Abstract
Initial growth studies in space have demonstrated microgravity has the potential to enhance antioxidant activity in fruit and vegetables. To examine whether a slow-rotating clinostat here on Earth can produce functional foods with enhanced antioxidant activity and related compounds, including carotenoids, phenolics and ascorbic acid, we grew mung bean seedlings under clinorotation (2 rpm) and measured the above components. The clinostat seedlings had higher antioxidant activity and accumulation of related compounds, compared to seedlings grown under normal gravity. To further explore the causes for these results, α-amylase activity and amount of starch in cotyledons were measured. Higher α-amylase activity and lower starch levels were observed in the cotyledons under clinorotation, suggesting the higher levels of sugars resulting from starch conversion were promoting the synthesis of antioxidant compounds and higher antioxidant activity in seedlings when grown under clinorotation. Our data indicate that a clinostat can enhance antioxidant activity in vegetables in the early developmental stage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many fruit and vegetables contain antioxidant compounds, such as vitamins, flavonoids, phenolics, alkaloids and carotenoids (Larson 1988). Given that these antioxidant compounds can reduce the risk of various diseases with less side effects than conventional drugs in a number of cases, fruit and vegetables enriched with these compounds are being viewed as potential health enhancing functional foods. However, early studies have shown that the activity and accumulation of antioxidant compounds in food can vary with growth conditions, such as temperature, light and cultural systems (Luthria et al. 2006; Paśko et al. 2009; Wang and Zheng 2001; Wang et al. 2002). Thus, if such functional foods are to be produced consistently and effectively, an understanding of the influences of growth conditions on antioxidant activity is important.
A unique growing condition that has been found recently to effect antioxidant activity is gravity. Plants have evolved under a constant gravitational environment here on the Earth, and altered gravitational conditions, such as microgravity, can induce changes in plant growth and development at morphological, molecular and cellular levels (Aarrouf et al. 1999a; Zheng et al. 2015). Previous space experiments have indicated that real microgravity enhances the accumulation of antioxidant compounds. For example, Musgrave et al. (2005) reported an increase in glucosinolate in Brassica rapa L., and De Micco and Aronne (2008) found an increase in phenolics in soybean. Although these results suggest that space environment can enhance antioxidant activity in foods, access to space is limited. Therefore, we hypothesize that cultivation of foods under simulated microgravity produced by ground-based simulation will also induce accumulation of antioxidant compounds, resulting in the production of enriched antioxidant foods.
A one axis clinostat (CL) can produce simulated microgravity here on the Earth by rotating plants, and has been used as a simulation device for space experiments. Although the influences of clinorotation are not always consistent with those of real microgravity, CL can be used to cultivate plants for a longer time than free-fall or parabolic flight, so this device is a useful tool to test our hypothesis. In CL experiments, researchers have employed two types of systems; a traditional CL that rotates the plants slowly at 1–10 rpm; or a fast-rotating CL running at 50–100 rpm (Brungs et al. 2016; Herranz et al. 2013). Recent studies have revealed that fast clinorotation can provide better weightlessness condition than traditional slow clinorotation (Horn et al. 2011; Krause et al. 2018). However, in this fast-rotating system, plants larger than a few millimeters or not placed very close to the rotation axis will be exposed to centrifugal forces (Krause et al. 2018). To cultivate larger plant, a slow clinorotation was used in this study.
To examine whether slow clinorotation has the potential to produce enriched functional foods, we measured antioxidant activity and accumulation of related compounds in mung bean seedlings grown under clinorotation. Additionally, starch accumulation, a predominant form of carbohydrate reserve in plant seeds, was examined as it begins to be hydrolyzed to sugars by α-amylase after germination (Swain and Dekker 1966). The converted sugars, such as glucose and sucrose, are a significant source of energy for plant growth and metabolite synthesis in germinating seedlings. The previous space experiment described above suggest that starch is also involved in the accumulation of antioxidant compounds: increases in phenolics in soybean grown in space were accompanied by starch decreases (De Micco and Aronne 2008). Since the sugars converted from starch are subsequently used for plant growth and not stored all in plant tissues, sugar levels are not useful indicators to investigate interactions between starch degradation and antioxidant activity in germinating seedlings. Thus, starch and α-amylase activity levels also were measured, in this study. In the Results and discussion, we explore the causes for changes in antioxidant activity under clinorotation.
