Abstract
Two new square-planar Ni(II) complexes, [NiL1(NCS)] (1) and [NiL2(N3)] (2) have been synthesized with the unsymmetrical tridentate Schiff base ligands [(CH3)2NCH2CH2N=C(CH3)CH=C(OH)(C6H5)], L 1 H, derived from benzoylacetone and 2-dimethylaminoethylamine and [(CH3CH2)2NCH2CH2N=C(CH3)CH=C(OH)(C6H5)], L 2 H, derived from benzoylacetone and 2-diethylaminoethylamine, respectively. The complexes have been characterized by elemental analysis, FT-IR, UV-Vis spectroscopy, electrochemical and thermal methods (where applicable). Structures have been established by the single-crystal X-ray diffraction technique which reveals the discrete nature of the complexes in which the metal centers adopt a distorted square planar geometry. Coordination environments of the metal ions in the complexes are satisfied with two different unsymmetrical Schiff base ligands having similar N2O donor sets and a terminal pseudohalide anion (thiocyanate for 1 and azide for 2).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multidentate Schiff base ligands play an important role in the development of coordination chemistry as they readily form complexes, which have reasonable stability, with most of the transition metal ions [1–3]. These Schiff base transition metal complexes have been of great interest for many years [4] due to their important roles in catalysis and enzymatic reactions, magnetism, and enhanced efficiency as therapeutic agents [5, 6]. They are once again topical in connection with self-assembling cluster complexes [7]. Metal complexes of Schiff bases derived from aromatic carbonyl compounds have been widely studied in connection with metalloprotein models because of the versatility of their steric and electronic properties, which can be modified by selecting the suitable amine precursors and ring substituents [8]. They are also known for their significant biological activities such as photosynthesis and transport of oxygen in mammalian and other respiratory systems [9, 10]. Transition metal complexes with oxygen and nitrogen donor Schiff bases are of particular interest [11, 12] for their ability to possess unusual configurations, structural lability and their sensitivity to molecular environments [13]. Amongst them, the enduring popularity of tridentate Schiff bases stems from the ease with which they can be synthesized, their versatility and their wide-ranging complexing ability [14]. Symmetrical Schiff base ligands, usually obtained by the condensation of a symmetrical diamine with two molecules of aldehyde/ketone providing identical moieties on each side of the diamine, have symmetrical electronic and steric contributions in the complex. However, unsymmetrical Schiff base ligands, usually obtained by an unsymmetrical combination of aldehydes/ketones with the diamine, allow for tuning of both electronic properties and steric effects on one side and/or the other side of the complex. Thus, the performance of unsymmetrical Schiff base catalysts may be maximised. Also, unsymmetrical Schiff bases are very important as they can bind one, two or more metal centers, involving various coordination modes, and allow successful synthesis of homo and/or heteronuclear metal complexes with interesting stereochemistries [15–18].
On the other hand, the chemistry of nickel complexes with multidentate Schiff base ligands has attracted attention because this metal can exhibit several oxidation states. Such complexes play an important role in bioinorganic chemistry and redox enzyme systems, and may provide the basis of models for active sites of biological systems or act as catalysts [19, 20]. Pseudohalides, especially azide, thiocyanate and cyanate, are useful as coligands for their versatile coordination modes in the formation of mono-, di- or poly-nuclear complexes [21]. However, it has been demonstrated that the azido group inhibits enzymatic reactions [22, 23]. Thus, the investigation of nickel–azide complexes recently has had special importance in understanding the role of the metal ion in biological reactions.
We have previously reported work on L 1 H in one mononuclear Ni(II) complex [24] and in di- and poly-nuclear Cu(II) complexes [25], with L 2 H in three mononuclear Cu(II) complexes [24, 26], and also with various other tridentate Schiff base ligands in the presence of coordinating NNN−, SCN− and OCN− anions in mononuclear Ni(II) complexes [19]. Here we discuss the coordination behavior of two unsymmetrical tridentate N2O donor ligands from the Schiff bases L 1 H and L 2 H (Scheme 1) in combination with pseudohalides (thiocyanate and azide) towards Ni(II) centers. This paper describes the synthesis, elemental analyses, spectroscopic studies, crystal structures, thermal and electrochemical investigations of two new mononuclear complexes [NiL1(NCS)] (1) and [NiL2(N3)] (2) in which the pseudohalides display terminal bonding mode.
