), Brunauer-Emmett-Teller (BET), X-ray powder diffraction (XRD), Temperature-programmed reduction (TPR), and H2 temperature-programmed desorption (H2-TPD) analyses. The results show that AuxPdy alloys are observed in Au–Pd/SBA-16 and Au–Pd/SBA-16-CeO2 catalysts. The catalytic results demonstrate that both Au–Pd/SBA-16 and Au–Pd/SBA-16-CeO2 catalysts exhibit higher activity and lower CO selectivity than the Au–Pd/CeO2 catalyst. This could be ascribed to the formation of AuxPdy alloys. The comparison of the Au–Pd/SBA-16 and Au–Pd/SBA-16-CeO2 catalysts reveals that the Au–Pd/SBA-16-CeO2 shows the lower CO selectivity, probably due to the presence of CeO2.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The polymer electrolyte fuel cell (PEMFC) using hydrogen is the most promising fuel cell for solving some of the serious energy-related problems [1, 2]. In order to avoid the technical difficulties of transportation, storage, and distribution of hydrogen, the fuel can be generated in an on-board steam reformer, which produces hydrogen from liquid fuels. Methanol is regarded as a primary candidate because of its abundance, high hydrogen to carbon (H/C) atomic ratio, and safe storage [3]. There are several reaction pathways for on-site hydrogen production from methanol, such as methanol decomposition (MD) [4], steam reforming of methanol (SRM) [5, 6], partial oxidation of methanol (POM) [7, 8], and oxidative steam reforming of methanol (OSRM) [9, 10]. Among them, POM is considered to be a suitable method due to its exothermic reaction, rapid reaction rate and using air or oxygen as oxidant instead of steam.
Supported Pd has been extensively utilized as a catalyst for H2 generation from methanol by POM [11]. However, the main disadvantage of these catalysts is the formation of considerable amounts of CO, which is a known poisonous component for the PEMFC anode. Recently, numerous attempts have been made to modify the catalytic performances of Pd catalysts by the addition of Au, Cu, Zn, and so on [12–14]. Among these additives, Au has attracted great attention, due to its excellent catalytic activity for CO oxidation at low temperature [15, 16]. Up to now, many Au–Pd systems have revealed the synergistic effect of Au and Pd leading to the formation of AuxPdy alloys, which were favorable to the improvement of the activity and selectivity for the POM reaction [12].
To obtain effective AuxPdy alloy catalysts for the POM reaction, the supports play a key role because of their surface areas and the interactions between the metals and supports, which affect the formation of AuxPdy alloys. Mesoporous silica, as a kind of support material, has recently been of great interest, because of its large surface area, high thermal stability, and well-ordered structure, which promotes the formation and dispersion of bimetallic alloys [14]. Based on the above-mentioned previous work, we focused on SBA-16 supports, which possess high surface area and 3-D cubic arrangement of cage-like mesopores [17–20]. Compared to the unidirectional pore mesoporous materials, SBA-16 could be more favorable for the reactants to access the catalytic sites, and the diffusion of products, owing to the unique 3-D channel network [17]. Additionally, we also noticed that CeO2 was a very active support for the further oxidation of CO into CO2, because it has high oxygen storage capacity and oxygen vacancies in the fluorite oxide structure [21–23]. Therefore, in this study, we prepared Au–Pd/SBA-16, Au–Pd/SBA-16-CeO2, and Au–Pd/CeO2 catalysts by deposition–precipitation technique using urea as precipitating agent. The activities for H2 production from POM reaction on these catalysts were investigated. Scanning electron micrographs (SEM), ICP-AES, XRD, BET, TPR, and H2-TPD analyses were used to identify the characteristics of these supports and catalysts. To the best of our knowledge, it is the first time that SBA-16 was utilized as a support for the POM reaction.
Experimental
Triblock copolymer Pluronic F127 was purchased from Aldrich. Other chemicals were obtained from Sinopharm Chemical Reagent co. All chemicals were analytical reagents. Deionized water was used for the catalyst preparation.
