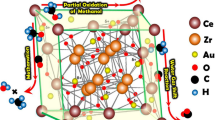
Abstract
The bimetallic x wt% Au–CuO/CeO2 catalysts with different Au contents (x = 0.5, 1, 2 wt%) and Au/CeO2, CuO/CeO2 samples are applied for CO2 hydrogenation to methanol. The catalysts are characterized by techniques such as XRD, Raman, XPS, H2-TPR and CO2-TPD measurements. It is shown that addition of 1 wt% of Au to CuO/CeO2 improves more significantly the catalyst activity in CO2 hydrogenation to methanol compared with other Au–CuO/CeO2 samples. The 1 wt% Au–CuO/CeO2 has a better ability to dissociatively adsorb hydrogen and enhance the number of oxygen vacancies, which leads to the highest methanol selectivity (T = 240 °C, P = 3 MPa, \(S_{{{\text{CH}}_{3} {\text{OH}}}}\) = 29.6%). The in situ DRIFTS reveals that a dual site character of the Au–CuO/CeO2 catalysts for CO2 hydrogenation, with CO2 being activated on sites of the CeO2 support, then stepwise hydrogenation of (bi)carbonate to formate and methoxy, finally methanol.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The atmospheric CO2 accumulation, due to human activities such as burning fossil fuels, deforestation, land use change and cement production, has become a serious problem for our environment on account of global warming, climate change, ocean acidification and glaciers melting. Hydrogenation of CO2 to methanol is an attractive and promising way to solve excess CO2 emission and energy shortage problems [1]. Methanol is a both a clean energy source and a versatile chemical for several chemical processes.
Due to the high thermodynamic stability of CO2, splitting of a C–O bond in the molecule is characterized by a high energy barrier. Thus, effective activation of CO2 is a critical step in improving the overall reaction kinetics of the process. Apart from CO2 activation, the number of active H atom is also a key parameter, which is necessary to promote hydrogenation of intermediate species [2].
Cu-supported catalysts have been widely used in methanol synthesis from CO2 hydrogenation [3, 4]. Providing more active hydrogen atom can probably further improve catalytic activity of Cu-based catalysts. It has been reported that the addition of gold metal to Cu-based catalysts, which can dissociate hydrogen efficiently [5, 6]. Au NPs have out-standing efficiency in a broad range of catalytic reactions [7]. The smaller Au NPs have more interfacial sites and low-coordinated Au atoms, which are believed to be the active sites to boost the chemical activity [7,8,9]. The Au-containing catalysts may not always be active for hydrogenation. Because of its unique plasmon character, gold has been extensively used for the photocatalytic conversion of CO2 [10, 11].
Compared to the traditional industrial Cu/ZnO/Al2O3 catalyst, which has been extensively characterized and studied, these Au catalysts are still barely understood. It has been found that the first subgroup B elements of the periodic table (such as Au and Cu) could produce strong interaction or form alloy with each other, which is the reason for more active on bimetallic Au–Cu catalyst compared to the individual Au and Cu catalysts [12]. It has shown that CeO2 is an appropriate support to react with CO2 due to its basic property [13]. Additionally, a synergy of the Au with copper to promote a direct and selective conversion of CO2 into methanol has not been identified. In this regard, CeO2-supported Au–CuO catalysts with different Au loading are employed to illustrate the role of Au in tuning CO2 hydrogenation selectivity.
2 Experimental
2.1 Catalyst Preparation
In the first step, the reducible oxide support CeO2 was prepared. The solution of precursor [Ce(NO3)3·6H2O] was continuously stirred and then co-precipitated using NaOH (0.2 mol/L) as a precipitant. The suspension was aged for 2 h. The resulting precipitate was obtained by filtration, washed with deionized water and ethanol for three times to remove the excess ions. Next, the samples were dried overnight at 80 °C in air and calcined at 500 °C for 4 h under air. Then the bimetallic Au–CuO/CeO2 catalysts were prepared by co-impregnation method with a amount of HAuCl4 (0.5, 1, 2 wt% Au) solution, Cu(NO3)2·3H2O (10 wt%) solution and the CeO2 support. After 30 min of ultrasonic, the obtained samples were dried and calcined at 400 °C for 4 h. The Au–CuO/CeO2 catalysts with different Au contents were denoted x wt% Au–CuO/CeO2 (x = 0.5, 1, 2 wt%).