Experimental
Materials
All the chemicals and solvents used for the synthesis were of reagent grade. 2-dimethylaminoethylamine (Fluka, Germany), 2-diethylaminoethylamine, benzoylacetone, sodium azide, sodium thiocyanate, nickel(II) acetate tetrahydrate, and Ni(ClO4)2 · 6H2O (Sigma-Aldrich, USA) were used as received without further purification.
Caution! Azido compounds of metal ions are potentially explosive especially in presence of organic ligands. Though no difficulties have been encountered during the preparation and characterization of our complexes, only a small amount of materials should be prepared and handled with care.
Physical measurements
Elemental analyses (C, H and N) were performed on a Perkin-Elmer 2400 II elemental analyzer. Nickel was analysed by atomic absorption with a Hitachi Z-8200 atomic absorption spectrophotometer. The infrared spectra of the complexes were recorded on a Perkin-Elmer RX I FT-IR spectrophotometer with KBr pellets in the range of 4,000–200 cm−1. The electronic spectra were measured on a Perkin-Elmer Lambda 40 (UV–Vis) spectrophotometer in dichloromethane. Electrochemical studies were performed on a CH 600A cyclic voltammeter instrument using acetonitrile as solvent. Thermal analyses were carried out at a heating rate of 10 °C/min with a Mettler-Toledo Star TGA/SDTA-851e thermal analyzer system in a dynamic atmosphere of N2 (flow rate 30 ml/min) in an alumina crucible for the range 25–350 °C.
Preparation of the ligands and complexes
Synthesis of the Schiff base L 1 H
The proligand L 1 H was prepared by refluxing benzoylacetone (0.811 g, 5 mmol) and 2-dimethylaminoethylamine (0.545 ml, 5 mmol) in 50 ml of methanol for half an hour. The resulting yellow solution containing the tridentate Schiff base was used without further purification.
Synthesis of the Schiff base L 2 H
The proligand L 2 H was prepared by the same procedure as used for L 1 H except that 2-diethylaminoethylamine was used instead of 2-dimethylaminoethylamine. Benzoylacetone (0.811 g, 5 mmol) was refluxed with 2-diethylaminoethylamine (0.711 ml, 5 mmol) in 50 ml of methanol for half an hour. The resulting mixture gave a yellow solution containing the tridentate Schiff base and was used without further purification.
Synthesis of the complex [NiL1(NCS)] (1)
To a methanolic solution (30 ml) of nickel(II) acetate tetrahydrate (0.248 g, 1 mmol), 10 ml methanolic solution of L 1 H (1 mmol) was added followed by the slow addition, with constant stirring, of 5 ml aqueous solution of sodium thiocyanate (0.081 g, 1 mmol). The mixture was stirred for 10 min and filtered. The filtrate was kept undisturbed at 5 °C in a refrigerator for four days. Red crystals of 1 suitable for X-ray diffraction were obtained on slow evaporation of the solvent. Crystals were isolated by filtration and air-dried. Yield: 65% with respect to the metal substrate. Anal.: Calc. for [C15H19N3NiOS]: C, 51.76; H, 5.50; N, 12.07; Ni, 16.86. Found: C, 51.72; H, 5.52; N, 12.05; Ni, 16.85%.
Synthesis of the complex [NiL2(N3)] (2)
10 ml solution of L 2 H (1 mmol) was added to 30 ml methanolic solution of Ni(ClO4)2 · 6H2O (0.365 g, 1 mmol), followed by mixing, with constant stirring for 10 min, with 10 ml of an aqueous solution of sodium azide (0.065 g, 1 mmol). This mixture was filtered and the filtrate was kept at room temperature for 3 days yielding deep red crystals of 2 suitable for X-ray diffraction. Crystals were isolated by filtration and air-dried. Yield: 68% with respect to metal substrate. Anal.: Calc. for [C16H23N5NiO]: C, 53.37; H, 6.44; N, 19.45; Ni, 16.30. Found: C, 53.30; H, 6.42; N, 19.43; Ni, 16.29%.