Support preparation
The mesoporous SBA-16 material was synthesized under acidic conditions, using Triblock copolymer Pluronic F127 as a structure-directing agent and TEOS as the silica source [24]. F127 (1.2 g) was added to HCl (1.75 mol/L, 60 g) aqueous solution and allowed to stir at 30 °C overnight, and then TEOS (4.0 mL) was added to this solution under vigorous magnetic stirring. After 10-min stirring, the mixture was kept under static conditions at 30 °C for 20 h. Finally, the solid products were collected by filtration, washed with ethanol, dried, and calcined at 550 °C with a heating rate of 2 °C/min in air for 5 h in order to remove the organic template.
CeO2 support was prepared by precipitation method. The appropriate quantities of (NH4)2Ce(NO3)6 (0.1 mol/L) were dissolved in deionized water with constant stirring. Into this solution, polyethylene glycol (PEG-4000) as protection agent was added. Then, NH3·H2O (5 vol.%) was slowly added to it until completely precipitated. The mixture was refluxed at 70 °C for 4 h, filtered, and the solid washed with ethanol thoroughly. The sample was dried at 70 °C overnight and calcined at 500 °C for 4 h. The binary support, SBA-16-CeO2, with a mass ratio of 5:5 was prepared in much the same way as CeO2 support except that a certain amount of SBA-16 was added to the (NH4)2Ce(NO3)6 (0.1 mol/L) solution.
Catalyst preparation
The Au–Pd/SBA-16, Au–Pd/SBA-16-CeO2, and Au–Pd/CeO2 catalysts were prepared by deposition–precipitation with urea method. The appropriate quantities of HAuCl4 (5.08 mL 0.01 mol/L) and PdCl2 (9.40 mL 0.01 mol/L) were dissolved in 100 mL deionized water containing the urea. After adding supports (1.0 g) to the solution (initial pH ≈ 2), the suspension was vigorously stirred at 80 °C for 8 h (pH increases), and then the solvents were removed by rotary evaporator. Afterward, the solids were washed several times to eliminate Cl−. Finally, the obtained samples were oven dried at 60 °C for 24 h and calcined at 400 °C for 2 h. The results of ICP-AES indicated that total load of the deposit noble metals was 2.0 wt% (Au: Pd = 5:5 wt/wt).
Characterization of samples
The composition of the catalysts was measured by ICP-AES using an American PE Corporation’s Optima 5300 DV. The textural characteristics of the supports and catalysts were analyzed by N2 physisorption method. N2 adsorption and desorption isotherms were performed at −196 °C on a Micromeritics ASAP2020C instrument. The samples were outgassed in vacuum (≈1 μm Hg) at 300 °C for 10 h before the measurement. The pore diameter was calculated from the analysis of desorption branch of the isotherm by the BJH (Barrett-Joyner-Halenda) method. XRD patterns were recorded on a Bruker AXS D8Focus diffractometer operating at 40 kV and 30 mA with Cu target Kα-ray irradiation. Diffraction data were collected between 0.5° and 10° with a step size of 0.01° and a step time of 2 s and 20°–70° with a scanning rate of 1°/min. The SEM was taken on a FEI SIRION electron microscope with an acceleration voltage of 5 kV. The sample was deposited on a sample holder with an adhesive carbon foil and sputtered with gold prior to imaging.
TPR experiments were carried out on a Chemisorb 2750 instrument over 0.1 g catalyst. The samples were heated from room temperature to 300 °C under flowing high-purity argon (50 mL/min) at a rate of 10 °C/min for 1 h in order to remove possible impurities. After cooling to room temperature, a reducing gas composed of 10% H2/Ar was employed at a flow rate of 50 mL/min, with a heating ramp of 10 °C/min. The H2 consumption was determined by gas chromatography equipped with a thermal conductivity detector.
H2-TPD was carried out on an in-house apparatus. Prior to the TPD experiment, 0.1 g of the samples was reduced at 350 °C for 2 h using a flow of high-purity H2 (30 mL/min) and then cooling to room temperature in argon (40 mL/min). H2 was pulsed until adsorption was saturated. Finally, the samples were heated to 400 °C at a rate of 10 °C/min to record the H2-TPD curves.