2.2 Catalyst Characterization
XRD patterns of the calcined catalysts were recorded using a D/max 22,000. The scanning angle was adjusted from 20° to 80° at 2θ Bragg angle with a step size of 0.02° and a time/step of 11 s. This technique was used to examine crystallite phase composition and mean particle size. The mean crystallite size was calculated by Debye–Scherrer equation.
Raman spectra were recorded on a Renishaw equipment. Room-temperature Raman were excited at 532 nm, the exposure time was 2 s and the accumulation number was 20 times.
The surface properties of pre-reduced catalysts were obtained by X-ray photoelectron spectroscopy (XPS). XPS was performed on K-Alpha + with 5 × 10–9 mbar analysis chamber vacuum. Spectra were obtained for the C 1 s, O 1 s, Au 4f, Ce 3d and Cu 2p regions.
The reducibility property of the samples was investigated by TPR on a PCA-1200 chemical adsorption instrument. The catalysts were pretreated in a home-made U-shape quartz reactor under an N2 flow. After this pretreatment, the catalysts were cooled to room temperature. A reducing gas of 10% (v/v) H2 in He was then exposed into the reactor at a flow rate of 120 mL/min and the samples were heated up to 500 °C at a heating rate of 10 °C/min.
H2/CO2 temperature programmed desorption were operated on a mass spectrometer (OmniStar 320). Before the TPD analysis, all catalysts were reduced with H2 (50 mL/min) at 300 °C for 3 h and went through the pretreatment under He at a rate of 60 mL/min at 310 °C for 0.5 h to clean the catalyst surface. After cooling to room temperature, the catalyst was saturated with a \(V_{{{\text{CO}}_{2} }} /V_{{{\text{He}}}}\) = 1:3 at room temperature for 0.5 h, followed by purging with He for 1 h to remove the physisorbed molecules. Afterwards, the TPD experiment was started in flowing He with a heating rate of 10 °C/min, and the desorbed CO2 data was detected by mass spectrometer (OmniStar 320). H2 temperature-programmed desorption (H2-TPD) were performed as the same procedure as CO2-TPD, and the difference is that the catalyst was saturated with \(V_{{{\text{H}}_{2} }} /V_{{{\text{He}}}}\) = 3:1.
The in situ DRIFTS measurements were done on a VERTEX equipment at the temperature of 240 °C and 3 MPa. The sample was placed in a ceramic crucible in an in situ DRIFTS cell fitted with ZnSe windows and connected to stainless steel gas lines. The in situ DRIFTS spectra were recorded with a resolution of 4 cm−1 and with an accumulation of 32 scans every 30 s during the temperature ramp. The IR data were analyzed by the OPUS software. The in situ DRIFTS spectra shown in this work were expressed in units of Kubelka–Munk. Similar to the reaction tests, the samples were also pretreated under H2 atmosphere at 300 °C. Subsequently, the catalysts were cooled down to the reaction temperature. The procedures of in situ DRIFTS experiment were as follows: (1) exposing to CO2/N2 mixture gas (60 mL/min); (2) treating in a N2 gas flow (60 mL/min); (3) changing to the reaction gas mixture CO2/H2 at a flow rate of 60 mL/min for CO2 hydrogenation; (4) exposing to H2/N2 atmosphere.
2.3 Catalytic Activity
Activity measurements for CO2 hydrogenation were carried out in a fixed bed reactor. 0.5 g of catalyst was placed in a stainless-steel tube reactor with diameter of 20 mm and length of 200 mm. Prior to the activity studies, the calcined catalyst was reduced at 300 °C for 3 h in pure H2 at a rate of 5 °C /min. After reduction, CO2 hydrogenation process was performed with a 1:3 molar ratio of CO2:H2, pressure of 3 MPa, temperature range between 200 and 300 °C. The activity test experiment keeps 2 h at each temperature. CO2 and CO were quantitatively analyzed using gas chromatograph (GC) equipped with a thermal conductivity detector and Porapak-Q column (GC7890T), the target product CH3OH was analyzed by using GC7900 equipped with flame ionization detector and a capillary column.