Materials and Methods
Chemicals
We purchased 1,1-diphenyl-2-picrylhydrazyl (DPPH), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) and ethylenediaminetetraacetic acid (EDTA) from Tokyo Chemical Industry Co., Ltd. We also purchased 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), sodium acetate, sodium carbonate (Na2CO3), gallic acid monohydrate, ammonium molybdate, metaphosphoric acid, Milli Q water, and HPLC grade acetonitrile from Wako Pure Chemical Industries, Ltd. Agar powder and starch were obtained from Kanto Chemical Co., Inc., and α-amylase from porcine pancreas (≥10 units/mg), amyloglucosidase from Aspergillus niger (≥70 units/mg), Folin-Ciocalteu reagent and potassium persulfate were obtained from Sigma-Aldrich.
Plant Material and Growth Conditions
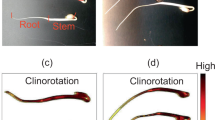
We cultivated seedlings on a horizontal CL as described in our previous study (Nakajima et al. 2017). The CL consisted of an AC servo motor (SGMAH-A5BAA21, Yasukawa Electric Co., Ltd.) and a cylindrical acrylic housing (8.4 cm diameter × 9.0 cm height) that was placed in an incubator (45 cm × 43 cm × 45 cm, 87,075 cm3). Prior to experimentation, we prepared 0.8% (w/w) agar medium (pH 5.8–6.1 adjusted with KOH, 2.9 cm depth) in this housing by melting agar powder in water during autoclaving. Twenty mung bean seeds were sterilized in 70% ethanol for 5 min and then 95% ethanol for 1 min, followed by two 1 min rinses in distilled water. Cultivation of seeds started immediately after washing, and the housings were covered with a transparent plastic film, which allows gas exchange, in order to avoid water loss. Control seedlings were cultivated under a stationary condition (Fig. 1a). In the CL experiments (Fig. 1b), the rotor axis and the seedlings axes were horizontal (i.e. perpendicular to the g-vector) and rotated at 2 rpm. At this rotation speed, a maximal centrifugal acceleration of 1.9 × 10−4 g was achieved. Seedlings in both conditions were grown at 26.0 ± 1.5 °C for 4 days and illuminated by a white LED (LDL-74 × 27SW2, CCS Inc.) under 18 h light/6 h dark, providing a light intensity of 40–60 μmol m−2 s−1 at the surface of the agar medium measured by a quantum sensor (MQ-200, Fujiwara Scientific Company Co., Ltd.). The light illuminated the control seedlings from above, and horizontally for the CL seedlings. Seedlings in both conditions were collected after 4 days cultivation from when the LED was turned on, and frozen in liquid nitrogen immediately after 4 days of cultivation and freeze-dried, except for the α-amylase assay. Lyophilized seedlings were ground to powder with a mortar and pestle, and kept at −80 °C until use. To obtain fundamental data, the length of shoot and root was also measured. Shoot sections was determined between the cotyledon/hypocotyl interface and the hypocotyl/root interface. Root sections were determined between the hypocotyl/root interface and the root tip.
Amylase Assay
Fresh cotyledons were homogenized in 50 mM K-phosphate buffer (pH 6.8) immediately after cultivation. After being centrifuged at 10,000 g for 10 min at 4 °C, the supernatant was used for enzyme assay. Amylase activity was measured according to a method Tripathi et al. (2007). A solution, containing 0.5 mL of 1% starch solution, 0.3 mL of 0.1 M sodium acetate buffer (pH 5.5) and 0.1 mL of Milli Q water, was incubated at 55 °C for 10 min for equilibration. The reaction was started by the addition of 0.1 mL of plant extracts, and then the mixture incubated for 5 min. The reaction was stopped by the addition of 0.5 mL of 1 M HCl and cooled at room temperature. A 0.2 mL aliquot of this mixture was diluted with distilled water to 10 mL, including 0.1 mL of 1 M HCl and 0.1 mL of 0.2% iodine solution. A blank was prepared by adding the enzyme solution after the reaction was stopped by the addition of HCl. The absorbance of the solution was measured at 610 nm.