Catalytic activity measurements
Partial oxidation of methanol was carried out at atmospheric pressure in a continuous micro-reactor, which was placed inside a programmable furnace. The catalyst (0.1 g) was reduced in situ first in H2 flow (30 mL/min) at 350 °C for 2 h and then cooled to the reaction temperature. Methanol was fed into the pre-heater by means of a Lab-Alliance liquid pump (Model: Series II-240). The oxygen and nitrogen (balanced) flows were adjusted by a mass flow controller. The molar ratio of O2/CH3OH was kept at 0.3, and the reaction was performed from 200 to 450 °C. The compositions were analyzed using an on-line gas chromatograph with a thermal conductor detector. A Porapak Q column was used to separate CH3OH, H2O, and CO2, and a 5Å molecular sieve column was employed to separate H2 and CO. The CH3OH conversion, O2 conversion, H2 selectivity, and CO selectivity were defined as below:
Results and discussion
Characterization of mesoporous SBA-16 support
Figure 1A shows the small-angle XRD pattern of calcined SBA-16. A pattern with three well-resolved peaks is observed at 2θ value of 0.88°, 1.36°, and 1.70° that matched well with the reported (110), (200), and (211) plane reflections of cubic mesoporous structure [25]. The d-spacing of (110) plane calculated from Bragg’s law is approximately 97 Å, which is also in the mesoporous range. The N2 adsorption–desorption isotherm and pore size distribution of SBA-16 are presented in Fig. 1B and Table 1. As shown in Fig. 1B, the adsorbent exhibits reversible type-IV behavior with a typical H2 hysteresis loop and a narrow pore size distribution, indicating that the mesopores have uniform cage-like pore structure. As listed in Table 1, the synthesized SBA-16 has a BET surface area of 594 m2/g, a pore volume of 0.69 cm3/g, and a narrow pore size distribution centered at 36.8 Å. Furthermore, the structure and morphology of the SBA-16 were analyzed by SEM (Fig. 1C), and it reveals many decaoctaheron-shape domains that aggregate into microstructures with relatively uniform size (2–6 μm), which is in good agreement with the previous reports [24].
Textural characteristics of catalysts
The N2-physisorption isotherms and pore size distributions for the catalysts are shown in Fig. 2. The Au–Pd/SBA-16 catalyst exhibits a typical type-IV isotherm with a pronounced hysteresis loop characteristic of cage-like materials with interconnected uniform mesopores [26]. For the Au–Pd/SBA-16-CeO2 catalyst, although the isotherm shows a slight difference as compared with SBA-16, it still indicates that the mesostructure is maintained. The change in the shape of the hysteresis loop is probably due to the obstruction of the pores by the CeO2 particles. Similar phenomenon has been observed in TiO2/SBA-16 catalyst [27]. The specific surface areas and pore volumes of the samples are summarized in Table 1. Compared to the corresponding supports, the BET surface areas and pore volumes of Au–Pd/SBA-16 and Au–Pd/SBA-16-CeO2 catalysts are smaller, which suggests that the metal particles are not only dispersed on the surface of supports but also inside the pores [28]. Interestingly, in spite of the obvious decrease in the pore volumes and BET surface areas of the two catalysts, their pore diameters do not show significant changes. This probably means that the pores are only partly blocked by the metal species, as described previously by Tsoncheva et al. [29]. For CeO2 support, after loading Au–Pd, the BET surface area increases, which is similar to the work reported by Hiroaki et al. [30].