3 Results and Discussion
3.1 Physico-chemical Properties
To determine the crystalline phase compositions of catalyst, XRD is then carried out and the patterns are shown in Fig. 1. The reflection peaks associated with Au element (Au oxide species or metallic Au species) are not observed in the XRD pattern of x wt% Au/CuO–CeO2 catalysts. The probable reason to explain is that the presence of the Au is in a form of highly dispersed nano-particles on the surface or the Au concentrations are below the detection limits. The diffraction peaks at 28.5°, 32.9°, 47.4°, 56.3°, 59.1°, 69.4°, 76.7°, and 78.9° are attributed to CeO2 with a face-centered cubic fluorite structure, and those at 35.5° and 38.7° are well consistent with CuO of monoclinic structure [14]. Additionally, the peaks associated with CuO species become broaden after adding gold to the catalysts, it is indicated that adding gold is beneficial to disperse the copper species and reduce the size of CuO species.
As depicted in Fig. 2, it is widely known that pure CeO2 sample presents a prominent peak at 464 cm−1 corresponding to F2g Raman active mode in CeO2 with fluorite-like structure [15], which can be viewed as a symmetric breathing mode of oxygen atoms surrounding cerium ions. For all the samples, the bands at 297 cm−1 and 632 cm−1 associated with oxygen vacancies appear [16]. The peak fitting for 632 cm−1 and 464 cm−1 is plotted in Fig. 2a, and the A632/A464 value for Au/CeO2 is the lowest (0.02). The value for CuO/CeO2 (0.18) is much higher than that of Au/CeO2. For the x wt% Au–CuO/CeO2 samples, the A632/A464 value increases compared with monometallic catalysts. The higher A632/A464 value of 0.36 for 1 wt% Au–CuO/CeO2 suggests the presence of larger number of oxygen vacancy sites, compared with the values of 0.23 and 0.25 for 0.5 wt% Au–CuO/CeO2 and 2 wt% Au–CuO/CeO2, respectively. The surface oxygen vacancies concentrations of all reduced samples are investigated by XPS. The changing trend of surface relative Ce3+/(Ce4+ + Ce3+) ratio is same to the variation of oxygen vacancies concentration (Table 2; Fig. 3b). The concentration of Ce3+/(Ce4+ + Ce3+) is in order: 1 wt% Au–CuO/CeO2 (39.4%) > 0.5 wt% Au–CuO/CeO2 (33.1%) > 2 wt% Au–CuO/CeO2 (30.6%) > CuO/CeO2 (32.1%) > Au/CeO2 (23.4%). The transformation of Ce4+ to Ce3+ can create the charge imbalance and oxygen vacancy on the catalyst according to the charge compensation. Meanwhile the relative amount of the defect oxygen species (Oβ) on the surface varies with the amount of the Au contents. 1 wt% Au–CuO/CeO2 has the highest Oβ/(Oα + Oβ + Oγ) concentration. Above all, the trend of surface oxygen vacancies concentration is same to that of the bulk oxygen vacancies concentration. And 1 wt% Au–CuO/CeO2 has the highest concentration in both reduced state and oxidized state.
Oxygen vacancies in a metal oxide support have been identified as one of the key components which can impact the chemical behavior of CO2 [17]. It is found that adding gold also promotes the formation of oxygen vacancies, especially for 1 wt% Au–CuO/CeO2 catalyst. The excess and insufficient gold content have a negative effect on the formation of defect oxygen, which may be associated with the different promotion ability of reduction of Ce4+ to Ce3+ for Au–CuO/CeO2 samples with different gold contents [18].
The H2-TPR profiles of the tested samples are shown in Fig. 4. The peak at the lower temperature of 215 °C for Au/CeO2 is ascribed to the reduction of ceria surface oxygen and/or the reduction of partially oxidized gold species [19]. This peak is observed at much higher temperatures (> 550 °C) over pure CeO2, implying that the addition of gold to CeO2 facilitates the reduction of surface oxygen species. CuO/CeO2 shows two reduction peaks, α peak (210–220 °C) is attributed to the reduction of small particle size of CuO species and/or the ceria surface oxygen, γ peak (255–265 °C) is attributed to large particle of CuO species [7]. The reduction temperature of Au–CuO/CeO2 samples is lower than that of CuO/CeO2 due to the addition of gold [20]. The 0.5 wt% Au–CuO/CeO2 has almost the same CuO reduction peaks to those of CuO/CeO2, because of the low concentration of gold. Differently, 1 wt% and 2 wt% Au–CuO/CeO2 samples show three H2 consumption peaks. Moreover it is found that more oxygen species could be reduced at lower temperatures on these both catalysts. Based on XRD result, CuO particle sizes of 1 wt% and 2 wt% Au–CuO/CeO2 samples are smaller, hence, new reduction peak β should be due to the presence of middle particle size of CuO. In addition, a upward shift of the reduction peak from 240 to 258 °C is observed when gold content increases from 1 to 2 wt%, because the larger CuO particles are observed over 2 wt% Au–CuO/CeO2 sample. The shift of binding energy of Cu 2p3/2 peak in all Au–CuO/CeO2 samples is observed (Fig. 3a), which suggests that gold addition promotes the CuO reduction and then shifts the reduction temperature to lower. The addition of the gold weakens the Cu–O bond, enhancing the reducibility of CuO [21].