Starch Quantification
Starch was converted to glucose as described by Smith and Zeeman (2006), and then quantified as glucose. Dry cotyledons were extracted in 80% ethanol, and incubated in a boiling bath for 5 min. After being centrifuged at 3000 g for 5 min, the supernatant was discarded and the residual extracted three times more in the same way. The insoluble residual was dried to remove ethanol, homogenized in 2.0 mL water, and then autoclaved at 121 °C for 30 min. After cooling, the sample was incubated at 37 °C overnight with 2.0 mL of 200 mM sodium acetate (pH 5.5) containing α-amylase (≥1 unit) and amyloglucosidase (≥10 units). After the reaction, the solution was filtered through a 0.45 μm membrane filter.
Glucose quantification was performed using a HPLC (Shimadzu Corp.) equipped with Luna NH2 column (4.6 mm × 250 mm) and a refractive index detector. Column temperature was maintained at 30 °C. The mobile phase was acetonitrile-water (70:30, v/v) at a flow rate of 1.0 mL/min. After analysis, the amount of starch was calculated as 90% of glucose. The results were expressed as the amount of starch in 2 cotyledons from a seedling.
Antioxidant Activity Assay
Antioxidant activity in seedlings under both growth conditions was determined by DPPH and ABTS radical scavenging assays. Dry seedlings (100 mg) were extracted in 2.5 mL of 80% acetone for 2 days at 4 °C, and filtered through a 0.45 μm membrane filter before analysis. The DPPH assay was performed according to the method (Xu and Chang 2007) with some modifications. The plant extracts (100 μL) were mixed with 3.9 mL of 100 μM DPPH solution. The mixture was shaken vigorously for 1 min and incubated for 30 min at room temperature in darkness. Absorbance at 517 nm was measured using a spectrometer (V-670, JASCO Corp.), and DPPH scavenging effect was calculated using following equation.
where, Asample: absorbance of reaction mixture with plant extracts; Acontrol: absorbance of reaction mixture without samples.
The ABTS assay was performed according to the method (Bai et al. 2016) with some modifications. The ABTS radical cation was prepared by mixing 7 mM ABTS and 2.45 mM potassium persulfate (final concentration). The mixture was incubated in darkness at room temperature for 12 h. The radical produced in this way is stable in this form for more than 2 days under the same conditions. The ABTS radical cation solution was then diluted with water to obtain an absorbance of 0.70 ± 0.02 at 734 nm. After adding 100 μL of plant extracts to 3.5 mL of ABTS, the mixture was shaken vigorously and incubated for 6 min at room temperature in darkness. The absorbance of the solution at 734 nm was measured, and ABTS scavenging effect was calculated using the following equation.
where, Asample: absorbance of reaction mixture with plans extracts; Acontrol: absorbance of reaction mixture without samples.
The results of DPPH and ABTS scavenging effect were expressed as Trolox equivalents.
Carotenoids, Phenolics and Ascorbic Acid Quantification
The major antioxidant compounds in fruit and vegetables are carotenoids, phenolics, and vitamin C (ascorbic acid) (Thaipong et al. 2006). Thus, we quantified these three compounds in the seedlings. For carotenoids quantification, dry plant samples (50 mg) were extracted in 8 mL of 80% acetone for 48 h at 4 °C. After being centrifuged and filtered, the absorbance of the solution was measured. Carotenoids content was calculated according to the equations (Lichtenthaler 1987).
The phenolics were measured according to the method (Erkan et al. 2008) with some modifications. Dry plant samples (100 mg) were extracted in 2.5 mL of 80% acetone for 48 h at 4 °C. After being filtered, 100 μL of the solution was mixed with 2.5 mL of 10% Folin-Ciocalteu reagent. After 5 min, 2.5 mL of 60 mg/mL aqueous Na2CO3 solution was added. The mixture was kept for 2 h at room temperature in darkness, and the absorbance of the solution measured at 725 nm. The results were expressed as gallic acid equivalents.
