Abstract
This study represents an optimized protocol for cell line culture of Matricaria chamomilla and the impact of clino-rotation on cell division, cell growth, and antioxidant enzyme activities for the first time. The cell suspension was transferred in the solid MS medium supplied with 2, 4-D, and KIN. Then the calli produced from a cell line were selected for callus subculture and clino-rotation treatment for 7 days by a 2D-clinostat. A significant rise of fresh and dry weights, cell division, total soluble sugar, reducing sugar, and starch contents were detected under clino-rotation. Protein content approximately unchanged in microgravity-treated calli. Antioxidant enzymes activities, such as peroxidase, catalase (CAT), and superoxide dismutase were elevated in calli exposed to microgravity. CAT activity showed a more than three-fold increase than that of control. According to native polyacrylamide gel electrophoresis, all the antioxidant enzymes isoforms were stronger in clino-rotated calli than that of the untreated control. Microgravity also stimulated H2O2 production and markedly adjusted lipid peroxidation in calli exposed to clino-rotation. These findings suggest that clino-rotation with stimulation of carbohydrate accumulation and antioxidant enzymes mitigates oxidative stress and improves growth and cell division.
Key message
The isolation of M. chamomilla cell line with high growth was conducted to study the impact of clino-rotation on some cellular and antioxidative enzyme responses. Clino-rotation stimulated the cell division and growth by induction of antioxidant enzyme activities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Living organisms have evolved for over a billion years in a 1 g gravity on Earth. Gravity is a critical environmental factor that can impact plant development, growth, and evolution. Alteration of gravity from 1 g to microgravity conditions would make notable physiological changes, which would stimulate the adaptive response pathways (Kamal et al. 2018; Soleimani et al. 2019). Investigation of microgravity biological impacts need to transfer samples on orbit, but access to space flight and the international space station is limited by the high cost and prepare the crew for the flight. To overcome these limitations, ground-based facilities such as different kinds of clinostats and magnetic levitation are used to apply microgravity. Clinostats are the analog devices which induce partial or reduced gravity similar to the Moon and Mars conditions (Kiss 2014). This device rotates the specimens around one or more axes, and the created microgravity by clinostat rotation (clino-rotation) alters with changing rotational speed (rpm) and sample placement radius (Dietlein et al. 2013; Kiss et al. 2019). Clino-rotated experiments are needed for complementarity and validity of the outcomes acquired in real microgravity (Kraft et al. 2000).
Cell division is a vital biological process that plays a crucial role in the growth and developmental processes and changes plant growth rate in reply to the stress (Cockcroft et al. 2000; Beemster et al. 2002). Recent studies exhibited that microgravity stimulated the changes in the cell cycle phases (Boucheron-Dubuisson et al. 2016), and decoupled cell proliferation and cell growth in the root meristematic cells of Arabidopsis thaliana (Manzano et al. 2013; Matia et al. 2010). Access to in vitro root meristem is restricted, so a homogenous population of cell suspension cultures with proliferating cells can provide a proper system to study cell growth and division (Menges and Murray 2002).
Microgravity and radiation are two major environmental stresses in space and can stimulate DNA injuries, including single or double-strand breaks, chromosome aberrations, and different mutations (Arena et al. 2014; Grimm et al. 2002). Proteomic analysis of A. thaliana seedlings on the international space station has marked the up-regulation of the oxidative stress response, cellular metabolism, cell wall-related, and other stress response proteins (Paul et al. 2011; Aubry‐Hivet et al. 2014; Ferl et al. 2015). Moreover, reduced gravity induced significant changes in amyloplast frequency, mitochondria dimension, number and size of nucleoli, and notable alteration in the regulation of cell cycle (Brykov 2011; Desiderio et al. 2019; Lang et al. 2017). Gravity modification may also influence the active transportation of ions and charges particles through protein carriers, and temporary membrane invaginations (Clément and Slenzka 2006).
Matricaria chamomilla is a medicinal plant belonging to the Asteraceae family, and has various pharmaceutical properties, including anti-microbial, anti-cancer, and antioxidant activities (Sebai et al. 2014; Silva et al. 2012; Patel et al. 2007; Hassanpour and Niknam 2020). Until now, little attention has been given to the association between antioxidative responses and cell division of medicinal plants under clino-rotaion. Many stress conditions make cellular redox imbalances and induce reactive oxygen species (ROS) production. ROS has been proposed to act an essential signaling role in reply to chemical and environmental stresses and affects cell viability, division, differentiation, apoptosis, and other physiological and biochemical responses (Zhao et al. 2007). However, there is still not much data about this subject on medicinal plant cells. So, the present study hypothesizes that clino-rotaion by induction of ROS could promote growth, cell division, and antioxidative capacity in Matricaria chamomilla cells. For all mentioned reasons, this research was managed to assess the effects of clino-rotaion on growth, antioxidant responses, and anatomical alteration in M. chamomilla.