Apart from CO2 activation, the supply of H adatoms is also important for the hydrogenation reaction in the methanol formation. Figure 5 shows the H2-TPD profiles of H2-reduced catalysts. No desorption peaks are observed over CeO2. Au/CeO2 has an extremely weak and broad desorption peak at around 150–300 °C, indicating that gold particles are responsible for dissociation of H2 over Au/CeO2. The CuO/CeO2 catalyst displays two stronger desorption peaks. The former desorption is attributed to the desorption of atomic hydrogen, and the high-temperature desorption peak presents the desorption of strongly adsorbed hydrogen on the bulk of metal particles [20]. It is demonstrated that monometallic gold and copper catalysts are likely to be responsible for H2 adsorption during CO2 hydrogenation. The spectra of 0.5 wt% Au–CuO/CeO2 sample is almost same to that of CuO/CeO2 because of low Au concentration. For 1 wt% Au–CuO/CeO2 sample, the H2 desorption temperature is lower and amount of H2 desorption is larger. However further increase the amount of gold, 2 wt% Au–CuO/CeO2 sample present a higher H2 desorption temperature and smaller H2 desorption compared to 1 wt% Au–CuO/CeO2 sample, which might be result of larger CuO particles.
The Cu 2p spectra for the reduced samples are presented in Fig. 3a. For all samples, a main peak around 932.4 eV shows that the copper species are mainly in Cu0 state after reduction. Cu 2p3/2 component at 933.7 eV and the shake-up peak indicate the presence of Cu2+ ions [22]. The ratio of Cu0/(Cu2+ + Cu0) over all samples is almost the same (46.4–47.8%). Generally, it is acknowledged that Cu0 is responsible for dissociation of H2. Based on the XRD and XPS results, the dispersion and the number of Cu0 is almost the same among all the samples. However, it has shown the samples have different H2 adsorption ability, which is probably due to the presence of Au.
The characteristic peaks of Au0 and Au+ species over 1 wt% Au–CuO/CeO2 are located at 83.8 eV and 85.7 eV in Au 4f7/2, while at 88.4 eV and 89.6 eV in Au 4f5/2. It can be observed that the gold species on the surface of Au–CuO/CeO2 samples is mainly Au+, with small amounts of Au0. When the H2 molecule interacts with Au+, the anti-bonding states will be less filled compared with metallic Au [14]. This will stabilize the binding between H2 and Au+, facilitating the splitting of the molecule. In this work, we configure that Au interaction with the Cu enables the ability to supply H adatoms to hydrogenate CO2. It has been demonstrated that the hydrogen spillover weakens and the amount of H2 consumed decreases due to inappropriate gold–copper interaction (too weak or strong), over 0.5 wt% and 2 wt% samples. Differently, the interaction between gold and copper over 1 wt% Au–CuO/CeO2 leads to the superior hydrogen spillover, therefore exhibiting better desorption properties.
3.2 Catalytic Activity
The catalytic performance results of the catalysts are shown in Table 1. CuO/CeO2 exhibits low catalytic performance toward methanol synthesis from CO2 hydrogenation. Specifically the CO2 conversion of which is as low as 3.8–9.6% from 200 to 300 °C. The methanol selectivity increases from 8.6 to 17.9% at 200–240 °C, then decreases to 4.3% at 300 °C. Au/CeO2 shows relatively high CO2 conversion (6.8–12.7% at 200–300 °C), but it favors the formation of CO rather than methanol. The methanol selectivity of Au/CeO2 keeps decreasing from 21.8 to 0.6% during whole process. Adding 1 wt% gold increases CO2 conversion (6.3–10.1% at 200–300 °C). The 1 wt% Au–CuO/CeO2 catalyst exhibits the highest methanol selectivity (29.6%) at 240 °C. The improvement of methanol selectivity over 1 wt% Au–CuO/CeO2 is even higher than that of CO2 conversion, especially for higher reaction temperature (≥ 240 °C). However methanol selectivity is found to drop with further addition of gold (2 wt%).