XRD of catalysts
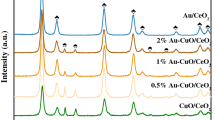
Figure 3 illustrates the XRD patterns of Au–Pd/SBA-16, Au–Pd/SBA-16-CeO2, and Au–Pd/CeO2 catalysts calcined at 400 °C. In Fig. 3A, the two patterns are similar and show the (110) reflection (0.92°) of the cubic mesopore structure, which is in accordance with the N2-physisorption analysis. Figure 3B depicts the wide-angle XRD patterns of the three catalysts. Peaks of 28.5°, 32.8°, 47.5°, and 57.5°, which are observed in Au–Pd/SBA-16-CeO2 and Au–Pd/CeO2 catalysts, are assigned to the diffraction patterns of CeO2 (111), (200), (220), and (311) planes, respectively [21]. A broad peak at 22.6° corresponds to the amorphous SiO2 reflection in Au–Pd/SBA-16 and Au–Pd/SBA-16-CeO2 catalysts [27]. It is interesting to note that the diffraction peaks at 38.9° and 44.8° attributed to the AuxPdy alloys are observed in Au–Pd/SBA-16 and Au–Pd/SBA-16-CeO2 catalysts [12]. Furthermore, the peak intensities of AuxPdy alloys in Au–Pd/SBA-16 catalyst are higher than those in Au–Pd/SBA-16-CeO2 catalyst. These results reveal that the SBA-16 promotes the formation of AuxPdy alloys while the CeO2 prohibits the process. Venezia et al. [31, 32] considered that gold had a trend to capture palladium into its lattice, forming AuxPdy alloys, which facilitated the improvement of catalytic performance.
TPR studies of catalysts
The TPR profiles of the pure CeO2, Au–Pd/SBA-16, Au–Pd/SBA-16-CeO2, and Au–Pd/CeO2 catalysts are depicted in Fig. 4. The low-temperature reduction peaks at ca. 52, 68 and, 91 °C are observed in Au–Pd/SBA-16, Au–Pd/SBA-16-CeO2, and Au–Pd/CeO2 catalysts, respectively, which may be attributed to the reduction of AuxOy, PdO, AuxPdyO and even part of superficial CeO2. The increase in the reduction temperature is probably due to the increase in the interactions between the Au, Pd particles and supports. Further inspection of these TPR profiles also reveals that both Au–Pd/SBA-16-CeO2 and Au–Pd/CeO2 catalysts exhibit higher H2 consumption than Au–Pd/SBA-16 catalyst, implying the reduction of superficial CeO2. It is noteworthy that the reduction temperatures of superficial CeO2 in Au–Pd/SBA-16-CeO2 (68 °C) and Au–Pd/CeO2 (91 °C) catalysts are much lower than the pure superficial CeO2 (447 °C), which could be owing to the metal-CeO2 interactions and the H2 spillover from metals to CeO2. Noticeably, another reduction peak at 526 °C is observed in Au–Pd/SBA-16-CeO2 catalyst, indicating the reduction of the bulk-phase lattice oxygen of CeO2. The reduction temperature of bulk CeO2 in Au–Pd/SBA-16-CeO2 catalyst is much lower than the temperature (over 750 °C) reported by Yao et al. [33], which could also be attributed to the H2 spillover. The comparison of Au–Pd/SBA-16-CeO2 and Au–Pd/CeO2 catalysts demonstrates that the bulk CeO2 reduction peak does not appear in the Au–Pd/CeO2 catalyst, suggesting that the SBA-16 promotes the dispersion of CeO2, which favors the reduction of the bulk CeO2.
H2-TPD profiles of the catalysts
Figure 5 presents the H2-TPD profiles of the Au–Pd/SBA-16, Au–Pd/SBA-16-CeO2, and Au–Pd/CeO2 catalysts. The comparison of the three TPD profiles indicates that the H2 desorption temperatures increase in the following order: Au–Pd/SBA-16 catalyst < Au–Pd/SBA-16-CeO2 catalyst < Au–Pd/CeO2 catalyst, probably owing to the existence of metal-CeO2 interactions. The lower desorption temperature makes desorption of reaction product H2 easier, which is beneficial to the POM reaction. On the basis of XRD as well as TPR and TPD results, we proposed that metal-CeO2 interactions can impede the formation of AuxPdy alloys, and the AuxPdy alloys exhibit lower H2 desorption temperature.