Material and methods
Callus culture and clino-rotation treatment
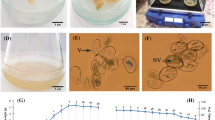
Seeds of M. chamomilla are given by Pakan Bazr Company (Isfahan, Iran). All chemicals are bought from Sigma-Aldrich®, St. Louis, MO, USA, except otherwise have shown. Seeds were disinfected in hypochlorite solution 15% (v/v) for 15 min and then in 70% (v/v) ethanol for 1 min, accompanied by three rinses of sterile distilled water. The disinfected seeds were cultivated on half-strength (½) Murashige and Skoog (MS) basal medium (Murashige and Skoog 1962) including 0.7% (v/w) agar and 0.3% (v/w) sucrose at a day-night temperature (25 °C), relative humidity (46%), and a 16-h photoperiod with white fluorescent light (46 μmol−1 m−2 s−1). For callus induction, 0.4–0.5 cm hypocotyl segments from 7-day-old seedling were put to solid MS medium supplied with 0.5 mg L−1 2, 4-D, and 1.5 mg L−1 KIN for three weeks. Then, the calli (0.6–0.7 g) were transferred to the same MS solid medium. The calli were put in the liquid MS medium with the same hormonal compositions and subcultured every two weeks (Hassanpour and Niknam 2020). After four times of subculture, about 1 mL of cell suspension was spread in the solid MS medium, and the cell lines were grown after three weeks. The cream callus lines with higher growth were subcultured in the center of Petri dish (the rotational radius (r) of 1 cm) and were located on a developed two-dimensional clinostat (designed and constructed by Paya Kesht Company, Iran) with 10 rpm rotational speed (ω), clockwise at 90° for one week (Fig. 1). The centrifugal force (g′) was measured with the equation of g′ = (2π/60)2rω2. The centrifugal acceleration was calculated from zero on the center to 1.12 × 10–3 g on the edge of the callus ring (Dietlein et al. 2013). At each test, three Petri dishes were fixed on the clinostat, and three of them were placed vertically (the same as clinostat samples) on the ground with g = 1 as control and kept near the equipment under similar situations in terms of the 25 ± 2 °C temperature and 57% humidity. In the preliminary experiments, the growth parameters of M. chamomilla calli were investigated after 0, 3 and, 7 days of clino-rotation. The 7-day clino-rotated calli showed significant difference with regards to fresh weight, dry weight and relative growth rate comparing to control. So, the one-week clino-rotaion selected for the next study. After 1 week, the calli were harvested for physiological and biochemical analyses. Five calli per treatment were applied for the measurement of fresh weight. These calli oven-dried (50 °C for 48 h), and the dry weight was calculated.
Microscopic analysis
The callus cells were fixed in ethanol: acetic acid solution (3:1) for 24 h, and then put into a 70% (v/v) ethanol solution and placed at 4 °C. The fixed calli were rinsed in distilled water, and hydrolyzed in HCl (1 N) at 60 °C for 15 min, and colored according to the Feulgen method for 45 min in the darkness. After staining, the cells were rinsed and digested in an enzyme mixture consisting of 1.5% (v/w) cellulase and 20% (v/w) pectinase at 37 °C for 1 h. The cells were then rinsed and examined under a Nikon E200 microscope.
Sugar contents
The contents of reducing sugars, soluble sugars and starch were evaluated in 20 mL of 80% (v/v) ethanol at 95 °C for 1 h from 0.5 g of fresh calli and centrifuged at 10,000g for 10 min. Total soluble sugars were examined by reacting 3 mL of the supernatant and 3 mL of anthrone reagent [anthrone (150 mg) in 100 ml H2SO4 (72%) (v/v)] (EMSURE®, MerckKGaA) and putting in boiling water bath (10 min). The absorbance was registered at 625 nm (Yemm and Willis 1956), and total sugar was calculated based on the method of Irigoyen et al. (1992). The starch content was quantified based on the method of Nomura et al. (1969). Reducing sugars were evaluated based on the method as defined by Parida et al. (2002). Absorbance was registered at 510 nm.
Hydrogen peroxide and lipid peroxidation contents
Hydrogen peroxide (H2O2) level in 1 g fresh calli was analyzed through the method by Velikova et al. (2000), and absorbance was calculated at 390 nm by a standard curve. Malondialdehyde content (MDA) as a result of lipid peroxidation was calculated spectrophotometrically at 532 and 600 nm through the method by Heath and Packer 1968.