Crystal structure determination and refinement
A deep red rectangular block shaped crystal of 1 was fixed on a glass fibre with epoxy resin and mounted on a Nonius CAD4 diffractometer equipped with scintillation counter and graphite monochromatized Mo-Kα radiation (λ = 0.71069 Å). The deep red prism crystal of 2 was mounted in oil on a glass fibre and fixed in the cold nitrogen stream on an Oxford Xcalibur-3 CCD diffractometer equipped with graphite monochromatized Mo-Kα radiation. Structures of both the complexes were determined by the direct methods routines in the SHELXS program [27] and refined by full-matrix least squares methods, on F2s, in SHELXL [27]. The non-hydrogen atoms were refined with anisotropic thermal parameters. Hydrogen atoms were included in idealised positions and their U isovalues were set to ride on the U eq values of the parent carbon atoms. The crystallographic data and the refinement results are listed in Table 1. All graphical works has been done using ORTEP [28] program.
Results and discussion
Infrared spectra
The solid-state FT-IR spectra of 1 and 2 are fully consistent with their structural data as revealed from X-ray studies. The weak-broad bands in the region 3250–3350 cm−1 due to the hydrogen bonded OH group in the free Schiff bases are absent from the IR spectra of 1 and 2, indicating the coordination of enolic oxygen atoms of the Schiff bases to the metal centers. The strong ν(C=N) bands in the range of 1617–1611 cm−1, observed for the free Schiff bases are shifted slightly towards lower frequencies (~1603 cm−1) indicating the coordination of azomethine nitrogen atom with the metal centers [29]. Coordination of the ligands is further substantiated by the bands at 459 and 462 cm−1, assignable to ν(Ni–N) observed for 1 and 2, respectively, and at 360 and 358 cm−1, assignable to ν(Ni–O) stretching frequencies. The ν(CN) absorption at 2103 cm−1 as a single peak for 1 suggests the presence of an N-coordinated terminal NCS group, and νa(NNN) at 2021 cm−1 as a single peak for 2 indicates the presence of a terminal azide ion [30].
Electronic spectra
The electronic spectra of 1 and 2 were recorded in acetonitrile solvent. Each spectrum consists of two low energy bands. Bands at 425 and 330 nm for 1, and 421 and 322 nm for 2, correspond to the d–d transition in square-planar nickel complexes [31].
Electrochemical studies
Electron transfer properties of the complexes were studied at room temperature using acetonitrile as solvent, a platinum electrode, a scan rate of 50 mVs−1 and tetrabutylammonium perchlorate as supporting electrolyte. The cyclic voltammograms of 1 and 2 show irreversible oxidative responses at +0.97 and +0.94 V, respectively due to Ni(II)-Ni(III) conversion, and indicate that the Ni(III) species is unstable and undergoes rapid decomposition in both complexes.
Thermal analysis
The thermal decomposition trace of 1 has been recorded. The thermal study of 2 was not carried out in order to avoid potential explosion of azide. The TGA experiment of 1 indicates that this is stable up to ~195 °C and then begins to lose the Schiff base in two steps between 195–240 and 240–330 °C. The intermediates could not be identified due to their immediate transformation but total mass loss corresponds to one equivalent of Schiff base. Further decomposition finally leads to a black residue of nickel(II) oxide.
Description of the crystal structures of 1 and 2
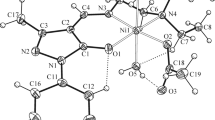
The crystal structures of 1 and 2, with the atom numbering schemes, are shown in Figs. 1 and 2, respectively. Selected bond lengths and angles for both complexes are given in Table 2. Each complex consists of one Ni(II) ion, one pseudohalide ligand, and one tridentate Schiff base anion ligand. The central Ni(II) ion displays a distorted square planar geometry in both complexes. The four coordination sites of 1 are occupied by a N2O donor set of the unsymmetrical Schiff base L 1 and the N-atom of a terminal thiocyanate ion; complex 2 is similarly coordinated by the unsymmetrical Schiff base L 2 and the N-atom of a terminal azide ion. Distortion of the ideal square-planar geometry in each case is evident from the bond distances, bond angles, and the deviations of the metal ions from the mean square-planes. The coordination bond lengths in 2 are longer than corresponding lengths in 1, resulting, in part, from the greater steric demand of the two ethyl substituents on N(7) in 2, compared to the two methyl substituents in 1. In each complex, the Ni-N(7) bond length (to the amino N-atom) is significantly longer than the other coordination bonds. There is considerable deviation of the cisoid and transoid angles from the ideal 90° and 180° values in both complexes. Deviations of the central Ni(II) ion from the least-squares planes through O(1), N(4), N(7), and N(8) for 1 and 2 are 0.040(2) and 0.0525(7) Å, respectively. The NCS− and NNN− ions in 1 and 2 remain almost linear, but coordination to the metal ion shows much greater deviation from linearity in 2 than in 1. The NNN− ligand in 2 is bent away from the ethyl groups and slightly out of the coordination plane. In both complexes, the phenyl ring remains almost coplanar with the coordination plane due to some conjugation with the pseudo aromatic system present.