A comparison of catalytic activity on Cu/Au-based catalysts for CO2 hydrogenation to methanol is summarized in Table 2. The monometallic Au-based catalysts with equivalent gold loading (1.1 wt%) to our work, which shows lower CO2 conversion and CH3OH selectivity compared with 1 wt% Au–CuO/CeO2 in our work [23, 24]. Though the monometallic Cu-based catalysts with higher copper contents, the catalytic performance of these samples is still lower than that of 1 wt% Au–CuO/CeO2 [25,26,27]. For bimetallic Au–Cu samples studied by other researchers [28], CO2 conversion of these samples is higher than that of 1 wt% Au–CuO/CeO2, however, CH3OH selectivity of these samples is much lower.
The 1 wt% Au–CuO/CeO2 shows the highest methanol selectivity among all samples. The CuO particle size of monometallic CuO/CeO2 and bimetallic Au–CuO/CeO2 samples is almost similar (22–26 nm), which indicates that particle size has little influence on catalytic performance. It is speculated that the excellent catalytic performance of 1 wt% Au–CuO/CeO2 sample can be attributed to larger amounts of oxygen vacancies and active H atoms.
3.3 In Situ DRIFTS Study
3.3.1 High Pressure In Situ DRIFTS of CO2 Adsorption
The 1 wt% Au–CuO/CeO2 sample with best catalytic performance is chosen as a representative one to illuminate the effect of Au addition on CO2 adsorption over 1 wt% Au–CuO/CeO2 sample. After CO2 adsorption, the methoxy species (1016, 1048 and 1078 cm−1) and bicarbonates (1240, 1286, 1646 and 1689 cm−1) are observed over CeO2 sample (Fig. S1a). For Au/CeO2 sample, the main adsorption species still are methoxy and bicarbonate species, additionally, one weak peak associated with formate species is detected (Fig. S1b). However, for CuO/CeO2 sample, only methoxy species are the main adsorption ones, indicating that the copper species on the CeO2 has an effect on adsorption of CO2 (Fig. S1c). Muttaqien et al. has demonstrated that pure copper species have ability to adsorb CO2 and the properties of copper species have an influence in CO2 adsorption capacity [29]. As is shown in Fig. S1d, the CO2 adsorption species on 0.5 wt% Au–CuO/CeO2 are similar to those of on CuO/CeO2. The low Au content has a little effect on CO2 adsorption. Figure 6 shows the spectra of CO2 adsorption ranging from 1 to 30 min over 1 wt% Au–CuO/CeO2 catalyst. The adsorption of CO2 on 1 wt% Au–CuO/CeO2 produces methoxy (1016, 1048 and 1078 cm−1), bicarbonates (1240, 1286, 1646 and 1689 cm−1) and carbonate species (1438 and 1521 cm−1) [30]. The higher concentration and more types of adsorption species are observed on 1 wt% Au–CuO/CeO2 catalyst. Hence, the addition of 1 wt% Au to CuO/CeO2 promotes CO2 adsorption due to the formation of the largest number of oxygen vacancies and excellent copper species properties. As adding 2 wt% Au to CuO/CeO2, the type and concentration of adsorption species are becoming smaller (Table 3).
Combining with CO2-TPD result (Fig. S3), pure CeO2 only shows CO2 desorption peaks ranging from 50 to 200 °C, which is assigned to weakly and moderately basic sites [31]. Additionally, a lager desorption peak at around 400 °C is observed on CuO/CeO2 and 1 wt% Au–CuO/CeO2 samples, which probably comes from the decomposition of carbonate species. As is confirmed by the result obtained from in situ DRIFTS of CO2 adsorption (Figs. 6, S1), carbonates species are only detected on these two samples. Hence, it is reasonable to speculate that weakly and moderately basic sites are active sites for CO2 adsorption and activation during CO2 hydrogenation to methanol over Au–CuO/CeO2 samples. Because 1 wt% Au–CuO/CeO2 with a higher density of basic sites readily activates CO2 and therefore it is reasonable to speculate that the hydrogenation activity is governed by the adsorption and dissociation of H2.