Total protein content and antioxidant enzymes activity
Fresh calli (0.5 g) was extracted using 2.5 mL extraction buffer of Tris–HCl (1 M, pH 6.8) (EMSURE®, MerckKGaA), the extract was used for protein and enzymes assays. Fresh calli (0.5 g) was extracted using extraction buffer (Tris–HCl (1 M), pH 6.8), and the extract was applied for protein and enzymes assays. Total protein content was calculated via Bradford (1976) method. Superoxide dismutase (SOD) activity was calculated by observing the inhibition of photochemical modification of nitroblue tetrazolium in a reaction compound, as specified by Giannopolitis and Ries (1977). POX and CAT activities were quantified via Abeles and Biles (1991) and Aebi (1984) methods, respectively. POX and CAT activities were estimated according to the spectrophotometric method according to Abeles and Biles (1991) and Aebi (1984) methods, respectively. Electrophoresis patterns of SOD and peroxidase (POX) were managed based on Laemmli (1970). SOD isoforms were monitored on gels based on the Beauchamp and Fridovich (1971) method. Three SOD isoforms, Fe-SOD, Cu/Zn-SOD, and Mn-SOD were recognized using inhibitors. Mn-SOD was visualized by its insensitivity to H2O2 (5 mM) and KCN (2 mM), while Cu/Zn-SOD reacted to KCN (2 mM). Fe-SOD was also inhibited by H2O2 (5 mM) according to Munns et al. (2003).
Statistical analysis
All experiments were performed with three or four times with three samples each to prove the reproducibility of the data and decrease errors. Statistical examination was performed by Student’s T test by the SPSS (version 18), and the data were showed as with as the mean ± the standard error (SE). Differences were assumed significant at a p value < 0.05.
Results
In the current study, some growth and biochemical parameters were examined to gain a better understanding of clino-rotation effect in M. chamomilla calli. The effects of clino-rotation on callus fresh and dry weights were presented in Fig. 2, and showed a significant increase compared to control. Clino-rotation resulted in a 112.86 and 64.55% increase of fresh and dry weights as compared to their controls, respectively. Calli staining with Schiff reagent showed two nucleoli in control and clino-rotated cells. However, the rate of cell division and amyloplast content were markedly increased in clino-rotated calli (Fig. 3). Amyloplasts distributed throughout the cytoplasm in clino-rotated cells.
Nuclear shape and cell division of M. chamomilla calli under control (a) and 2D-clinorotation. Control cells (a), clinostat treated cells (b), cell division calculated with nuclei. Bars are equaled to 60 µm in a and b. Data are mean ± SE, n = 4. *Indicate significant differences at p ≤ 0.05, according to Student’s t test
Statistical analysis of soluble and reducing sugars in clino-rotated and control groups showed a significant increase of the soluble and reducing sugars in the calli grown under clino-rotation. Starch concentration in the clino-rotated samples was approximately 2 times over the control (Table 1).
To estimate oxidative damage, the H2O2 and MDA contents were measured in calli. clino-rotation induced significantly H2O2 content (40.45%) comparing to the control group, although MDA content showed a 21.69% decrease in clinostat-treated calli (Fig. 4).
Soluble protein content unchanged approximately under clino-rotation, but the activity of antioxidant enzymes increased significantly comparing to control (Table 1 and Fig. 5). The activity of SOD (51.02%) and POX (72.38%) increased in clinostat-exposed calli comparing to the control (Fig. 5a, b). The more increment of antioxidant enzyme activity was observed in CAT activity and showed a 3.54 folds increase in clino-rotation condition over the control (Fig. 5c).
Various isoforms of antioxidant enzymes (SOD and POX) in treated calli and control groups have been identified by native polyacrylamide gel electrophoresis (PAGE). After gel putting of gels in KCN (3 mM) to inactivate Cu/Zn-SOD and H2O2 (5 mM) for inhibition of Fe-SOD and Cu/Zn-SOD, different SOD isoforms were determined. As exhibited in Fig. 5d, three isoforms of SOD: one Mn-SOD isoform, and two Cu/Zn-SOD isoforms were determined in 10% PAGE gel. Microgravity treatment caused a significant increase in bands intensity of Cu/Zn-SOD isoform (Fig. 5d). The results are in agreement with the results of the spectrophotometric analysis of SOD activity in control and clino-rotated calli. PAGE analysis of POX activity exhibited two isoforms (POX1 and POX2) in the treated calli and control group (Fig. 5e). The increase in POX activity in treated calli seems to be largely due to the rise of band intensity of both isoforms.