The ascorbic acid was measured according to a method by Bajaj and Kaur (1981) with some modifications. Dry plant samples (200 mg) were extracted in 7.5 mL of oxalic acid-EDTA solution overnight at room temperature. The extract was filtered, and 5.0 mL of solution mixed with 0.5 mL of metaphosphoric acid-acetic acid solution, 1.0 mL of 5% sulfuric acid solution and 2 mL of 5% ammonium molybdate solution. After 15 min, the (blue) solution was filtered again and absorbance measured at 760 nm.
Statistical Analysis
Seedling cultivation was replicated independently at least three times. Over 15 seedlings were used for length measurements. Ten samples in both growth conditions were used for other measurements. All data were represented as means ± standard error and the difference between control and CL samples was tested by Student t test. Significant difference was accepted at p < 0.05.
Results and Discussion
Growth of Seedlings
The seedlings grown with clinorotation had increased shoot and root length (Table 1). These results show clinorotation promotes the elongation of mung bean seedlings in comparison to those grown under normal gravity.
Amylase Activity and Amount of Starch
The α-amylase activity in cotyledons under clinorotation was 42% higher and the amount of starch in CL cotyledons decreased by 23% compared to control seedlings (Table 2). Since starch is an important energy source for growth and development, measurements of starch accumulation and enzyme activity involving starch degradation have been a focus of early microgravity researchers. The lower starch and higher activity of starch degradation enzymes during spaceflight were reported in various plants as described by Kuznetsov et al. (2001) and references therein. There are also reports of starch decrease for plants grown both in space and under clinorotation; pepper leaves during spaceflight (Johnson and Tibbitts 1968), soybean cotyledons during spaceflight and under clinorotation (1 rpm) (Brown and Piastuch 1994; De Micco and Aronne 2008), and cress roots under clinorotation (2 rpm) (Hensel and Sievers 1980). The results obtained for mung bean cotyledons under clinorotation in this study are consistent with the above.
Other studies, though, have found no significant difference in starch accumulation in sweet potato stem during spaceflight (Mortley et al. 2008), or Brassica napus L. cotyledons under clinorotation (1 rpm) (Aarrouf et al. 1999b). These varying results were sometimes reported in microgravity experiments, due to differences in not only biotic factors, such as plant species, cultivar and age, but also abiotic factors, such as hardware and experimental protocols.
Antioxidant Activity and Accumulation of Related Compounds
The antioxidant activity of mung bean seedlings is shown in Table 3. Both DPPH and ABTS assays showed that antioxidant activity in the seedlings grown with clinorotation was significantly higher than that of control seedlings. Additionally, concentration of carotenoids, phenolics and ascorbic acid in the seedlings under clinorotation were significantly increased by 32%, 14% and 40%, respectively (Table 4). Thus, higher antioxidant activity in the CL seedlings is mainly due to enhanced accumulation of carotenoids, phenolics and ascorbic acid. These results indicate that slow-rotating CL is a useful tool to enhance antioxidant activity in mung bean seedlings.
The data obtained in this study shows slow clinorotation positively promotes both growth and antioxidant activity in the early developmental stage of mung bean seedlings. These changes under clinorotation may be due to higher carbohydrate (sugar) availability (energy) derived from starch conversion. In germinating seedlings, sugars transported from cotyledons are necessary for cell proliferation and emergence of organs before starting photosynthesis. The removal of cotyledons blocks this sugar translocation and strongly suppresses plant growth in the early developmental stage (Wheeler 1966). The higher α-amylase activity and lower starch in cotyledons of CL seedlings (Table 2) imply enhanced starch degradation, suggesting higher levels of sugars under clinorotation. Hilaire et al. (1996) previously found an enhanced growth of soybean roots under clinorotation (1 rpm), suggesting that lower starch reserves may be due to enhanced conversion to and translocation of sugars from the cotyledons. Elongation of both shoot and root under clinorotation (Table 1) may be due to higher energy supply via enhanced starch conversion (Fig. 2).