Catalytic activity
CH3OH conversion, O2 conversion, H2 selectivity, and CO selectivity for POM reaction were studied on Au–Pd/SBA-16, Au–Pd/SBA-16-CeO2, and Au–Pd/CeO2 catalysts from 200 to 450 °C (Figs. 6, 7). As shown in Fig. 6, Au–Pd/SBA-16 and Au–Pd/SBA-16-CeO2 catalysts exhibit higher CH3OH conversion and H2 selectivity when compared to Au–Pd/CeO2 catalyst, probably due to the formation of AuxPdy alloys and their larger BET surface areas. It is noteworthy that the BET surface area of Au–Pd/SBA-16-CeO2 catalyst is lower than that of Au–Pd/SBA-16 catalyst, but the two catalysts show similar catalytic activity. This demonstrates that the AuxPdy alloys play a more important role in the POM reaction than BET surface area. In all catalytic systems, oxygen is completely consumed throughout the examined temperature range. CH3OH conversion and H2 selectivity increase with increasing reaction temperatures. This could be explained here.
Initially, CH3OH quickly consumes O2 leading to highly exothermic methanol combustion (MC) reaction (Eq. (1)) with production of H2O and CO2 [34]. Since O2 has been completely consumed, the other CH3OH consumption reactions have taken place during POM reaction (Eq. (2)).
When the temperature increases from 250 to 300 °C, the H2O formed via MC reaction may be consumed by SRM reaction (Eq. (3)), leading to the increase in H2 selectivity with consequent decrease in H2O selectivity [35].
With the further increasing of the reaction temperatures, no more O2 is available and the MD reaction predominates, thus leading to more H2 and CO formation (Eq. (4)).
CO produced by the side reactions is subsequently transformed into CO2 by the WGS reaction (Eq. (5)) or CO oxidation (Eq. (6)).
Formerly, supported Au–Pd catalyst has been reported to be an efficient catalyst for low-temperature CO oxidation [32], which explains the low amount of CO during POM reaction over the three catalysts. The comparison of CO selectivity for the three catalysts demonstrates that both Au–Pd/SBA-16 and Au–Pd/SBA-16-CeO2 catalysts show lower CO selectivity than Au–Pd/CeO2 catalyst, which indicates that the AuxPdy alloys can promote CO oxidation [32]. Further inspection of Au–Pd/SBA-16 and Au–Pd/SBA-16-CeO2 catalysts reveals that CO selectivity of Au–Pd/SBA-16-CeO2 catalyst is lower than that of Au–Pd/SBA-16 catalyst, owing to the good activity of CeO2 to oxidize CO into CO2. The above results suggest that both the AuxPdy alloys and CeO2 can facilitate the oxidation of CO. As revealed by the Figs. 6, 7, the Au–Pd/SBA-16-CeO2 exhibits 100% CH3OH conversion, 99.2% H2 selectivity, and 3.9% CO selectively at 450 °C.
Conclusion
Au–Pd/SBA-16, Au–Pd/SBA-16-CeO2, and Au–Pd/CeO2 catalysts were prepared by deposition–precipitation technique and examined by BET, XRD, TPR, and H2-TPD analyses. The results show that the SBA-16 promotes the formation of AuxPdy alloys while the metal-CeO2 interactions impede this process. The three catalysts were also studied for partial oxidation of methanol to produce H2. Au–Pd/SBA-16 and Au–Pd/SBA-16-CeO2 catalysts showed higher activity and selectivity than Au–Pd/CeO2 catalyst. Compared with Au–Pd/SBA-16 catalyst, Au–Pd/SBA-16-CeO2 catalyst showed the lower CO selectivity. The results reveal that the AuxPdy alloys play an important role in the POM reaction. Both the AuxPdy alloys and CeO2 facilitate the oxidation of CO.