Discussion
This study was carried out to sufficiently understand the clino-rotation impacts on some antioxidative enzyme activity and growth parameters of M. chamomilla calli. This finding shows the defense mechanisms of M. chamomilla calli after exposed to clino-rotation for the first time. Clino-rotation induced the fresh and dry weights of M. chamomilla calli. Cell division also raised markedly under clino-rotation (Figs. 2, 3). Growth alterations are the most obvious replies of a plant under stress situations, and microgravity can have extensive impacts on plant cell growth and development (Chen et al. 2015; Dauzart et al. 2016). It is well understood that cell processes, including cell division, growth, and other parameters are modified under clino-rotation (Matía et al. 2010). Plant cell growth is associated with the proper coordination of many processes at the cellular level, such as cell proliferation, activation of ROS scavenger enzymes, stimulation of transcription factors, expression of genes, and etc. under stress situations (Matía et al. 2009; Shabrangi et al. 2015; Foyer and Shigeoka 2011; Hassanpour et al. 2017) (Fig. 6). Some studies demonstrated positive effects of clino-rotation and real space flight on plant cell proliferation, which in turn affects growth and development (Herranz and Medina 2014). Boucheron-Dubuisson et al. (2016) showed growth alterations and reduction of ribosome biogenesis under clino-rotation in root meristem cells of Arabidopsis. Moreover, the previous examination exhibited that parameters can be related to the rate of ribosome biogenesis, namely nucleolar structure, nucleolar size, and nucleolin levels in clino-rotated A. thaliana (Matía et al. 2009). However, in M. chamomilla cells, clinostat-treated cells showed a higher potential of cell division, which resulted in increasing of growth rate and biomass. This may show the importance of gravity force on the cell cycle by the gravitational signal perception and its transport to nuclei.
Schematic representation of clino-rotation effects on defensive system and growth induction. Clino-rotation causes osmotic and oxidative stresses in plants. Clino-rotation promotes H2O2 generation through NAD(P)H oxidase. Mitogen-activated protein kinases (MAPKs) may also stimulate, and following transcription factors activate the related genes with antioxidative enzyme (Sugimoto et al. 2014). On the other hand, sugars accumulate and prepare carbon and energy source for growth and cell division under microgravity. Induction of antioxidative enzymes activities and sugar accumulation inhibit oxidative stress and help to induce cell division and growth
The number of amyloplast and accumulation of total reducing sugars, soluble sugars, and starch increased significantly in clinostat-treated M. Chamomilla calli comparing to control (Table 1). Accumulation of different carbohydrate constitutes has been previously reported under microgravity conditions (Mortley et al. 2008; Stutte et al. 2006). Carbohydrates play a significant role in osmoprotectant, carbon and energy storages, production of compatible solutes, and radical scavenging in plant cells that support plant growth under stress (Parida and Das 2005). Cells utilize energy to keep status homeostasis versus gravity due to sending torque to microgravity-treated cells from the earth gravity (Nace 1983). In the present study, increased soluble sugars showed that the M. chamomilla cells saved more carbon and energy under clino-rotation, as a precursor of Krebs cycle for more production of ATP. On the other hand, the accumulation of total sugars can be a protection mechanism for the prevention of oxidative damage and improve membrane stabilization (Hernandez-Marin and Martínez 2012; Hosseini et al. 2015) (Fig. 6). Starch is a key molecule in mediating plant responses to stress conditions (Wang and Messing 2012). The previous studies revealed that plant growth is affected by the presence of an energy-saving system similar to starch. Moreover, starch may have a protective mechanism and serves as an alternative source of carbon and energy following stress situations (Mishra and Prakash 2010). There are various results of starch content in plants grown under space condition and clinorotation. For example, Mortley et al. (2008) reported starch accumulation increased in sweet potato stem during spaceflight. Increased starch content has also reported in A. thaliana calli under clinorotation (10 rpm) (Wang et al. 2006). However, Nakajima et al. (2019) marked slow clinorotation (2 rpm) induced the activity of a-amylase and following the starch content decreased in Mung Bean Seedlings. Lower starch and higher soluble sugar concentrations were also reported in pepper leaves in plants grown on board Biosatellite II (Johnson and Tibbitts 1968). The various results may be associated with the type of plant tissue, explant used for callus induction, cell line, age, genetic, duration and speed of clino-rotation and/or environmental conditions of plant growth hardware.