Schematic diagram of growth and antioxidant activity in mung bean seedlings under clinorotation. Clinorotation promotes α-amylase activity and starch degradation, resulting in higher levels of sugars derived from starch conversion. These sugars may act as not only nutrition for elongation of seedlings, but also signal and precursor for the synthesis of antioxidant compounds. As a result, the clinostat seedlings had increased shoot and root length, and enhanced antioxidant activity
Sugars derived from starch conversion may also be involved in enhanced accumulation of carotenoids, phenolics and ascorbic acid under clinorotation. Sugars play important roles in not only nutrition, but also as signal molecules that regulate gene expression and metabolite synthesis. The first step in the carotenoids biosynthetic pathway is the synthesis of phytoene from geranylgeranyl pyrophosphate, which is regulated by phytoene synthase. The expression of this enzyme is the main factor for determining carotenoids accumulation (Zhou et al. 2011). Sucrose has been shown to activate phytoene synthase and result in enhanced carotenoids accumulation (Télef et al. 2006). Phenolics synthesis and related genes can be also stimulated by glucose and sucrose. For instance, these sugars enhance the activity of phenylalanine ammonia lyasein, the key enzyme in phenolics biosynthesis, in several germinating seedlings (Guo et al. 2011; Solfanelli et al. 2006). This phenolics enzyme is involved in the formation of phenylpropanoids, flavonoids, proanthocyanindins, hydroxystilbenes, coumarins and lignins. In addition, Dicko et al. (2006) reported that germinating sorghum with higher amylase activity exhibited increased total phenolics, because of the enhanced activity of phenylalanine ammonia lyasein. This study suggests that higher amylase activity induces accumulation of total phenolics in germinating seedlings. Furthermore, glucose may also contribute to enhanced accumulation of ascorbic acid under clinorotation. Previous biochemical studies have identified the biosynthetic pathways of ascorbic acid (Valpuesta and Botella 2004), and have shown that labelled glucose is converted to ascorbic acid via several intermediates in the pathway (Loewus et al. 1956). Thus, the increased ascorbic acid found in the seedlings under clinorotation in this study may be due to higher precursor levels than in control seedlings. The detailed mechanism of enhanced antioxidant activity and accumulation of related compounds in seedlings under clinorotation is far from complete. However, from the results here we suggest that higher supply of sugars derived from starch conversion promote the synthesis of carotenoids, phenolics and ascorbic acid, resulting in enhanced antioxidant activity under clinorotation (Fig. 2).
Conclusions
In this study, mung bean seedlings cultivated for 4 days under slow clinorotation had DPPH and ABTS assay results that indicate enhanced antioxidant activity in the seedlings, compared to those cultivated under normal gravity. The higher observed α-amylase activity and lower starch levels in the CL seedlings suggest higher supply of sugars derived from starch conversion under clinorotation. These sugars may play significant roles in enhanced antioxidant activity of the seedling under clinorotation.