References
Appleby AJ, Foulkes FR (1989) Fuel cell hand book. Van Nostrand Reinhold, NewYork
Granovskii M, Dincer I, Rosen MA (2006) Int J Hydrogen Energy 31:337
Cheng WH (1995) Appl Catal A Gen 130:13
Yong ST, Hidajat K, Kawi S (2008) Catal Today 131:188
Matter PH, Ozkan US (2005) J Catal 234:463
Lin SSY, Thomson WJ, Hagensen TJ, Ha SY (2007) Appl Catal A Gen 318:121
Kulprathipanja A, Falconer JL (2004) Appl Catal A Gen 261:77
Cubeiro ML, Fierro JLG (1998) J Catal 179:150
Turco M, Bagnasco G, Costantino U, Marmottini F, Montanari T, Ramis G, Busca G (2004) J Catal 228:56
Turco M, Bagnasco G, Cammarano C, Senese P, Costantino U, Sisani M (2007) Appl Catal B 77:46
Cubeiro ML, Fierro JLG (1998) Appl Catal A Gen 168:307
Wang JX, Luo LT (2008) Catal Lett 126:325
Schuyten S, Guerrero S, Miller JT, Shibata T, Wolf EE (2009) Appl Catal A Gen 352:133
Eswaramoorthi I, Dalai AK (2009) Int J Hydrogen Energy 34:2580
Haruta M, Yamada N, Kobayashi T, Iijima S (1989) J Catal 115:301
Haruta M, Tsubota S, Kobayashi T, Kageyama H, Genet MJ, Delmon B (1993) J Catal 144:175
Sakamoto Y, Kaneda M, Terasaki O, Zhao DY, Kim JM, Stucky GD, Shim HJ, Ryoo R (2000) Nature 408:449
Mesa M, Sierra L, Patarin J, Guth JL (2005) Solid State Sci 7:990
Yu CZ, Fan J, Tian BZ, Zhao DY (2004) Chem. Mater 16:889
Yu CZ, Tian BZ, Fan J, Stucky GD, Zhao DY (2002) J Am Chem Soc 124:4556
Chen QB, Luo LT (2008) Indian J Chem Sect A 47:1317
Andreeva D, Idakiev V, Tabakova T, Ilieva L, Falaras P, Bourlinos A, Travlos A (2002) Catal Today 72:51
Fu Q, Deng WL, Saltsburg H, Stephanopoulos MF (2005) Appl Catal B 56:57
Jin HX, Wu QY, Chen C, Zhang DL, Pang WQ (2006) Microporous Mesoporous Mater 97:141
Kleitz F, Solovyov LA, Anikumar GA, Choi SH, Ryoo R (2004) Chem Commun 13:1536
Huo QS, Leon R, Petroff PM, Stucky GD (1995) Science 268:1324
Ma J, Qiang LS, Tang XB, Li HY (2010) Catal Lett 138:88
Hess C, Hoefelmeyer JD, Tilley TD (2004) J Phys Chem B 108:9703
Tsoncheva T, Linden M, Areva S, Minchev C (2006) Catal Commun 7:357
Sakurai H, Tsubota S, Haruta M (1993) Appl Catal A Gen 102:125
Venezia AM, Parola VL, Deganello G (2002) J Catal 212:56
Venezia AM, Liotta LF, Pantaleo G (2003) Appl Catal A Gen 251:359
Yao HC, Yao YFY (1984) J Catal 86:254
Chang FW, Roselin LS, Ou TC (2008) Appl Catal A Gen 334:147
Agrell J, Birgersson H, Boutonnet M, Cabrera IM, Navarro RM, Fierro JLG (2003) J Catal 219:389
Acknowledgments
This work was supported by the National Natural Science Foundations of China (20701018 and 21062013) and the Foundation of Educational Department of Jiangxi Province (GJJ08024).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, L., Wang, S., Chen, C. et al. Catalytic partial oxidation of methanol over Au–Pd bimetallic catalysts: a comparative study of SBA-16, SBA-16-CeO2, and CeO2 as supports. Transition Met Chem 36, 387–393 (2011). https://doi.org/10.1007/s11243-011-9481-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-011-9481-3