Stress conditions can cause unsuitable effects on the growth and cell membrane by the ROS generation (Choudhary et al. 2007; Yadav 2010; Merati et al. 2016). These products cause adverse modifications to cell components (lipids, proteins, and DNA) and result in lipid peroxidation as a reliable sign of oxidative stress (Halliwell 1989). In this work, the H2O2 level increased significantly in M. chamomilla calli under clino-rotation. An increase in H2O2 level has been previously observed under stress conditions (Chao et al. 2010; Merati et al. 2016). Moreover, the high production of H2O2 in the clino-rotated calli may be shown the level of ROS as a promoter of cell signaling pathways or the inhibition of the H2O2-scavenging enzyme activities (Chao et al. 2010; Hayat et al. 2010). Space flight could stimulate mitogen-activated protein kinases (MAPKs) activation and the expression of NADPH oxidase genes (Sugimoto et al. 2014), which in turn might activate transcription factors for induction of stress-related genes (Fig. 6). On the other hand, MDA content declined in clino-rotated calli against to control (Fig. 4). It has been previously reported that ROS accumulation could regulate the activity of antioxidant enzymes, and following the growth of M. chamomilla cell suspension culture increased under stress conditions (Hassanpour and Niknam 2020). Reduction of MDA content, along with an increase of fresh and dry weights, suggests that clino-rotation stimulates ROS accumulation for induction of cell signaling and growth.
A trivial reduction in protein content was recognized in clinostat-treated calli (Table 1). A reduction of protein content has been previously observed in Pisum sativum L. (Kozeko and Kordyum 2006) and A. thaliana affected by microgravity (Link et al. 2014). Diversely, Wang et al. (2006) indicated that the proteome modifications induced by clinostat rotation. Decreased protein content in clino-rotated calli might be attributed to a decrease in the protein synthesis, the deficiency of amino acids or denaturation of enzymes required in protein synthesis (Maas et al. 1979; Hall and Flowers 1973).
Antioxidative enzymes, including SOD, POX, and CAT are the main enzymatic antioxidants associated with plant resistance and play pivotal roles in decreasing the H2O2 and MDA contents and maintaining cell membrane integrity under oxidative stresses (Chen et al. 2015). Application of clino-rotation induced significantly antioxidative enzyme activities against to the control group. CAT enzyme showed the higher activity than that of the other enzymes (Fig. 5c), indicating that CAT was more sensitive to gravity changes and plays a vital role in the scavenging of H2O2 free radicals. Induction of SOD and POX activities has been previously reported in Stevia (Choi et al. (2011) and tomato (Chen et al. 2015) under clino-rotation. SOD is an initial line of protection against ROS-induced injuries, and it causes the dismutation of superoxide into H2O2 that must be convert to O2 and H2O by other antioxidative enzymes like APX, CAT, and POX (Ozkur et al. 2009). These results suggest that enhanced antioxidative enzymes might provide better protection to the cells against oxidative stress triggered by clino-rotation (Fig. 6).
PAGE analysis showed the quantitative differences in clino-rotated calli and control (Fig. 5), and revealed that all the antioxidative enzymatic bands in treated calli were stronger than the control. These data indicate all isoforms are involved in more efficiently against clinorotation-induced oxidative damage. Moreover, intensities of the SOD and POX isoforms established the spectrophotometric results. As Gaspar et al. (1982) reported, the POX is in various isoforms which are developmentally controlled and respond to exogenous stimulations. Clino-rotation increased band intensity of SOD isoforms especially, Cu/Zn–SOD isoform, which was more prominent than that of the Mn-SOD isoform (Fig. 5d). Cu/Zn–SOD isoforms are commonly located in chloroplast or cytosol, and Mn-SOD in mitochondria (Amor et al. 2005). Our results showed that the chloroplast or cytosolic isoforms of SOD (Cu/Zn–SOD) could be the main part of total SOD activity, and protected calli under clino-rotation.
Conclusion
This study prepares a new perception of M. chamomilla calli responses to clino-rotation through the analysis of enzymatic antioxidative pathways. The increase in antioxidant enzyme activity, accumulation of sugars, and growth parameters were observed under clino-rotation condition. The rise of cell growth, cell division proves that clino-rotation is not a limiting factor for the plant cell growth and development. CAT activity changes more obviously than POX and SOD, which means that CAT is more sensitive to clino-rotation than POX and SOD. A lower MDA content along with the increment of dry and fresh weights in clinostat treated-calli shows that calli are equipped with a free radical scavenging system. Though, the complicated mechanism in which antioxidant enzymes act a critical role following clino-rotation conditions stays obscure and needs to be examined further.