Abbreviations
- CL:
-
Clinostat
References
Aarrouf, J., Schoëvaërt, D., Maldiney, R., Perbal, G.: Changes in hormonal balance and meristematic activity in primary root tips on the slowly rotating clinostat and their effect on the development of the rapeseed root system. Physiol. Plant. 105, 708–718 (1999a)
Aarrouf, J., Darbelley, N., Demandre, C., Razafindramboa, N., Perbal, G.: Effect of horizontal clinorotation on the root system development and on lipid breakdown in rapeseed (Brassica napus) seedlings. Plant Cell Physiol. 40, 396–405 (1999b)
Bai, Y., Xu, Y., Chang, J., Wang, X., Zhao, Y., Yu, Z.: Bioactives from stems and leaves of mung beans (Vigna radiata L.). J. Funct. Foods. 25, 314–322 (2016)
Bajaj, K.L., Kaur, G.: Spectrophotometric determination of L-ascorbic acid in vegetables and fruits. Analyst. 106, 117–120 (1981)
Brown, C.S., Piastuch, W.C.: Starch metabolism in germinating soybean cotyledons is sensitive to clinorotation and centrifugation. Plant Cell Environ. 17, 341–344 (1994)
Brungs, S., Egli, M., Wuest, S.L., Christianen, P.C.M., van Loon, J.J.W.A., Anh, T.J.N., Hemmersbach, R.: Facilities for simulation of microgravity in the ESA ground-based facility programme. Microgravity Sci. Technol. 28, 191–203 (2016)
De Micco, V., Aronne, G.: Biometric anatomy of seedlings developed onboard of Foton-M2 in an automatic system supporting growth. Acta Astronaut. 62, 505–513 (2008)
Dicko, M.H., Gruppen, H., Zouzouho, O.C., Traoré, A.S., van Berkel, W.J.H., Voragen, A.G.J.: Effects of germination on the activities of amylases and phenolic enzymes in sorghum varieties grouped according to food end-use properties. J. Sci. Food Agric. 86, 953–963 (2006)
Erkan, N., Ayranci, G., Ayranci, E.: Antioxidant activities of rosemary (Rosmarinus Officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem. 110, 76–82 (2008)
Guo, R., Yuan, G., Wang, Q.: Sucrose enhances the accumulation of anthocyanins and glucosinolates in broccoli sprouts. Food Chem. 129, 1080–1087 (2011)
Hensel, W., Sievers, A.: Effects of prolonged omnilateral gravistimulation on the ultrastructure of statocytes and on the graviresponse of roots. Planta. 150, 338–346 (1980)
Herranz, R., Anken, R., Boonstra, J., Braun, M., Christianen, P.C.M., de Geest, M., Hauslage, J., Hilbig, R., Hill, R.J.A., Lebert, M., Medina, F.J., Vagt, N., Ullrich, O., van Loon, J.W.A., Hemmersbach, R.: Ground-based facilities for simulation of microgravity: organism-specific recommendations for their use, and recommended terminology. Astrobiology. 13, 1–17 (2013)
Hilaire, E., Peterson, B.V., Guikema, J.A., Brown, C.S.: Clinorotation affects morphology and ethylene production in soybean seedlings. Plant Cell Physiol. 37, 929–934 (1996)
Horn, A., Ullrich, O., Huber, K., Hemmersbach, R.: PMT (Photomultiplier) clinostat. Microgravity Sci. Technol. 23, 67–71 (2011)
Johnson, S.P., Tibbitts, T.W.: The liminal angle of a plagiogeotropic organ under weightlessness. Bioscience. 18, 655–661 (1968)
Krause, L., Braun, M., Hauslage, J., Hemmersbach, R.: Analysis of statoliths displacement in Chara rhizoids for validating the microgravity-simulation quality of clinorotation modes. Microgravity Sci. Technol. 30, 229–236 (2018)
Kuznetsov, O.A., Brown, C.S., Levine, H.G., Piastuch, W.C., Sanwo-Lewandowski, M.M., Hasenstein, K.H.: Composition and physical properties of starch in microgravity-grown plants. Adv. Space Res. 28, 651–658 (2001)
Larson, R.A.: The antioxidants of higher plants. Phytochem. 27, 969–978 (1988)
Lichtenthaler, H.: K.: chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 148, 350–382 (1987)
Loewus, F.A., Jang, R., Seegmiller, C.G.: The conversion of C14-labeled sugars to L-ascorbic acid in ripening strawberries. J. Biol. Chem. 222, 649–664 (1956)