View of 1, with atom numbering scheme; displacement ellipsoids are drawn at the 50% probability level (There is disorder in the ethylene bridge; this has been resolved, as shown, with alternative sites C(6a) and C(6b), but the corresponding alternative sites for the methyl atoms C(71) and C(72) have not been located)
The principal intermolecular contacts in both compounds appear to be C–H···π interactions. In 1, the C(31)-H(31c) bond is directed towards the centre of the C(11–16) ring of an adjacent molecule and these contacts link molecules in pairs about centres of symmetry; also, the C(71)-H(71b) bond points from the opposite side to one end of the C(11–16) ring and links molecules around a twofold-screw symmetry axis parallel to the b axis. In compound 2, C(31)–H(31a)···C(11–16) contacts link molecules by translation along the a axis, and the methylene C(71)–H(71b) bond makes close contact with a neighbouring chelate ring and thus forms chains along a 21 symmetry axis parallel to the b axis.
A comparison of 1 and 2 with our previously reported Cu(II) and Ni(II) complexes [24] indicates that the differences in bond distances, bond angles, and in the deviations of the metal ions from the mean square-planes between 1 and 2 arise not only from the presence of more bulky ethyl substituents in 2 compared to the less-steric methyl substituents in 1, but also from electronic effects of the Schiff base ligands and metal ion; the alignment and mode of bonding of the pseudohalogens, also play important roles.
Conclusion
In this paper, we have reported two new square-planar Ni(II) complexes with unsymmetrical tridentate Schiff base ligands having N2O donor sets. Fourfold coordination is completed in each complex by a monodentate pseudohalide ligand. Both complexes have been characterized by micro-analytical, spectral, electrochemical and thermal methods. Structural characterizations by single-crystal diffraction analysis have also been discussed. Structural analyses reveal that a combined effect of the steric and electronic nature of the ligands and metal ion, in addition to the alignment and the bonding mode of the pseudohalogens, control the stability of the complexes.
Supplementary data
CCDC-699998 (1) and 699999 (2) contain the supplementary crystallographic data for this paper. These data may be obtained free of charge at www.ccdc.cam.ac.uk [or from Cambridge Crystallographic Data Center, 12, Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336 033; e-mail: deposit@ccdc.cam.ac.uk].