References
Abeles FB, Biles CL (1991) Characterization of peroxidases in lignifying peach fruit endocarp. Plant Physiol 95:269–273
Aebi H (1984) Catalase in vitro. Method enzymol. Elsevier, pp 121–126
Amor NB, Hamed KB, Debez A, Grignon C, Abdelly C (2005) Physiological and antioxidant responses of the perennial halophyte Crithmum maritimum to salinity. Plant Sci 168:889–899
Arena C, De Micco V, Macaeva E, Quintens R (2014) Space radiation effects on plant and mammalian cells. Acta Astronaut 104:419–431
Aubry-Hivet D, Nziengui H, Rapp K, Oliveira O, Paponov IA, Li Y, Hauslage J, Vagt N, Braun M, Ditengou FA, Dovzhenko A, Palme K (2014) Analysis of gene expression during parabolic flights reveals distinct early gravity responses in Arabidopsis roots. Plant Biol 16:129–141
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Beemster GT, De Vusser K, De Tavernier E, De Bock K, Inzé D (2002) Variation in growth rate between Arabidopsis ecotypes is correlated with cell division and A-type cyclin-dependent kinase activity. Plant Physiol 129:854–864
Boucheron-Dubuisson E, Manzano AI, Le Disquet I, Matía I, Sáez-Vasquez J, Van Loon JJ, Herranz R, Carnero-Diaz E, Medina FJ (2016) Functional alterations of root meristematic cells of Arabidopsis thaliana induced by a simulated microgravity environment. J Plant Physiol 207:30–41
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brykov V (2011) Clinorotation affects the ultrastructure of pea root mitochondria. Microgravity Sci Tec 23:215–219
Chao YY, Chen CY, Huang WD, Kao CH (2010) Salicylic acid-mediated hydrogen peroxide accumulation and protection against Cd toxicity in rice leaves. Plant Soil 329:327–337
Chen Y, Lu J, Li H, Sun Q, Zhao Y, Su L, Liu M (2015) Effects of spaceflight and simulated microgravity on cell sub-microstructure and antioxidant enzyme activity in tomato. Sci Chain Technol Sci 58:338–345
Choi YS, Jung MY, Soh WY, Han KS, Yeo UD (2011) Changes of antioxidant enzymes in Stevia Plants under clinorotation, shaking, and low temperature stresses. Korean J Plant Res 24:343–350
Choudhary M, Jetley UK, Khan MA, Zutshi S, Fatma T (2007) Effect of heavy metal stress on proline, malondialdehyde, and superoxide dismutase activity in the cyanobacterium Spirulinaplatensis-S5. Ecotoxicol Environ Saf 66:204–209
Clement G, Slenzka K (2006) Fundamentals of space biology: research on cells, animals, and plants in space. Springer Sci Business Media, p 18
Cockcroft CE, den Boer BG, Healy JS, Murray JA (2000) Cyclin D control of growth rate in plants. Nature 405:575–579
Dauzart AJ, Vandenbrink JP, Kiss JZ (2016) The effects of clinorotation on the host plant, Medicagotruncatula, and its microbial symbionts. Front Astron Space Sci 3:3
Desiderio A, Salzano AM, Scaloni A, Massa S, Pimpinella M, De Coste V, Pioli C, Nardi L, Benvenuto E, Villani ME (2019) Effects of simulated space radiations on the tomato root proteome. Front Plant Sci 10:1334
Dietlein I, Doi T, Haubold H, Hauslage J, Hemmersbach H, Hoson T, Sarah Lammens S, Li Y, Long M, Van Loon JJWA, Niu A, Takahashi H, Ochiai M, Osman A, Steffens H (2013) Teacher’s guide to plant experiments in microgravity. Human Space Technology Initiative, United Nations, New York
Ferl RJ, Koh J, Denison F, Paul AL (2015) Spaceflight induces specific alterations in the proteomes of Arabidopsis. Astrobiology 15(1):32–56
Foyer CH, Shigeoka S (2011) Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol 155(1):93–100
Gaspar T, Penel C, Thorpe T, Greppin H (1982) A survey of their biochemical and physiological roles in higher plants. Peroxidases. Univ. of Geneva, Geneva, pp 1970–1980
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol 59:309–314
Grimm D, Bauer J, Kossmehl P, Shakibaei M, Schöberger J, Pickenhahn H, Schulze-Tanzil G, Vetter R, Eilles C, Paul M, Cogoli A (2002) Simulated microgravity alters differentiation and increases apoptosis in human follicular thyroid carcinoma cells. FASEBJ 16:604–606
Hall J, Flowers T (1973) The effect of salt on protein synthesis in the halophyte Suaeda maritima. Planta 110:361–368
Halliwell B (1989) Free radicals, reactive oxygen species and human disease: a critical evaluation with special reference to atherosclerosis. Br J Exp Pathol 70:737