3.3.2 High Pressure In Situ DRIFTS of CO2 Hydrogenation
The in situ DRIFT spectra of 1 wt% Au–CuO/CeO2 catalyst under CO2 + H2 is displayed in Fig. 7. Since no peaks corresponding to methanol are observed. The support CeO2 itself is not active for CO2 hydrogenation to methanol. These results indicate that the presence of active metal is necessary for the CO2 hydrogenation to methanol [32]. Neither Au/CeO2 nor CuO/CeO2 shows good methanol selectivity. Gold is demonstrated to be more selective and active for the formation of CO/CH4 rather than CH3OH product in CO2 hydrogenation reaction [33]. For a series of Au–CuO/CeO2 samples, the type and concentration of intermediate species on 0.5 wt% Au–CuO/CeO2 is almost identical to those of on CuO/CeO2 sample (Fig. S4d). For 2 wt% Au–CuO/CeO2, only a small concentration of methanol is observed (Fig. S4e). The interaction between gold and copper of high/low gold loading catalyst is both unfavorable for providing active H* species.
Differently, compared to other samples, the intensities of methoxy (1078 and 1498 cm−1) and formate (1390 and 2703 cm−1) on 1 wt% Au–CuO/CeO2 become stronger, suggesting an improvement of H2 dissociation. And a larger number of methanol is detected on 1 wt% Au–CuO/CeO2. Evidently, the interaction between gold and copper over 1 wt% Au sample leads to a remarkable enhancement of methanol selectivity, while the improvement is very little on their single counterparts. It is reported that the hydrogen spillover is beneficial to enhance methanol formation rate, resulting in higher methanol selectivity [34]. It has shown that the superior methanol selectivity of CuO–ZnO–ZrO2–GO is attributed to a promotional effect of GO nanosheet serving as a bridge between mixed metal oxides which enhances a hydrogen spillover from the copper surface [35]. The hydrogen spillover improves methanol yield over Au/Cu–Zn–Al catalyst for methanol synthesis from CO2 [28]. Thus it is reasonable to suggest that the fine interaction between Au and Cu promotes the hydrogen spillover over 1 wt% Au–CuO/CeO2, resulting in higher methanol selectivity.
It is known that the reaction temperature also plays a significant role in the evolution of intermediate species, resulting in different catalytic performance of CO2 hydrogenation to methanol. Figure 8 shows in situ DRIFT spectra of CO2 hydrogenation over 1 wt% Au–CuO/CeO2 from 240 to 300 °C. The peaks assigned to bicarbonates (1240, 1274, 1646, 1689 cm−1) decrease from 240 to 300 °C, simultaneously, the intensities of methoxy (1016, 1078, 1498 cm−1), formate (1390, 2703 cm−1) intermediates start to increase 240 °C to 300 °C. Additionally, methanol (2834 and 2948 cm−1) and by-products CO (2127 cm−1) as well as methane (3016 cm−1) increase at 240–300 °C. Obviously, these observations illustrate that bicarbonates adsorbed on CeO2 are firstly hydrogenated into formate, then methoxy and finally methanol, as is shown in Scheme 1. The findings proposed in the work would be helpful for designing complex catalysts with multiple active components.
4 Conclusions
This work investigates the effect of Au addition on catalytic performance of Au–CuO/CeO2 catalysts for CO2 hydrogenation to methanol. Tuning Au loading can affect the formation and evolution of the surface species, therefore controlling the catalytic performance. The 1 wt% Au–CuO/CeO2 is able to activate both H2 and CO2 for the efficient production of methanol. Additionally, in situ DRIFT experiments suggest the formate pathway for the reaction mechanism on 1 wt% Au–CuO/CeO2, and CO2 molecular firstly is adsorbed and activated on CeO2 forming (bi)carbonates, and then formate and methoxy species, finally methanol.