References
Ueno T, Ohashi M, Kono M, Kondo K, Suzuki A, Yamane T, Watanabe Y (2004) Inorg Chem 43:2852. doi:10.1021/ic0498539
Pal S, Barik AK, Gupta S, Hazra A, Kar SK, Peng S-M, Lee G-H, Butcher RJ, Fallah MSEl, Ribas J (2005) Inorg Chem 44:3880. doi:10.1021/ic0501420
Hou N-N (2005) Acta Cryst E61:m1197
Yamada S (1999) Coord Chem Rev 190–192:537. doi:10.1016/S0010-8545(99)00099-5
Ali MA, Haroon CM, Nazimuddin M, Majumder SMM, Tarafder MTH, Khair MA (1992) Trans Met Chem 17:133. doi:10.1007/BF02910804
Hossain ME, Alam MN, Ali MA, Nazimuddin M, Smith FE, Hynes RC (1996) Polyhedron 15:973. doi:10.1016/0277-5387(95)00310-X
Chattopadhyay S, Bocelli G, Cantoni A, Ghosh A (2006) Inorg Chim Acta 359:4441. doi:10.1016/j.ica.2006.06.009 and references therein
Marchetti F, Pettinari C, Pettinari R, Cingolani A, Leonesi D, Lorenzotti A (1999) Polyhedron 18:3041. doi:10.1016/S0277-5387(99)00230-2
Ramesh M, Chandrasekar KB, Reddy KH (2000) Indian J Chem 39A:1337
Coughlin PK, Lippard SJ (1984) J Am Chem Soc 106:2328. doi:10.1021/ja00320a018
You Z-L, Zhu H-L, Liu W-S (2004) Z Anorg Allg Chem 630:1617. doi:10.1002/zaac.200400125
You Z-L, Zhu H-L (2004) Z Anorg Allg Chem 630:2754. doi:10.1002/zaac.200400270
Golcu A, Tumer M, Demirelli H, Wheatley RA (2005) Inorg Chim Acta 358:1785. doi:10.1016/j.ica.2004.11.026
Coles SJ, Hursthouse MB, Kelley DG, Toner AJ, Walker NM (1998) J Chem Soc Dalton Trans 3489. doi:10.1039/a805764h
Zanello P, Tamburini S, Vigato PA, Mazzocchin GA (1987) Coord Chem Rev 77:165. doi:10.1016/0010-8545(87)85034-8
Timken MD, Marrit WA, Hendrickson DN, Gagne RR, Sinn E (1985) Inorg Chem 24:4202. doi:10.1021/ic00218a044
Pouralimardan O, Chamayou A-C, Janiak C, Hassan H-M (2007) Inorg Chim Acta 360:1599. doi:10.1016/j.ica.2006.08.056
Ikawa K, Nagata T, Aruyama K (1993) Chem Lett 1049 doi:10.1246/cl.1993.1049
Mondal N, Mitra S, Gramlich V, Ghodsi SO, Malik KMA (2001) Polyhedron 20:135. doi:10.1016/S0277-5387(00)00601-X and references therein
Amirnasr M, Schenk KJ, Meghdadi S, Morshedi M (2006) Polyhedron 25:671. doi:10.1016/j.poly.2005.07.040
Dey SK, Mondal N, Fallah MSEl, Escuer A, Solans X, Matsushita T, Gramlich V, Mitra S (2004) Inorg Chem 43:2427. doi:10.1021/ic0352553
Youn H-D, Kim E-J, Roe J-H, Hah YC, Kang S-O (1996) Biochem J 318:1452
Watt RK, Ludden PW (1999) J Bacteriol 181:4554
Basak S, Sen S, Mitra S, Marschner C, Sheldrick WS (2008) Struct Chem 19:115. doi:10.1007/s11224-007-9260-0
Talukder P, Datta A, Mitra S, Rosair G, El Fallah MS, Ribas J (2004) Dalton Trans 4161. doi:10.1039/b413084g
Talukder P, Datta A, Mitra S, Rosair G (2004) Z Naturforsch 59b:655 (and references therein)
Sheldrick GM, SHELX97-Programs for Crystal Structure Analysis: Structure Determination and Refinement (SHELX-S/L) (2008) Acta Cryst A64:112
Farrugia LJ, ORTEP-3 for Windows (1997) J Appl Cryst 30:565. doi:10.1107/S0021889897003117
Tanaka M, Kitaoka M, Ökawa H, Kida S (1976) Bull Chem Soc Jpn 42:677
Nakamoto K (1997) Infrared and Raman spectra of inorganic and coordination compounds, 5th edn Parts A and B. Wiley, New York
Lever ABP (1984) Inorganic electronic spectroscopy, 2nd edn. Elsevier, Amsterdam
Acknowledgement
This work has been financially supported by Defense Research and Development Organisation, University Grants Commission and Council of Scientific and Industrial Research, New Delhi, Goverment of India. S. Sen is grateful to CSIR (New Delhi, India) for the award of Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shit, S., Sen, S., Mitra, S. et al. Syntheses, characterization and crystal structures of two square-planar Ni(II) complexes with unsymmetrical tridentate Schiff base ligands and monodentate pseudohalides. Transition Met Chem 34, 269–274 (2009). https://doi.org/10.1007/s11243-009-9189-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-009-9189-9