Hassanpour H, Niknam V (2020) Establishment and assessment of cell suspension cultures of Matricaria chamomilla as a possible source of apigenin under static magnetic field. Plant Cell Tiss Organ Cult. https://doi.org/10.1007/s11240-020-01885-4
Hassanpour H, Niknam V, Haddadi BH (2017) High-frequency vibration improve callus growth via antioxidant enzymes induction in Hyoscyamus kurdicus. Plant Cell Tissue Organ Cult 128(1):1–11. https://doi.org/10.1007/s11240-016-1103-5
Hayat Q, Hayat S, Irfan M, Ahmad A (2010) Effect of exogenous salicylic acid under changing environment: a review. Environ Exp Bot 68:14–25
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hernandez-Marin E, Martínez A (2012) Carbohydrates and their free radical scavenging capability: a theoretical study. J Phys Chem B 116:9668–9675
Herranz R, Medina FJ (2014) Cell proliferation and plant development under novel altered gravity environments. Plant Biol 16:23–30
Hosseini SM, Hasanloo T, Mohammadi S (2015) Physiological characteristics, antioxidant enzyme activities, and gene expression in 2 spring canola (Brassica napus L.) cultivars under drought stress conditions. Turk J Agric For 39:413–420
Irigoyen J, Einerich D, Sánchez-Díaz M (1992) Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativd) plants. Physiol Plant 84:55–60
Johnson SP, Tibbitts TW (1968) The liminal angle of a plagiogeotropic organ under weightlessness. Bioscience 18:655–661
Kamal KY, Herranz R, Van Loon JJ, Medina FJ (2018) Simulated microgravity, Mars gravity, and 2 g hypergravity affect cell cycle regulation, ribosome biogenesis, and epigenetics in Arabidopsis cell cultures. Sci Rep 8:1–16
Kiss JZ (2014) Plant biology in reduced gravity on the Moon and Mars. Plant Biol 16:12–17. https://doi.org/10.1111/plb.12031
Kiss JZ, Wolverton C, Wyatt SE, Hasenstein KH, Van Loon JJWA (2019) Comparison of microgravity analogs to spaceflight in studies of plant growth and development. Front Plant Sci 10:1577
Kozeko L, Kordyum E (2006) The stress protein level under clinorotation in context of the seedling developmental program and the stress response. Microgravity Sci Technol 18:254
Kraft TF, Van Loon JJ, Kiss JZ (2000) Plastid position in Arabidopsis columella cells is similar in microgravity and on a random-positioning machine. Planta 211(3):415–422
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lang T, Van Loon JJWA, Bloomfield S, Vico L, Chopard A, Rittweger J, Kyparos A, Blottner D, Vuori I, Gerzer R, Cavanagh PR (2017) Towards human exploration of space: the theseus review series on muscle and bone research priorities. npj Microgravity 3(1):1–8
Link BM, Busse JS, Stankovic B (2014) Seed-to-seed-to-seed growth and development of Arabidopsis in microgravity. Astrobiology 14:866–875
Maas EV, Ogata G, Finkel MH (1979) Salt-induced inhibition of phosphate transport and release of membrane proteins from barley roots. Plant Physiol 64:139–143
Manzano AI, Larkin OJ, Dijkstra CE, Anthony P, Davey MR, Eaves L, Hill RJ, Herranz R, Medina FJ (2013) Meristematic cell proliferation and ribosome biogenesis are decoupled in diamagnetically levitated Arabidopsis seedlings. BMC Plant Biol 13(1):124
Matia I, van Loon JW, Carnero-Díaz E, Marco R, Medina FJ (2009) Seed germination and seedling growth under simulated microgravity causes alterations in plant cell proliferation and ribosome biogenesis. Microgravity Sci Tec 21:169
Matía I, González-Camacho F, Herranz R, Kiss JZ, Gasset G, van Loon JJ, Marco R, Medina FJ (2010) Plant cell proliferation and growth are altered by microgravity conditions in spaceflight. J Plant Physiol 167:184–193
Menges M, Murray JA (2002) Synchronous Arabidopsis suspension cultures for analysis of cell cycle gene activity. Plant J 30(2):203–212
Merati MJ, Niknam V, Hassanpour H, Mirmasoumi M (2016) Comparative effects of salt stress on growth and antioxidative responses in different organs of pennyroyal (Mentha pulegium L.). J Plant Res 28(5):1097–1107
Mishra P, Prakash V (2010) Response of non-enzymatic antioxidants to zinc Induced stress at different pH in Glycine max L. cv. Merrill Acad J Plant Sci 3:1–10
Mortley DG, Bonsi CK, Hill WA, Morris CE, Williams CS, Davis CF, Williams JW, Levine LH, Petersen BV, Wheeler RM (2008) Influence of microgravity environment on root growth, soluble sugars, and starch concentration of sweetpotato stem cuttings. J Am Soc Hortic Sci 133:327–332