References
Roy S, Cherevotan A, Peter SC (2018) Thermochemical CO2 hydrogenation to single carbon products: scientific and technological challenges. ACS Energy Lett 3:1938–1966
Yang CS, Pei CL, Luo R et al (2020) Strong electronic oxide-support interaction over In2O3/ZrO2 for highly selective CO2 hydrogenation to methanol. J Am Chem Soc 46:19523–19531
Din IU, Shaharun MS, Naeem A et al (2019) Revalorization of CO2 for methanol production via ZnO promoted carbon nanofibers based Cu–ZrO2 catalytic hydrogenation. J Energy Chem 39:68–76
Din IU, Alotaibi MA, Abdulrahman IA (2020) Green synthesis of methanol over zeolite based Cu nano-catalysts, effect of Mg promoter. Sustain Chem Pharm 16:100264
Bracey CL, Ellis PR, Hutchings GJ (2009) Application of copper–gold alloys in catalysis: current status and future perspectives. Chem Soc Rev 8:2231–2243
Martin O, Mondelli C, Curulla-Ferre D (2015) Zinc-rich copper catalysts promoted by gold for methanol synthesis. ACS Catal 5:5607–5616
Reina TR, Ivanova S, Centeno MA (2016) The role of Au, Cu and CeO2 and their interactions for an enhanced WGS performance. Appl Catal B 187:98–107
Campbell CT (2004) The active site in nanoparticle gold catalysis. Science 5694:234–235
Sankar M, He Q, Engel RV (2020) Role of the support in gold-containing nanoparticles as heterogeneous catalysts. Chem Rev 120:3890–3938
Laszlo B, Baan K, Varga E (2016) Photo-induced reactions in the CO2–methane system on titanate nanotubes modified with Au and Rh nanoparticles. Appl Catal B 199:473–484
Xue HR, Wang T, Gong H (2018) Constructing ordered three-dimensional TiO2 channels for enhanced visible-light photocatalytic performance in CO2 conversion induced by Au nanoparticles. Chem Asian J 5:577–583
Li Y, Na W, Wang H et al (2017) Hydrogenation of CO2 to methanol over Au–CuO/SBA15 catalyst. J Porous Mater 24:591–599
Zhu JD, Su YQ, Chai JC et al (2020) Mechanism and nature of active sites for methanol synthesis from CO/CO2 on Cu/CeO2. ACS Catal 19:11532–11544
Jing GJ, Zhang L, Ma YR et al (2019) Comparison of Au–Ce and Au–Cu interaction over Au/CeO2–CuO catalysts for preferential CO oxidation. CrystEngComm 21:363
Jeon S, Ham H, Suh Y et al (2015) Raman scattering study of cubic–tetragonal phase transition in Zr1−xCexO2 solid solution. RSC Adv 5:54806–54815
Reddy BM, Khan A, Lakshmanan P et al (2005) Structural characterization of nanosized CeO2–SiO2, CeO2–TiO2, and CeO2–ZrO2 catalysts by XRD, Raman, and HRTEM techniques. J Phys Chem B 8:3355–3363
Ye J, Liu C, Mei D et al (2013) Active oxygen vacancy site for methanol synthesis from CO2 hydrogenation on In2O3(110): a DFT study. ACS Catal 3:1296–1306
Liao X, Chu W, Dai X et al (2013) Bimetallic Au–Cu supported on ceria for PROX reaction: effects of Cu/Au atomic ratios and thermal pretreatments. Appl Catal B 142–143:25–37
Vourros A, Garagounis I, Kyriakou V et al (2017) Carbon dioxide hydrogenation over supported Au nanoparticles: effect of the support. J CO2 Util 19:247–256
Gamboa-Rosales NK, Ayastuy JL, Gonzalez-Marcos MP et al (2012) Oxygen-enhanced water gas shift over ceria-supported Au–Cu bimetallic catalysts prepared by wet impregnation and deposition-precipitation. Int J Hydrog Energy 37:7005–7016
Pongstabodee S, Monyanon S, Luengnaruemitchai A (2012) Hydrogen production via methanol steam reforming over Au/CuO, Au/CeO2, and Au/CuO–CeO2 catalysts prepared by deposition-precipitation. J Ind Eng Chem 18:1272–1279
An B, Zhang J, Cheng K et al (2017) Confinement of ultrasmall Cu/ZnOx nanoparticles in metal–organic frameworks for selective methanol synthesis from catalytic hydrogenation of CO2. J Am Chem Soc 139:3834–3840
Nevanperä TK, Ojala S, Laitinen T et al (2019) Catalytic oxidation of dimethyl disulfide over bimetallic Cu–Au and Pt–Au catalysts supported on γ-Al2O3, CeO2, and CeO2–Al2O3. Catalysts 9:603