Luthria, D.L., Mukhopadhyay, S., Krizek, D.T.: Content of total phenolics and phenolic acids in tomato (Lycopersicon esculentum mill.) fruits as influenced by cultivar and solar UV radiation. J. Food Compost. Anal. 19, 771–777 (2006)
Mortley, D.G., Bonsi, C.K., Hill, W.A., Morris, C.E., Williams, C.S., Davis, C.F., Williams, J.W., Levine, L.H., Petersen, B.V., Wheeler, R.M.: Influence of microgravity environment on root growth, soluble sugars, and starch concentration of sweetpotato stem cuttings. J. Am. Soc. Hortic. Sci. 133, 327–332 (2008)
Musgrave, M.E., Kuang, A., Tuominen, L.K., Levine, L.H., Morrow, R.C.: Seed storage reserves and glucosinolates in Brassica rapa L. grown on the international space station. J. Am. Soc. Hortic. Sci. 130, 848–856 (2005)
Nakajima, S., Shiraga, K., Suzuki, T., Kondo, N., Ogawa, Y.: Chlorophyll, carotenoid and anthocyanin accumulation in mung bean seedling under clinorotation. Microgravity Sci. Technol. 29, 427–432 (2017)
Paśko, P., Bartoń, H., Zagrodzki, P., Gorinstein, S., Fołta, M., Zachwieja, Z.: Anthocyanins, total polyphenols and antioxidant activity in amaranth and quinoa seeds and sprouts during their growth. Food Chem. 115, 994–998 (2009)
Smith, A.M., Zeeman, S.C.: Quantification of starch in plant tissues. Nat. Protoc. 1, 1342–1345 (2006)
Solfanelli, C., Poggi, A., Loreti, E., Alpi, A., Perata, P.: Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol. 140, 637–646 (2006)
Swain, R.R., Dekker, E.E.: Seed germination studies II. Pathways for starch degradation in germinating pea seedlings. Biochim. Biophys. Acta. 122, 87–100 (1966)
Télef, N., Stammitti-Bert, L., Mortain-Bertrand, A., Maucourt, M., Carde, J.P., Dominique, R., Gallusci, P.: Sucrose deficiency delays lycopene accumulation in tomato fruit pericarp discs. Plant Mol. Biol. 62(3), 453–469 (2006)
Thaipong, K., Boonprakob, U., Crosby, K., Cisneros-Zevallos, L., Hawkins, B.D.: Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compost. Anal. 19, 669–675 (2006)
Tripathi, P., Leggio, L.L., Mansfeld, J., Ulbrich-Hofmann, R., Kayastha, A.M.: α-Amylase from mung beans (Vigna radiata)-correlation of biochemical properties and tertiary structure by homology modelling. Phytochem. 68, 1623–1631 (2007)
Valpuesta, V., Botella, M.A.: Biosynthesis of L-ascorbic acid in plants: new pathways for an old antioxidant. Trends Plant Sci. 9, 573–577 (2004)
Wang, S.Y., Zheng, W.: Effect of plant growth temperature on antioxidant capacity in strawberry. J.Agric. Food Chem. 49, 4977–4982 (2001)
Wang, S.Y., Zheng, W., Galletta, G.J.: Cultural sysyem affects fruit quality and antioxidant capacity in strawberries. J.Agric. Food Chem. 50, 6534–6542 (2002)
Wheeler, A.W.: Effect of removing cotyledons, apical growing region, or trifoliate leaves on the growth and growth-substance content of dwarf French bean (Phaseolus vulgaris). J. Exp. Bot. 17, 621–626 (1966)
Xu, B.J., Chang, S.K.C.: A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 72, 159–166 (2007)
Zheng, H.Q., Han, F., Le, J.: Higher plants in space: microgravity perception, response, and adaptation. Microgravity Sci. Technol. 27, 377–386 (2015)
Zhou, X., Mcquinn, R., Fei, Z., Wolters, A.M.A., van Eck, J., Brown, C., Giovannoni, J.J., Li, L.: Regulatory control of high levels of carotenoid accumulation in potato tubers. Plant Cell Environ. 34, 1020–1030 (2011)
Acknowledgements
The authors thank Prof. Garry John Piller (Kyoto University) for reading this report and helpful suggestions. This work was supported by JSPS KAKENHI grant number 16H05010 and 17 J08363.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nakajima, S., Ogawa, Y., Suzuki, T. et al. Enhanced Antioxidant Activity in Mung Bean Seedlings Grown under Slow Clinorotation. Microgravity Sci. Technol. 31, 395–401 (2019). https://doi.org/10.1007/s12217-019-9699-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12217-019-9699-9