Munns R, Rebetzke GJ, Husain S, James RA, Hare RA (2003) Genetic control of sodium exclusion in durum wheat. Aust J Agric Res 54:627–635
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nace GW (1983) Gravity and positional homeostasis of the cell. Adv Space Res 3(9):159–168
Nakajima S, Ogawa Y, Suzuki T, Kondo N (2019) Enhanced antioxidant activity in Mung Bean seedlings grown under slow clinorotation. Microgravity Sci Technol 31:395–401
Nomura T, Kono Y, Akazawa T (1969) Enzymic mechanism of starch breakdown in germinating rice seeds II. Scutellum as the site of sucrose synthesis. Plant Physiol 44(5):765–769
Ozkur O, Ozdemir F, Bor M, Turkan I (2009) Physiochemical and antioxidant responses of the perennial xerophyte Capparis ovata Desf. to drought. Environ Exp Bot 66:487–492
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf 60:324–349
Parida A, Das AB, Das P (2002) NaCl stress causes changes in photosynthetic pigments, proteins, and other metabolic components in the leaves of a true mangrove, Bruguiera parviflora, in hydroponic cultures. J Plant Biol 45:28–36
Patel D, Shukla S, Gupta S (2007) Apigenin and cancer chemoprevention: progress, potential and promise. Int J Oncol 30:233–245
Paul AL, Manak MS, Mayfield JD, Reyes MF, Gurley WB, Ferl RJ (2011) Parabolic flight induces changes in gene expression patterns in Arabidopsis thaliana. Astrobiology 11:743–758
Sebai H, Jabri M-A, Souli A, Rtibi K, Selmi S, Tebourbi O, El-Benna J, Sakly M (2014) Antidiarrheal and antioxidant activities of chamomile (Matricaria recutita L.) decoction extract in rats. J Ethnophar 152:327–332
Shabrangi A, Hassanpour H, Majd A, Sheidai M (2015) Induction of genetic variation by electromagnetic fields in Zea mays L. and Brassica napus L. Caryologia 68(4):1–8. https://doi.org/10.1080/00087114.2015.1109920
Silva N, Barbosa L, Seito L, Junior AF (2012) Antimicrobial activity and phytochemical analysis of crude extracts and essential oils from medicinal plants. Nat Prod Res 26:1510–1514. https://doi.org/10.1080/14786419.2011.564582
Soleimani M, Ghanati F, Hajebrahimi Z (2019) The role of phenolic compounds in growth improvement of cultured tobacco cells after exposure to 2-D clinorotation. Plant Physiol 9:2921–2929
Stutte G, Monje O, Hatfield R, Paul AL, Ferl R, Simone C (2006) Microgravity effects on leaf morphology, cell structure, carbon metabolism and mRNA expression of dwarf wheat. Planta 224:1038–1049
Sugimoto M, Youko Oono Y, Matsumoto GO, T, Yazawa T, Margarita A, Levinskikh MA, Sychev VN, Bingham GE, Wheeler R and Hummerick M, (2014) Genome-wide expression analysis of reactive oxygen species gene network in Mizuna plants grown in long-term spaceflight. BMC Plant Biol 14:4–15
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66
Wang W, Messing J (2012) Analysis of ADP-glucose pyrophosphorylase expression during turion formation induced by abscisic acid in Spirodela polyrhiza (greater duckweed). BMC Plant Biol 12:5
Wang H, Zheng HQ, Sha W, Zeng R, Xia QC (2006) A proteomic approach to analysing responses of Arabidopsis thaliana callus cells to clinostat rotation. J Exp Bot 57:827–835
Yadav SK (2010) Cold stress tolerance mechanisms in plants. A review. Agron Sustain Dev 30:515–527
Yemm E, Willis A (1956) The respiration of barley plants IX. The metabolism of roots during the assimilation of nitrogen. New Phytol 55:229–252
Zhao MG, Zhao X, Wu YX, Zhang LX (2007) Enhanced sensitivity to oxidative stress in Arabidopsis nitric oxide synthesis mutant. J Plant Physiol 164:737–745
Acknowledgements
The financial support of this study was provided by Aerospace Research Institute, Ministry of Science Research and Technology of Iran. The authors thank Ali Darvishi to build the clinostat device.
Author information
Authors and Affiliations
Contributions
HH designed, performed the experiments and supervised the whole work. MG analyzed the data. Both authors help to write and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Christophe Hano.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hassanpour, H., Ghanbarzadeh, M. Induction of cell division and antioxidative enzyme activity of Matricaria chamomilla L. cell line under clino-rotation. Plant Cell Tiss Organ Cult 146, 215–224 (2021). https://doi.org/10.1007/s11240-021-02060-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-021-02060-z