Tan Q, Shi Z, Wu D (2018) CO2 Hydrogenation to methanol over a highly active Cu–Ni/CeO2-nanotube catalyst. Ind Eng Chem Res 57:10148–10158
Patil P, Nakate UT, Harish K et al (2020) Au sensitized La–CeO2 catalyst coated ceramics monoliths for toluene catalysis application. Mater Chem Phys 240:12269
Piqueras CM, Puccia V, Vega DA et al (2016) Selective hydrogenation of cinnamaldehyde in supercritical CO2 over Me–CeO2 (Me = Cu, Pt, Au): insight of the role of Me–Ce interaction. Appl Catal B 185:265–271
Pongstabodee S, Monyanon S, Luengnaruemitchai A (2012) Hydrogen production via methanol steam reforming over Au/CuO, Au/CeO2, and Au/CuO–CeO2 catalysts prepared by deposition–precipitation. J Ind Eng Chem 18:1272–1279
Wang G, Chen L, Sun Y et al (2015) Carbon dioxide hydrogenation to methanol over Cu/ZrO2/CNTs: effect of carbon surface chemistry. RSC Adv 5:45320–45330
Muttaqien F, Hamamoto YJ, Hamada I et al (2017) CO2 adsorption on the copper surfaces: van der Waals density functional and TPD studies. J Chem Phys 147:094702
Fisher IA, Bell AT (1997) In-situ infrared study of methanol synthesis from H2/CO2 over Cu/SiO2 and Cu/ZrO2/SiO2. J Catal 172:222–237
Sloczynski J, Grabowski R, Kozlowska A et al (2004) Catalytic activity of the M/(3ZnO–ZrO2) system (M = Cu, Ag, Au) in the hydrogenation of CO2 to methanol. Appl Catal A 278:11
Wang YH, Gao WG, Wang H et al (2017) Structure–activity relationships of Cu–ZrO2 catalysts for CO2 hydrogenation to methanol: interaction effects and reaction mechanism. RSC Adv 7:8709
Liu X, Guo Q, Guo D et al (2016) Methanol synthesis from CO2 hydrogenation over copper catalysts supported on MgO-modified TiO2. J Mol Catal A 425:86–93
Fujitani T, Saito M, Kanai Y et al (1995) Development of an active Ga2O3 supported palladium catalyst for the synthesis of methanol from carbon dioxide and hydrogen. Appl Catal A 125:199–202
Pasupulety N, Driss H, Alhamed YA et al (2015) Studies on Au/Cu–Zn–Al catalyst for methanol synthesis from CO2. Appl Catal A 504:308–318
Acknowledgements
This work was financially supported by the National Nature Science Foundation of China (21876019), Fundamental Research Funds for the Central Universities (DUT19LAB10), Dalian Science and Technology Innovation Fund (2019J12SN74) and the Fund of the State Key Laboratory of Catalysis in DICP (N-18-08).
Author information
Authors and Affiliations
Author notes
Weiwei Wang and Desiree Wager Kimpouni Tongo have contributed equally to this work.
Contributions
WW: Conceptualization, Methodology, Formal analysis, Data curation, Investigation, Writing-Original draft preparation. DWKT: Methodology, Formal analysis, Data curation, Investigation, Writing-Original draft preparation. LS: Visualization, Investigation, Software, Methodology. ZQ: Supervision, Funding acquisition, Project administration, Resources Validation, Conceptualization, Writing-Reviewing and Editing.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest when submitting this manuscript, and all authors have approved the publication of the manuscript. And the authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Approval
I would like to state on behalf of my co-authors that the work described is the original study, has not been published before, and has not been considered for publication elsewhere. All the authors listed have approved the accompanying manuscript while being considered by Topics in Catalysis.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, W., Tongo, D.W.K., Song, L. et al. Effect of Au Addition on the Catalytic Performance of CuO/CeO2 Catalysts for CO2 Hydrogenation to Methanol. Top Catal 64, 446–455 (2021). https://doi.org/10.1007/s11244-021-01414-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-021-01414-3